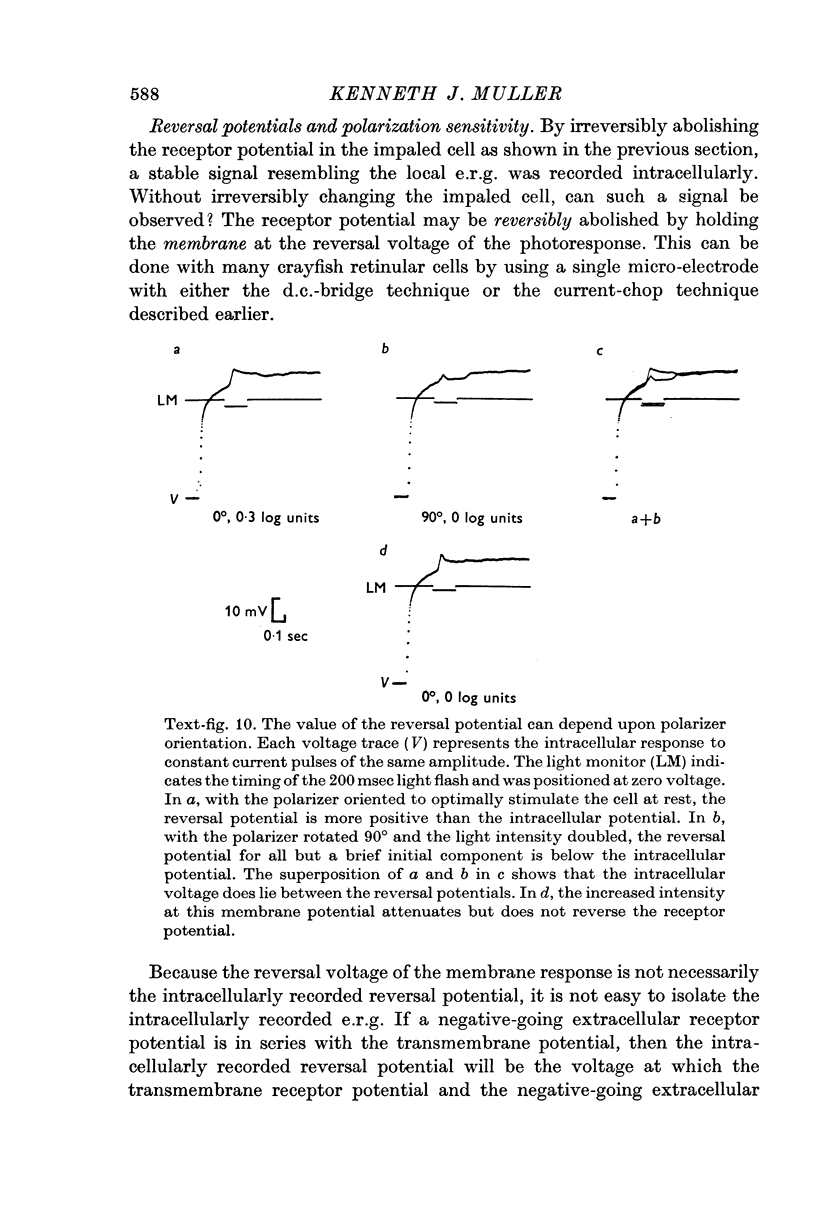
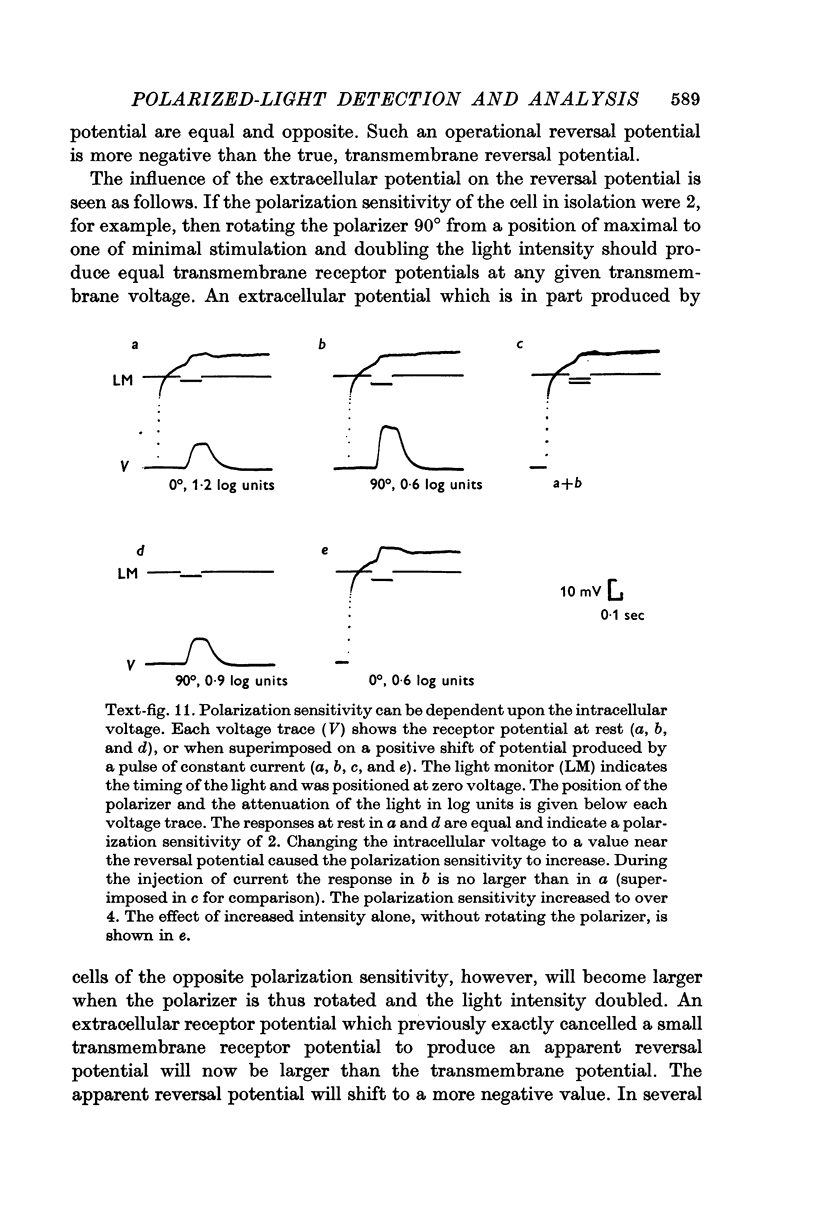
Abstract
1. The sensitivity to plane-polarized light and the electrical interactions of photoreceptors were examined with intracellular and extracellular micro-electrodes in excised compound eyes of the crayfish.
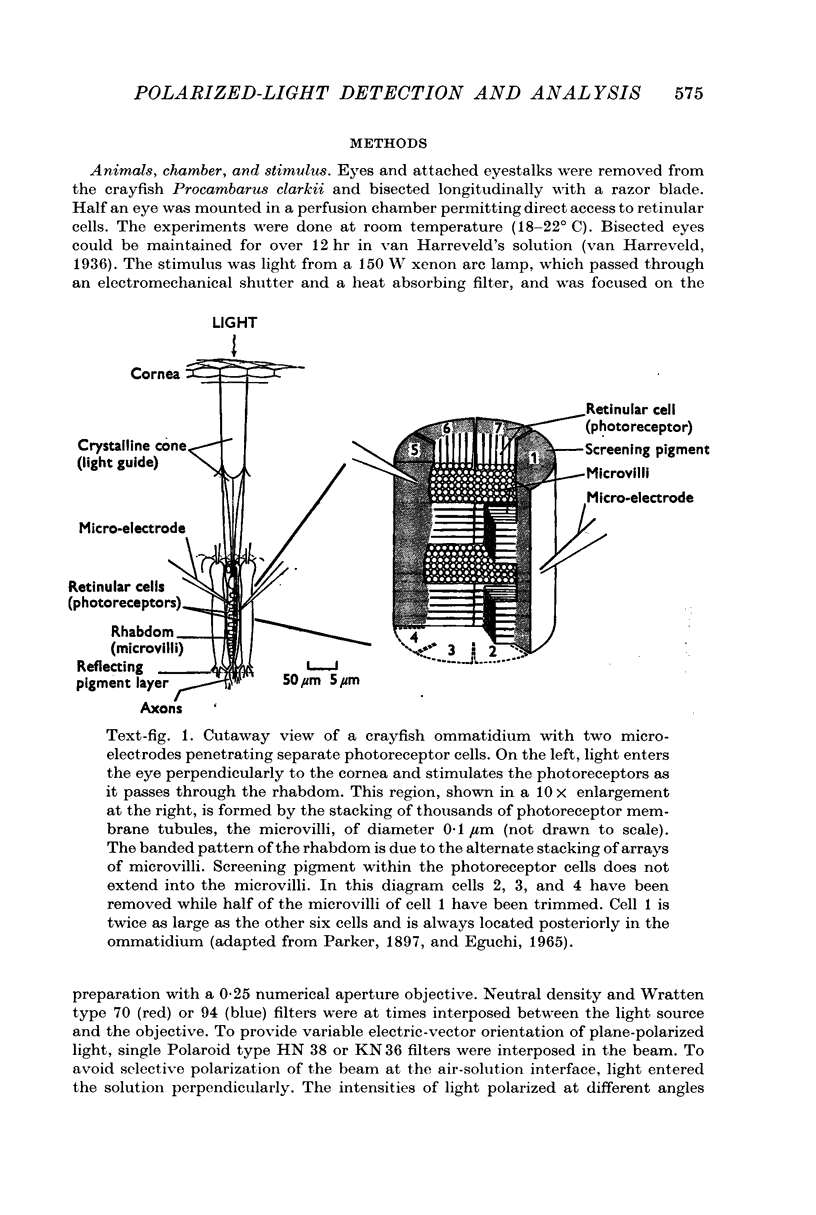
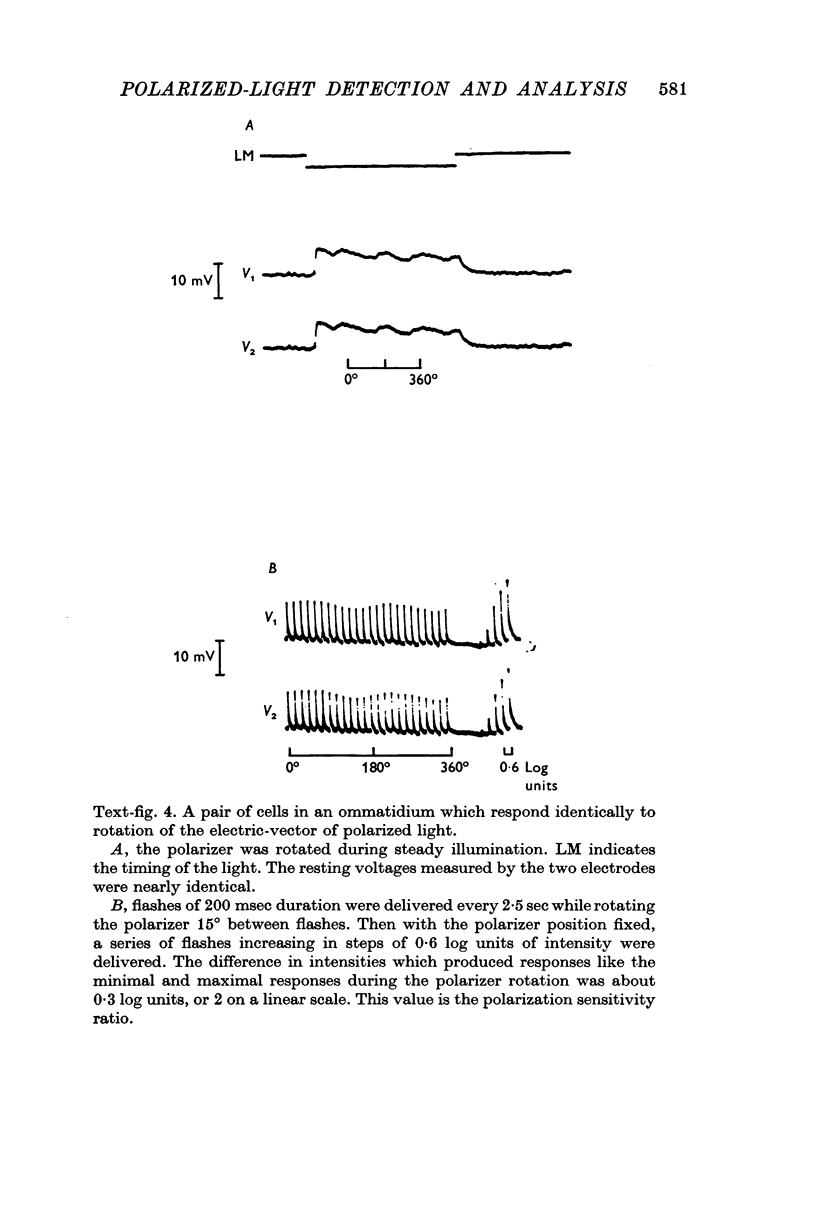
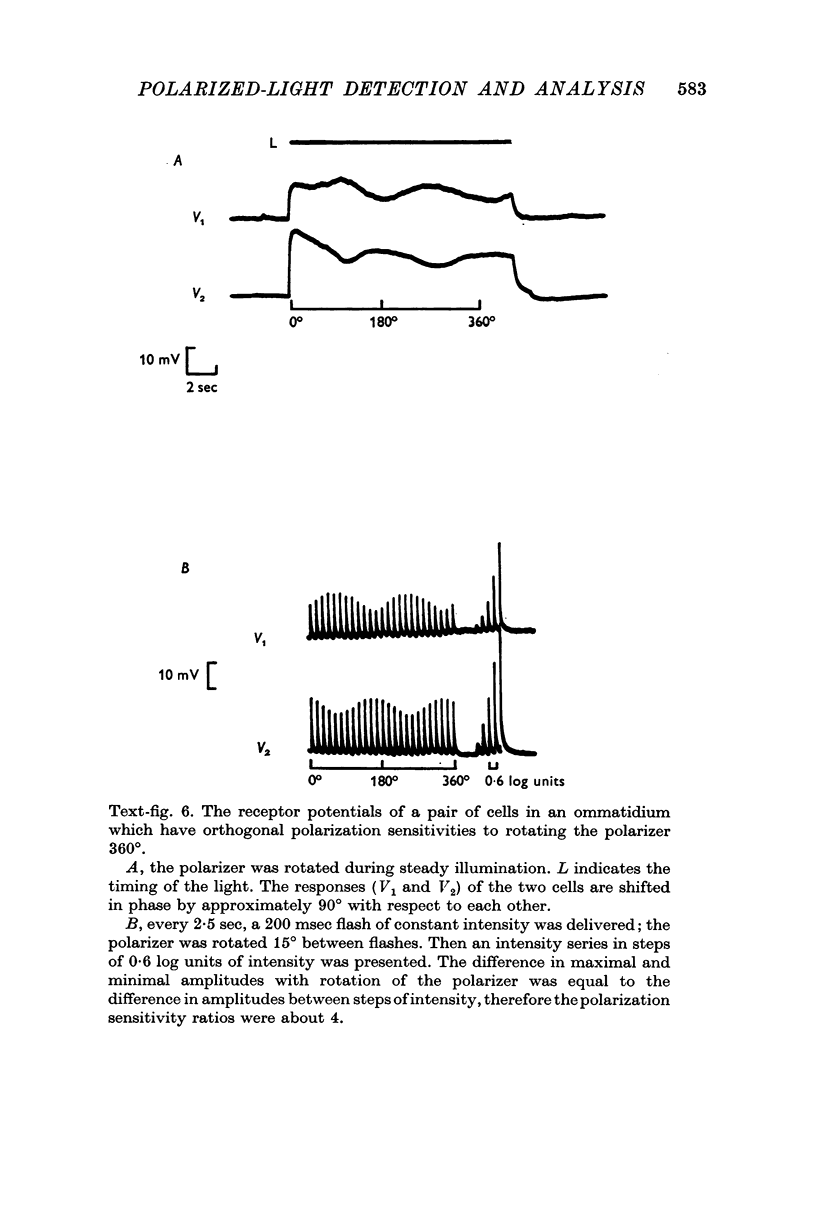
2. There are two types of photoreceptor: each photoreceptor cell responds best to polarized light when the electric-vector of the light is oriented in one of two orthogonal directions. Seven cells, representing each type, are grouped together to form ommatidia.
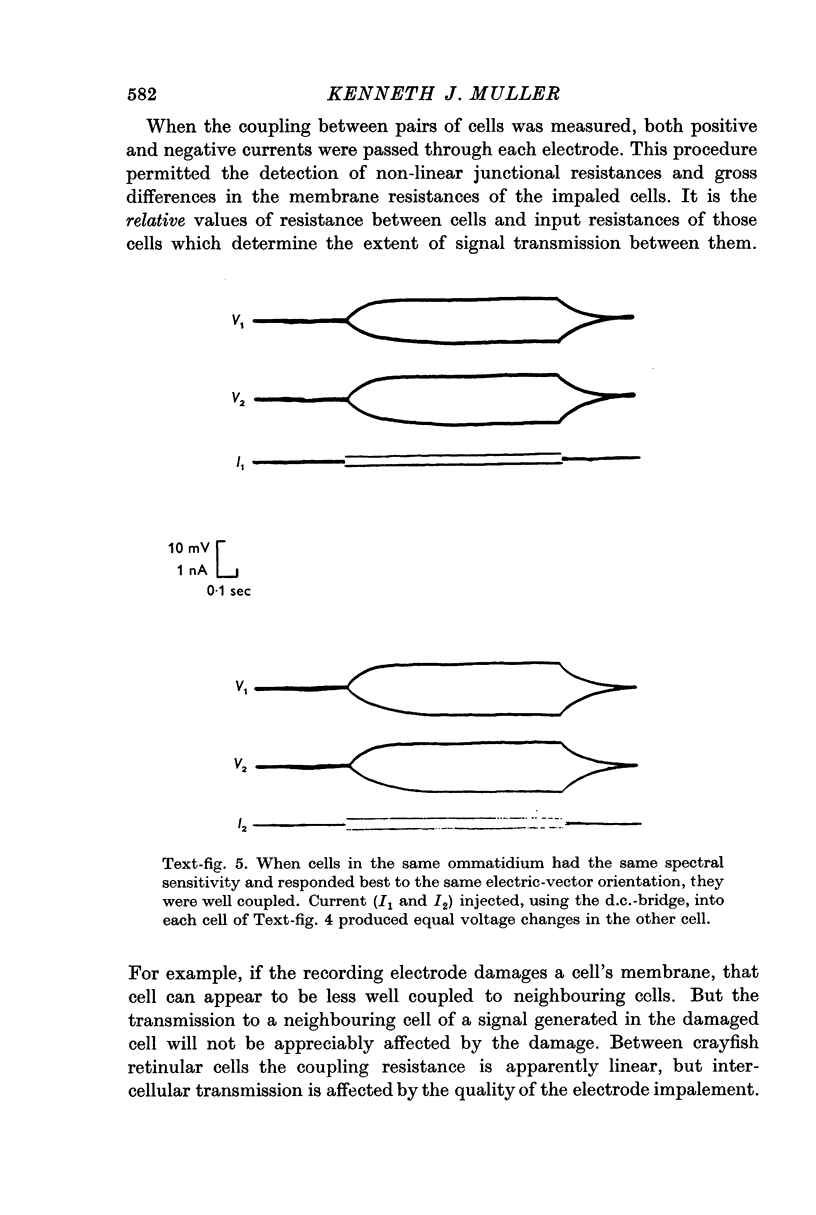
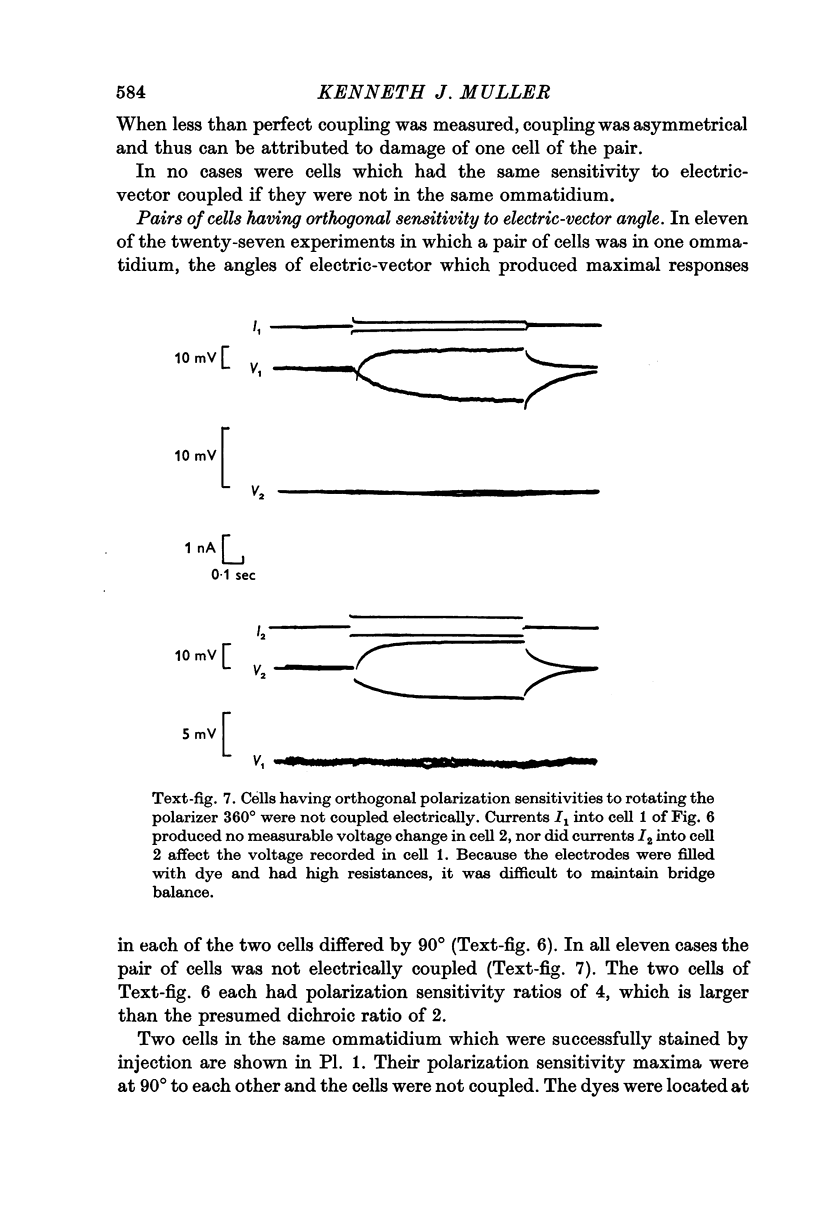
3. In each ommatidium, cells that are sensitive to the same orientation of the electric-vector of polarized light are coupled electrically. Cells having orthogonal polarized-light sensitivities are not coupled.
4. Nearly all cells studied were sensitive to orange light. A few cells of both types were found that were sensitive to blue light. Blue-sensitive cells were not coupled to orange-sensitive cells.
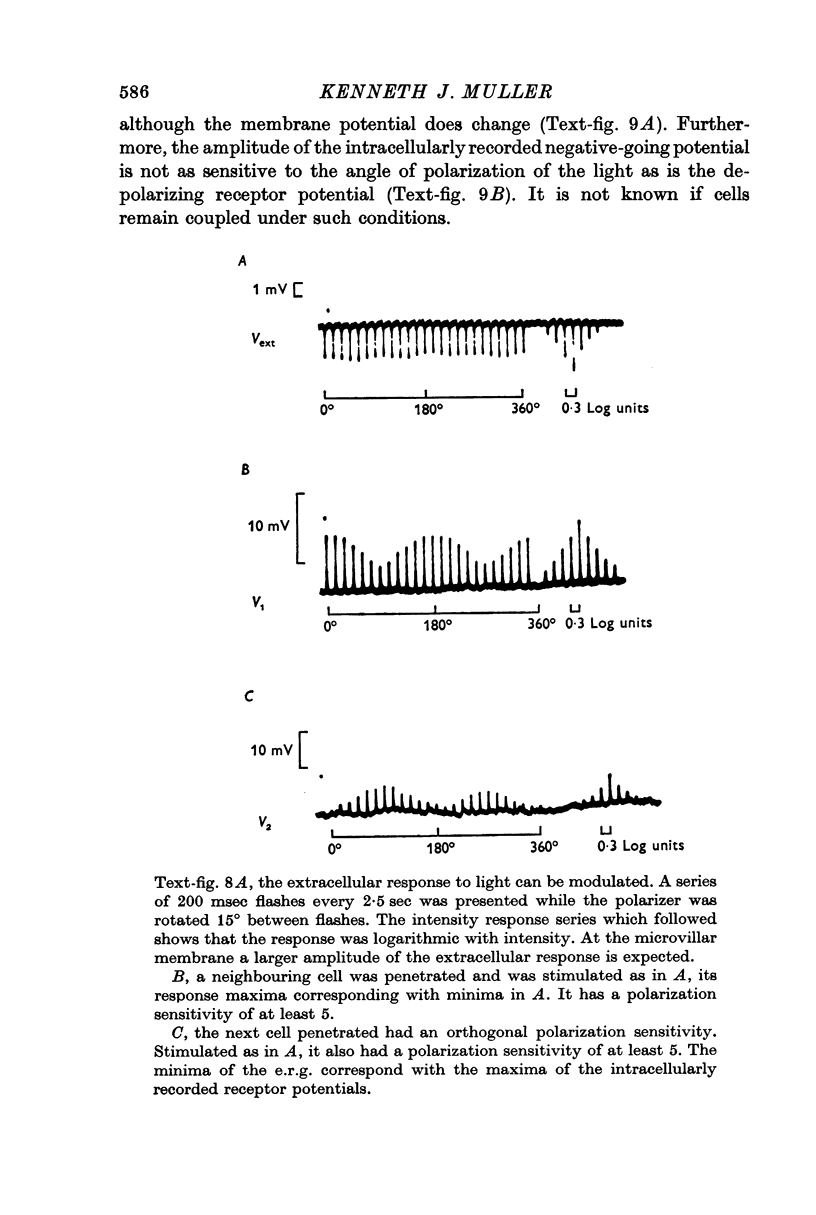
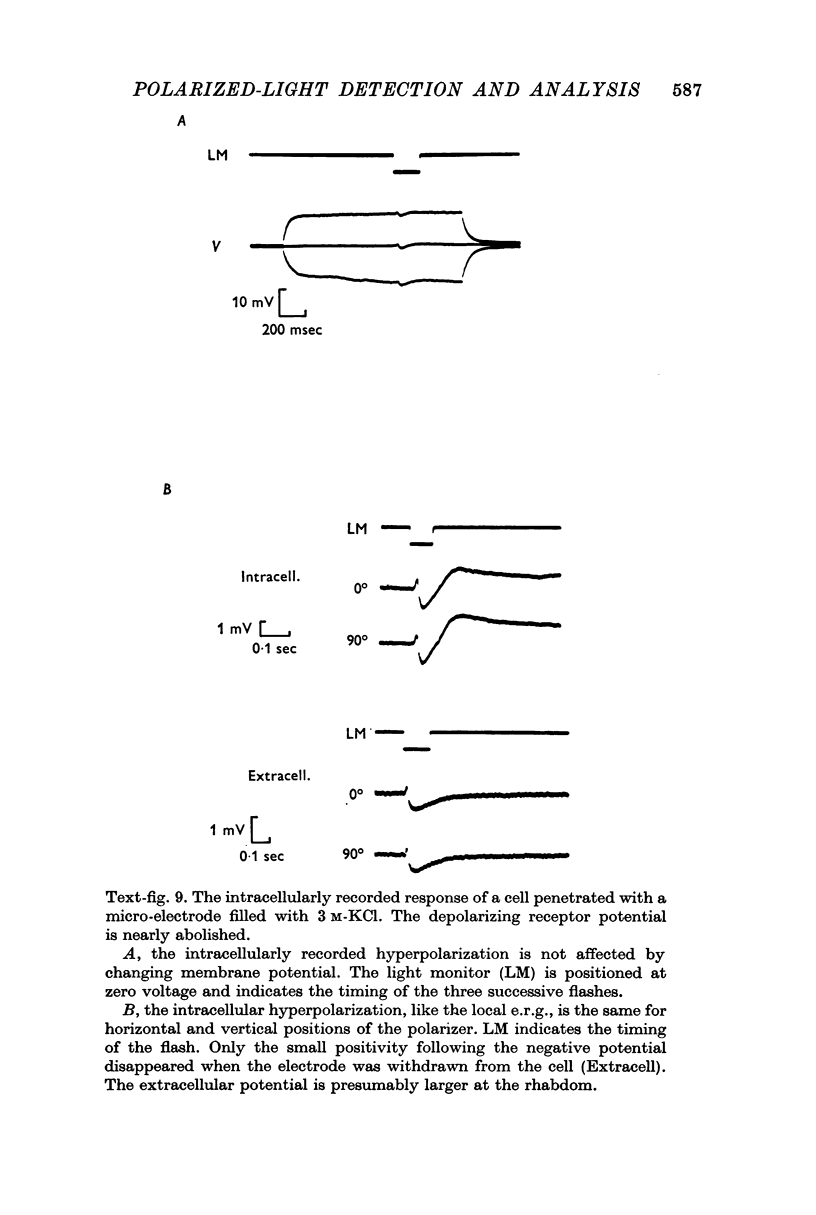
5. The photocurrents of both cell types produce negative extracellular potentials which can be greater than 10 mV when measured near the photoreceptive membranes within ommatidia. Evidence suggests that the extracellular potentials produced by one type of cell can effectively reduce the receptor potentials recorded in the other cell type. It is proposed that such a mutual non-synaptic interaction can make a cell more sensitive to the orientation of polarized-light than would be predicted from the cell's differential absorption of polarized light (i.e. its dichroic ratio).
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benolken R. M. Regenerative transducing properties of a graded visual response. Cold Spring Harb Symp Quant Biol. 1965;30:445–450. doi: 10.1101/sqb.1965.030.01.043. [DOI] [PubMed] [Google Scholar]
- Eguchi E. Rhabdom structure and receptor potentials in single crayfish retinular cells. J Cell Physiol. 1965 Dec;66(3):411–429. doi: 10.1002/jcp.1030660314. [DOI] [PubMed] [Google Scholar]
- FUORTES M. G., POGGIO G. F. Transient responses to sudden illumination in cells of the eye of Limulus. J Gen Physiol. 1963 Jan;46:435–452. doi: 10.1085/jgp.46.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenbach W. H. The morphology of the eyes of Limulus. II. Ommatidia of the compound eye. Z Zellforsch Mikrosk Anat. 1969;93(4):451–483. doi: 10.1007/BF00338531. [DOI] [PubMed] [Google Scholar]
- Glantz R. M. Light adaptation in the photoreceptor of the crayfish, Procambarus clarki. Vision Res. 1968 Nov;8(11):1407–1421. doi: 10.1016/0042-6989(68)90087-4. [DOI] [PubMed] [Google Scholar]
- KUWABARA M., NAKA K. Response of a single retinula cell to polarized light. Nature. 1959 Aug 8;184(Suppl 7):455–456. doi: 10.1038/184455a0. [DOI] [PubMed] [Google Scholar]
- Lasansky A. Cell junctions in ommatidia of Limulus. J Cell Biol. 1967 May;33(2):365–383. doi: 10.1083/jcb.33.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKA K. I., EGUCHI E. Spike potentials recorded from the insect photoreceptor. J Gen Physiol. 1962 Mar;45:663–680. doi: 10.1085/jgp.45.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S. R. Interreceptor coupling in ommatidia of drone honeybee and locust compound eyes. Vision Res. 1969 Sep;9(9):999–1029. doi: 10.1016/0042-6989(69)90044-3. [DOI] [PubMed] [Google Scholar]
- Shaw S. R. Optics of arthropod compound eye. Science. 1968 Jul 4;165(3888):88–90. [PubMed] [Google Scholar]
- Shaw S. R. Polarized light responses from crab retinula cells. Nature. 1966 Jul 2;211(5044):92–93. doi: 10.1038/211092a0. [DOI] [PubMed] [Google Scholar]
- Shaw S. R. Sense-cell structure and interspecies comparisons of polarized-light absorption in arthropod compound eyes. Vision Res. 1969 Sep;9(9):1031–1040. doi: 10.1016/0042-6989(69)90045-5. [DOI] [PubMed] [Google Scholar]
- Stretton A. O., Kravitz E. A. Neuronal geometry: determination with a technique of intracellular dye injection. Science. 1968 Oct 4;162(3849):132–134. doi: 10.1126/science.162.3849.132. [DOI] [PubMed] [Google Scholar]
- Varela F. G., Wiitanen W. The optics of the compound eye of the honeybee (Apis mellifera). J Gen Physiol. 1970 Mar;55(3):336–358. doi: 10.1085/jgp.55.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman T. H., Fernández H. R., Goldsmith T. H. Dichroism of photosensitive pigment in rhabdoms of the crayfish Orconectes. J Gen Physiol. 1969 Sep;54(3):415–432. doi: 10.1085/jgp.54.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- v FRISCH K. Die Sonne als Kompass im Leben der Bienen. Experientia. 1950 Jun 15;6(6):210–221. doi: 10.1007/BF02173654. [DOI] [PubMed] [Google Scholar]