Abstract
1. The intracellularly recorded responses of goldfish Mauthner neurones to iontophoretically applied pulses of amino acids have been analysed: their time courses have been compared with each other, and with those predicted from diffusion theory.
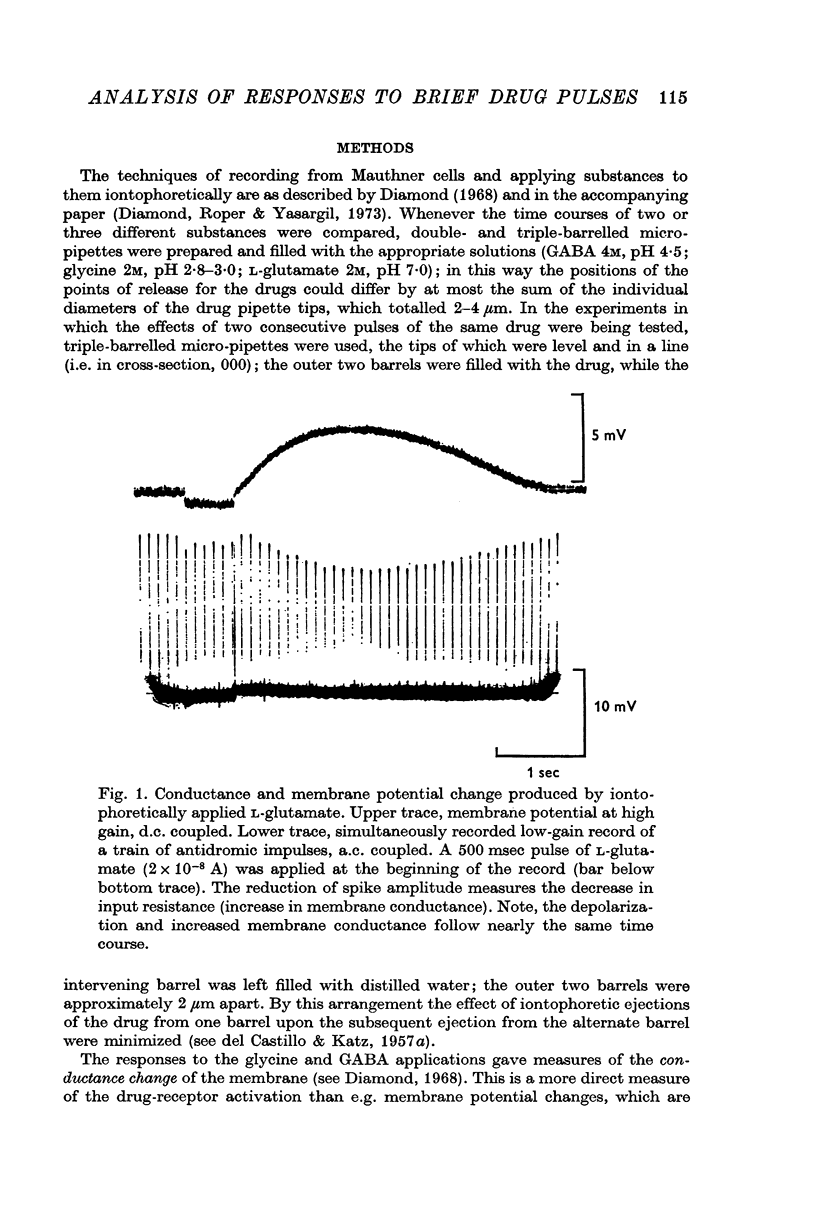
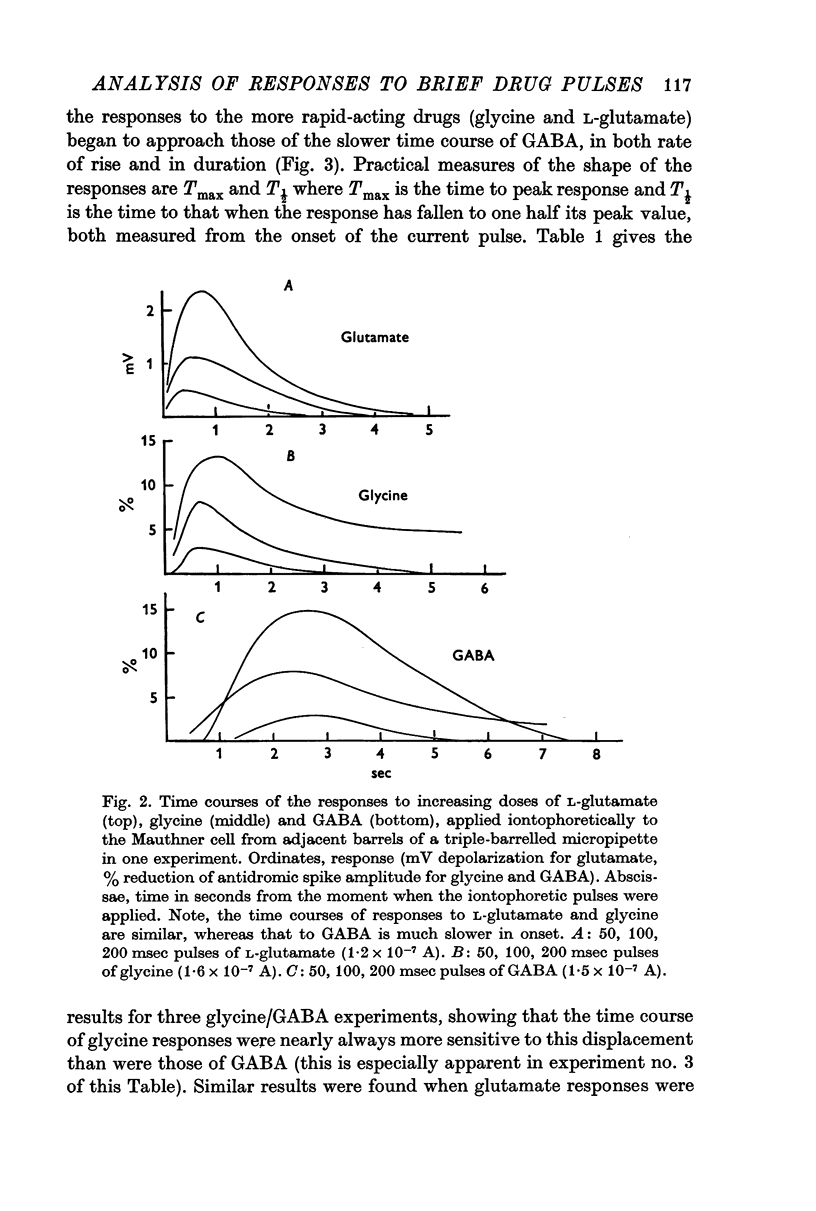
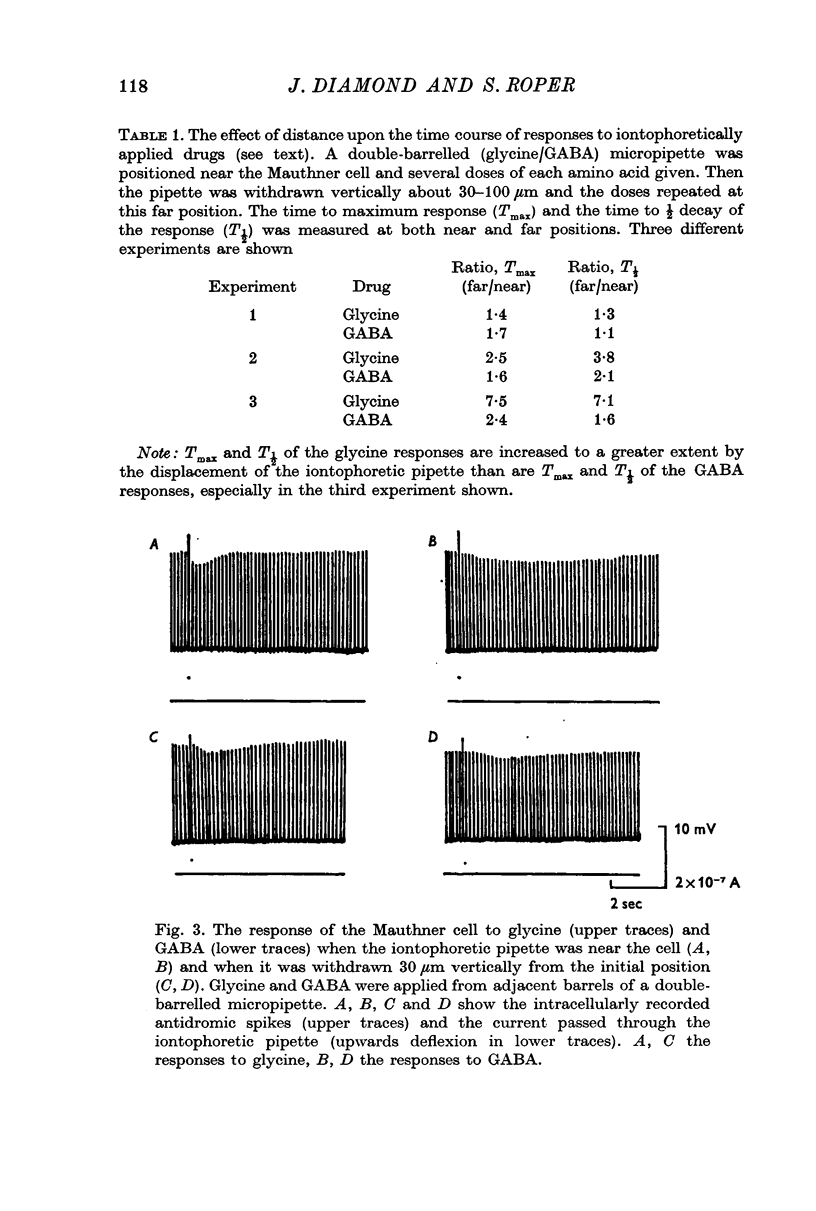
2. The rise time of the response to GABA is slower than that to glycine or L-glutamate. The response curves of the latter substances were very similar, and unlike that of GABA were markedly affected by increasing the distance of pipette-tip from the membrane. The results suggest that the time course of the responses to glycine and L-glutamate are determined mainly by free diffusion in the brain tissue (at least within about 200 μm of the cell), while that to GABA must be rate-limited by other factors, e.g. drug-receptor activation time.
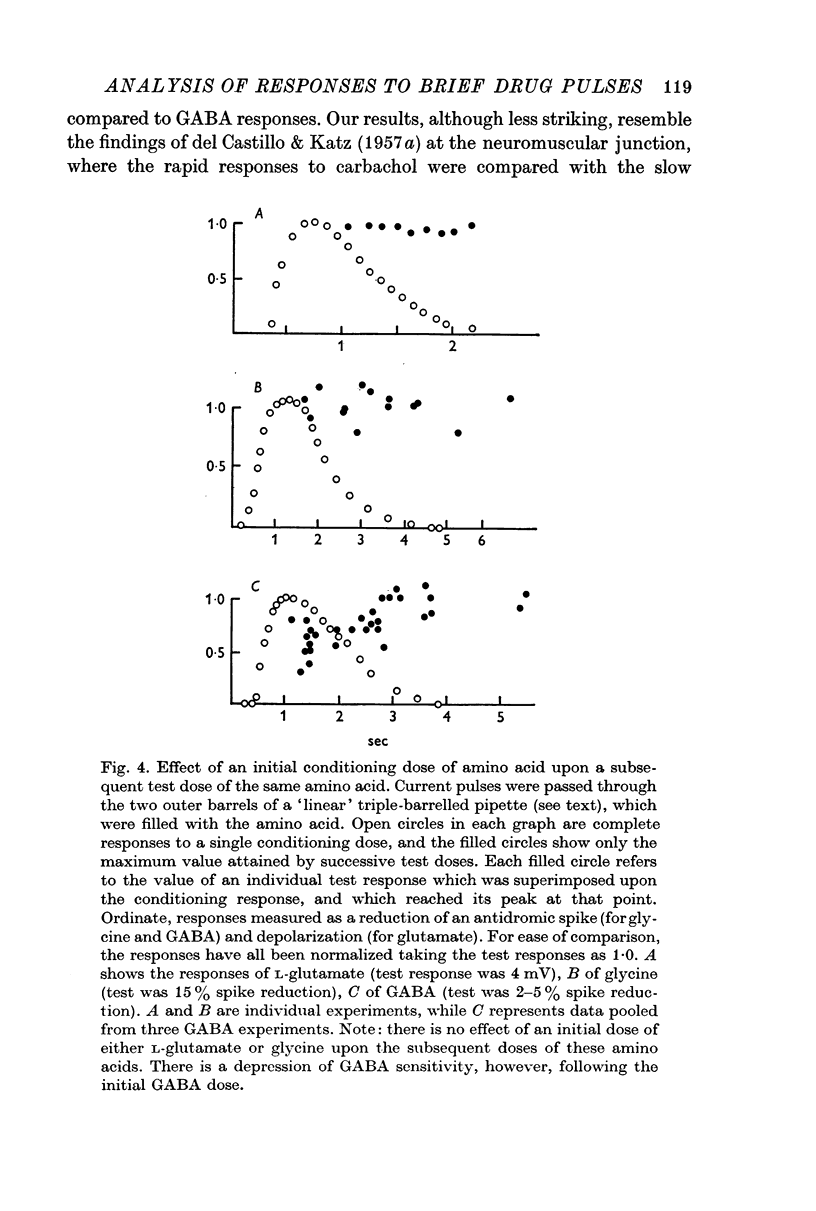
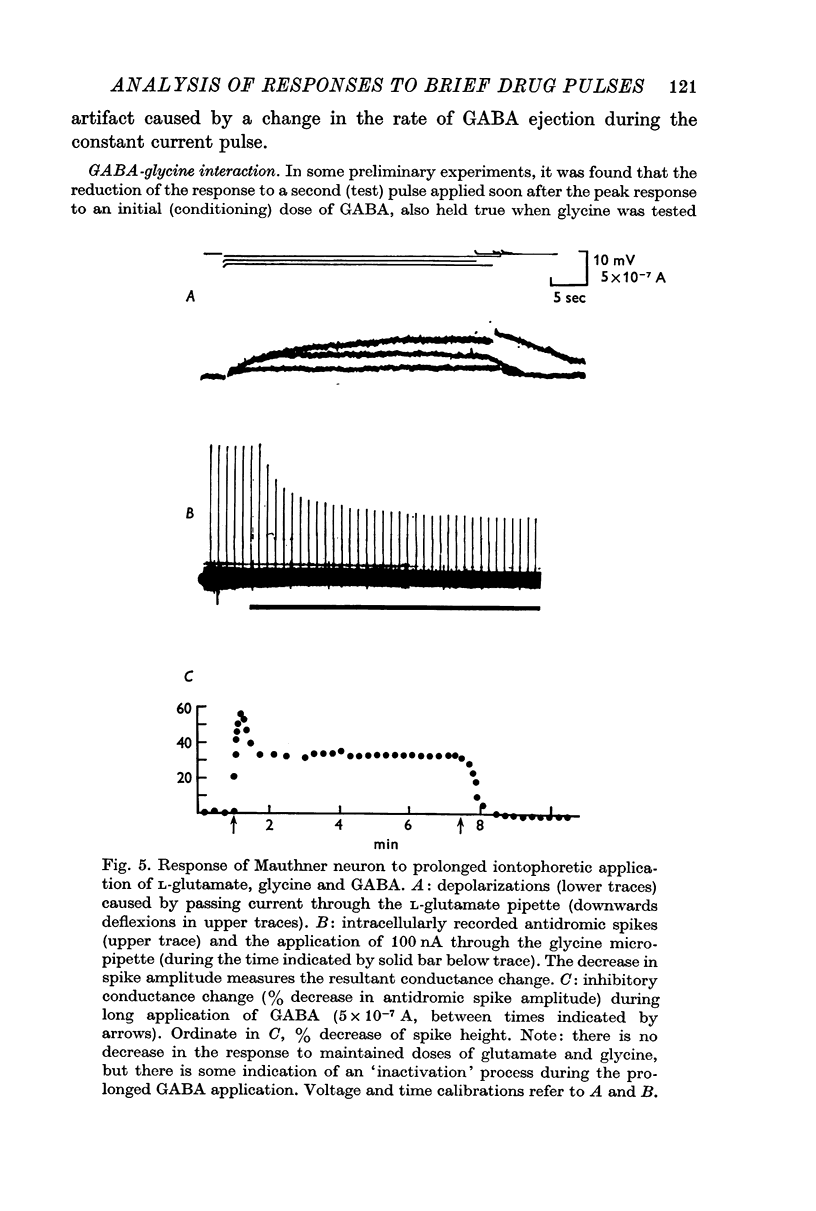
3. The possibility that the responses are influenced by some desensitizing process was investigated by applying a second (test) drug pulse during the response to a prior conditioning one. In the case of glycine and of L-glutamate there was no attenuation of the response to a second pulse at any time. With GABA, however, the second response was reduced during the period of the conditioning response; the reduction was progressively less marked the later the test pulse occurred. A similar effect with GABA was seen when glycine was used as the test pulse. The responses to long-maintained drug pulses also indicated that for GABA, but not for glycine or glutamate, there seems to be some desensitizing process present.
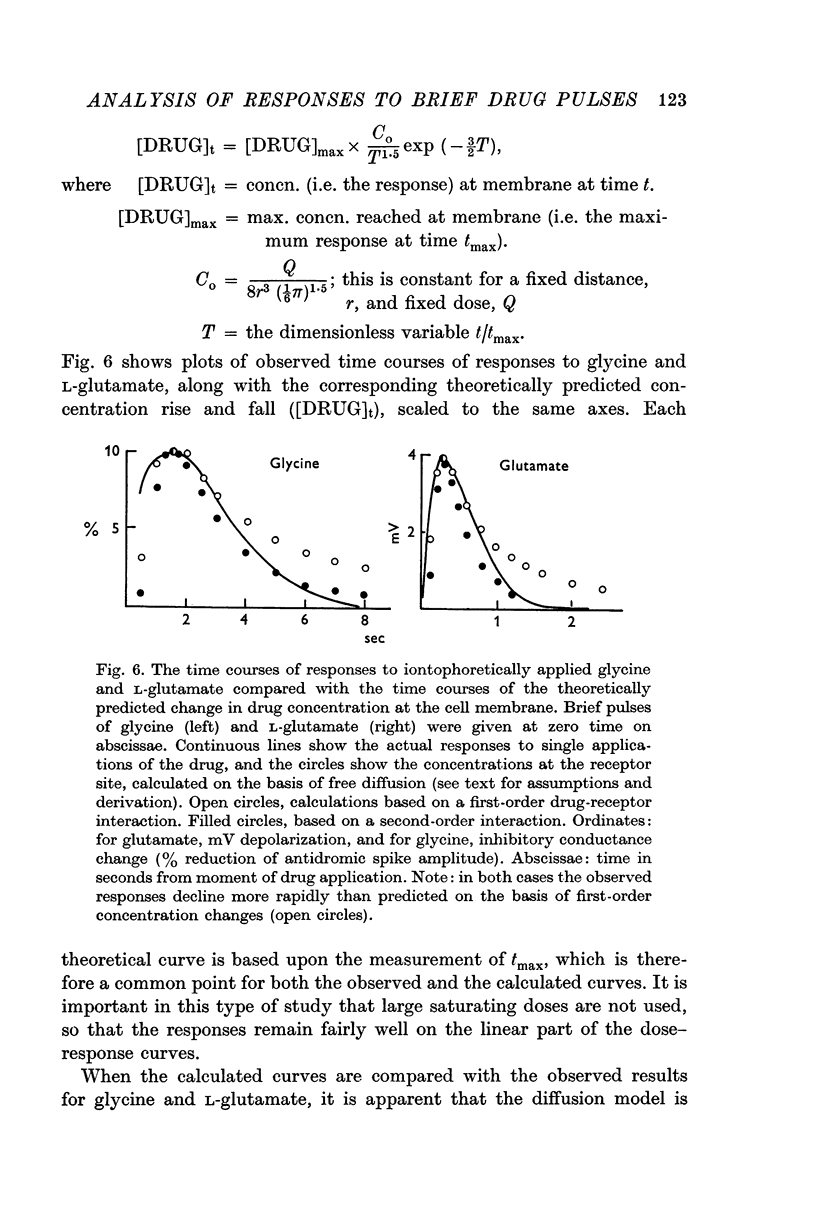
4. Calculated time courses of responses to brief pulses of glycine and of L-glutamate (based upon diffusion theory) differed somewhat from the observed curves, largely during the falling phase. However, when the calculations were based upon second-order reactions (two molecules of drug per receptor) the diffusion model gave results very like the observed ones.
5. Possible implications of these results for the role these three amino acids may have as neuro-transmitters are mentioned.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtis D. R., Hösli L., Johnston G. A., Johnston I. H. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res. 1968;5(3):235–258. doi: 10.1007/BF00238666. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. A comparison of acetylcholine and stable depolarizing agents. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):362–368. doi: 10.1098/rspb.1957.0017. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The identity of intrinsic and extrinsic acetylcholine receptors in the motor end-plate. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):357–361. doi: 10.1098/rspb.1957.0016. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO L., KATZ B. A study of curare action with an electrical micromethod. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):339–356. doi: 10.1098/rspb.1957.0015. [DOI] [PubMed] [Google Scholar]
- Diamond J., Huxley A. F. The activation and distribution of GABA and L-glutamate receptors on goldfish Mauthner neurones: an analysis of dendritic remote inhibition. J Physiol. 1968 Feb;194(3):669–723. doi: 10.1113/jphysiol.1968.sp008432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J., Roper S., Yasargil G. M. The membrane effects, and sensitivity to strychnine, of neural inhibition of the Mauthner cell, and its inhibition by glycine and GABA. J Physiol. 1973 Jul;232(1):87–111. doi: 10.1113/jphysiol.1973.sp010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S., Krnjević K. Cortical inhibition and gamma-aminobutyric acid. Exp Brain Res. 1969;9(2):137–154. doi: 10.1007/BF00238327. [DOI] [PubMed] [Google Scholar]
- FATT P., KATZ B. The effect of inhibitory nerve impulses on a crustacean muscle fibre. J Physiol. 1953 Aug;121(2):374–389. doi: 10.1113/jphysiol.1953.sp004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUKAWA T., FURSHPAN E. J. Two inhibitory mechanisms in the Mauthner neurons of goldfish. J Neurophysiol. 1963 Jan;26:140–176. doi: 10.1152/jn.1963.26.1.140. [DOI] [PubMed] [Google Scholar]
- Feltz A., Mallart A. An analysis of acetylcholine responses of junctional and extrajunctional receptors of frog muscle fibres. J Physiol. 1971 Oct;218(1):85–100. doi: 10.1113/jphysiol.1971.sp009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Kuffler S. W., Dennis M. J. Differential chemosensitivity of synaptic and extrasynaptic areas on the neuronal surface membrane in parasympathetic neurons of the frog, tested by microapplication of acetylcholine. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):541–553. doi: 10.1098/rspb.1971.0046. [DOI] [PubMed] [Google Scholar]
- Herz A., Zieglgänsberger W., Färber G. Microelectrophoretic studies concerning the spread of glutamic acid and GABA in brain tissue. Exp Brain Res. 1969;9(3):221–235. doi: 10.1007/BF00234456. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Neal M. J. The uptake of [3H]GABA by slices of rat cerebral cortex. J Neurochem. 1968 Oct;15(10):1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. The interaction between edrophonium (tensilon) and acetylcholine at the motor end-plate. Br J Pharmacol Chemother. 1957 Jun;12(2):260–264. doi: 10.1111/j.1476-5381.1957.tb00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R., Wickelgren W. O., Ber1anek R. Effects of iontophoretically applied drugs on spinal interneurons of the lamprey. J Physiol. 1970 May;207(3):653–665. doi: 10.1113/jphysiol.1970.sp009086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani E., Gerschenfeld H. M. Comparative study of acetylcholine and 5-hydroxytryptamine receptors on single snail neurons. J Neurophysiol. 1969 Jan;32(1):64–74. doi: 10.1152/jn.1969.32.1.64. [DOI] [PubMed] [Google Scholar]
- Waud D. R. On diffusion from a point source. J Pharmacol Exp Ther. 1968 Jan;159(1):123–128. [PubMed] [Google Scholar]
- Waud D. R. The rate of action of competitive neuromuscular blocking agents. J Pharmacol Exp Ther. 1967 Oct;158(1):99–114. [PubMed] [Google Scholar]
- Werman R. An electrophysiological approach to drug-receptor mechanisms. Comp Biochem Physiol. 1969 Sep 15;30(6):997–1017. doi: 10.1016/0010-406x(69)91038-x. [DOI] [PubMed] [Google Scholar]


