Abstract
1. The experiments correlate certain changes in the ultrastructure of cat hypogastric nerves constricted at two points with the distribution of a mitochondrial enzyme (cytochrome oxidase), noradrenaline (stored in some of the vesicles with an electron dense core, i.e. granular vesicles) and adenosine triphosphate (ATP) (present in noradrenaline storage granules, mitochondria and the soluble fraction of the axon).
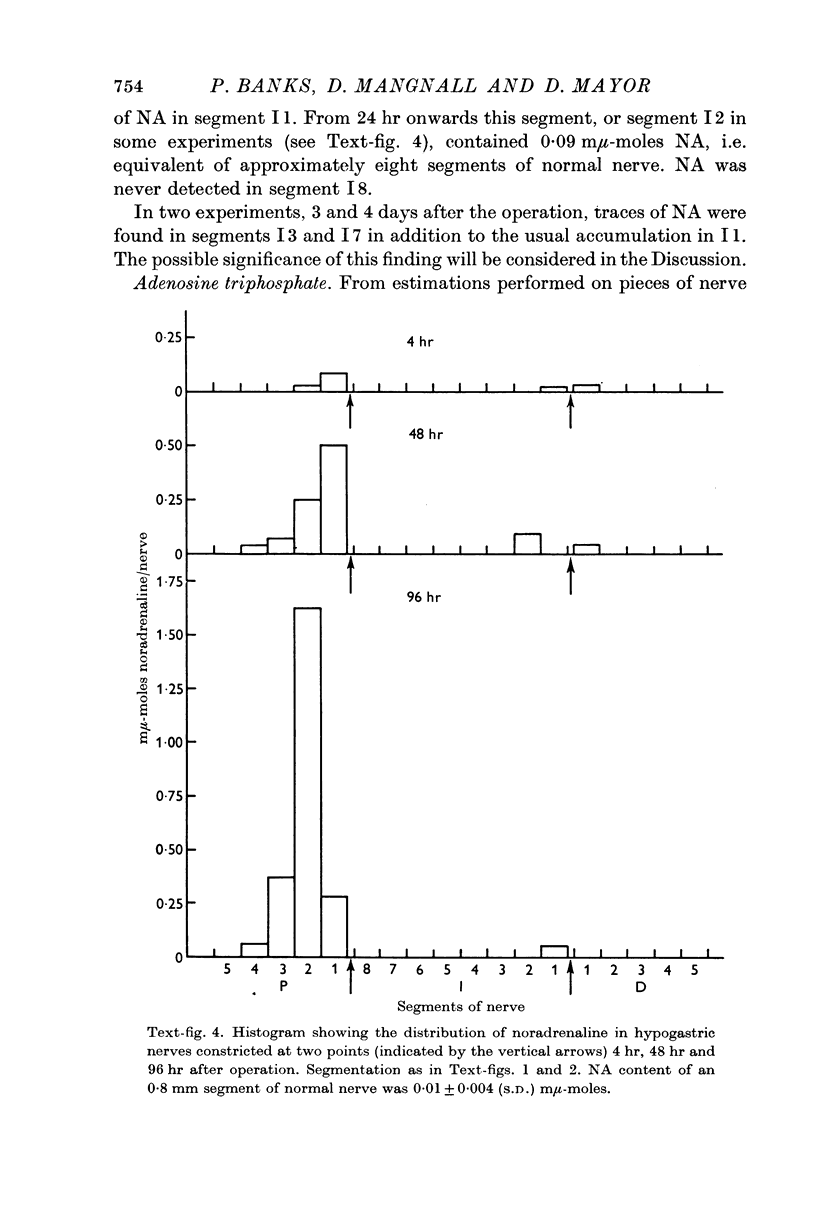
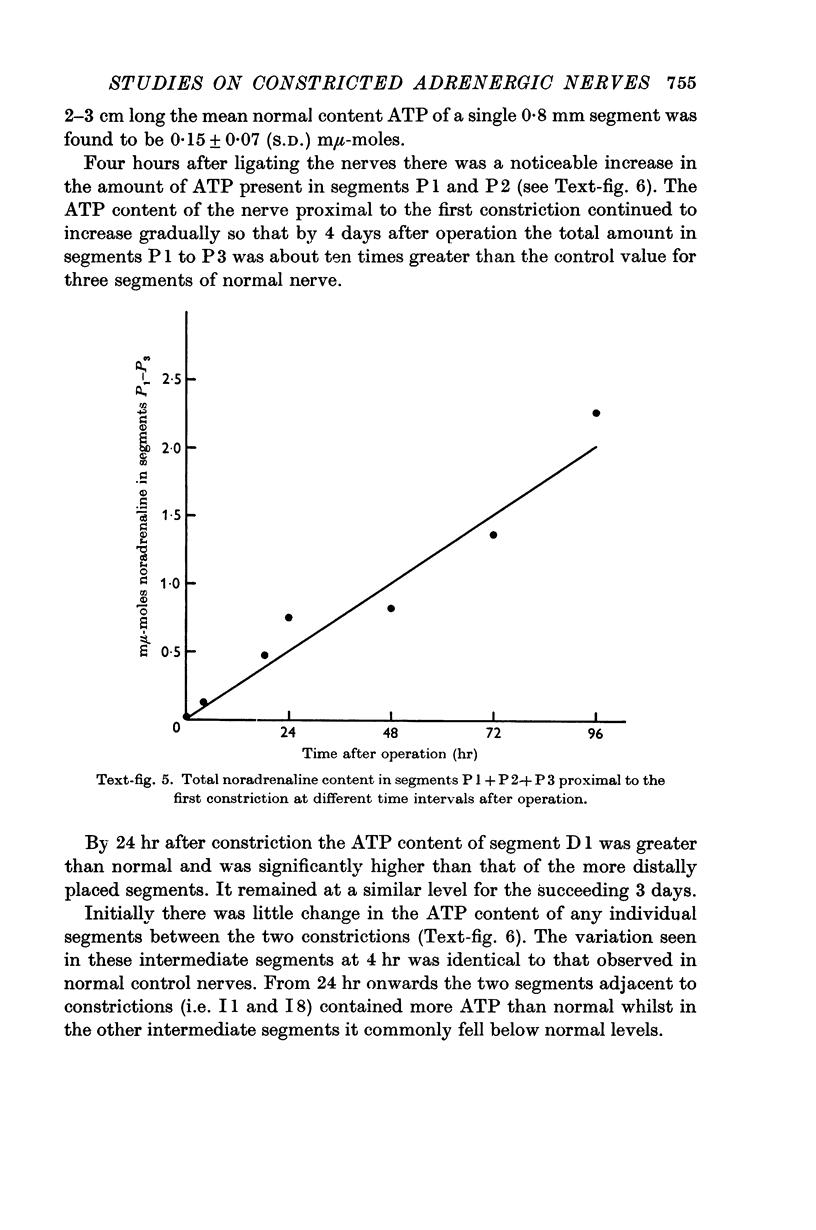
2. Noradrenaline (NA) and granular vesicles accumulated proximal but not distal to both constrictions. The total amount of NA and the concentration of granular vesicles above the first constriction was greater than that present in a similar piece of normal nerve, indicating that the cell body was continuing to produce the transmitter despite injury to its axon. The granular vesicles proximal to the first constriction were found in swollen or distorted axons and in new axonal outgrowths. It was concluded that the movement of NA in these constricted nerves was only centrifugal in direction.
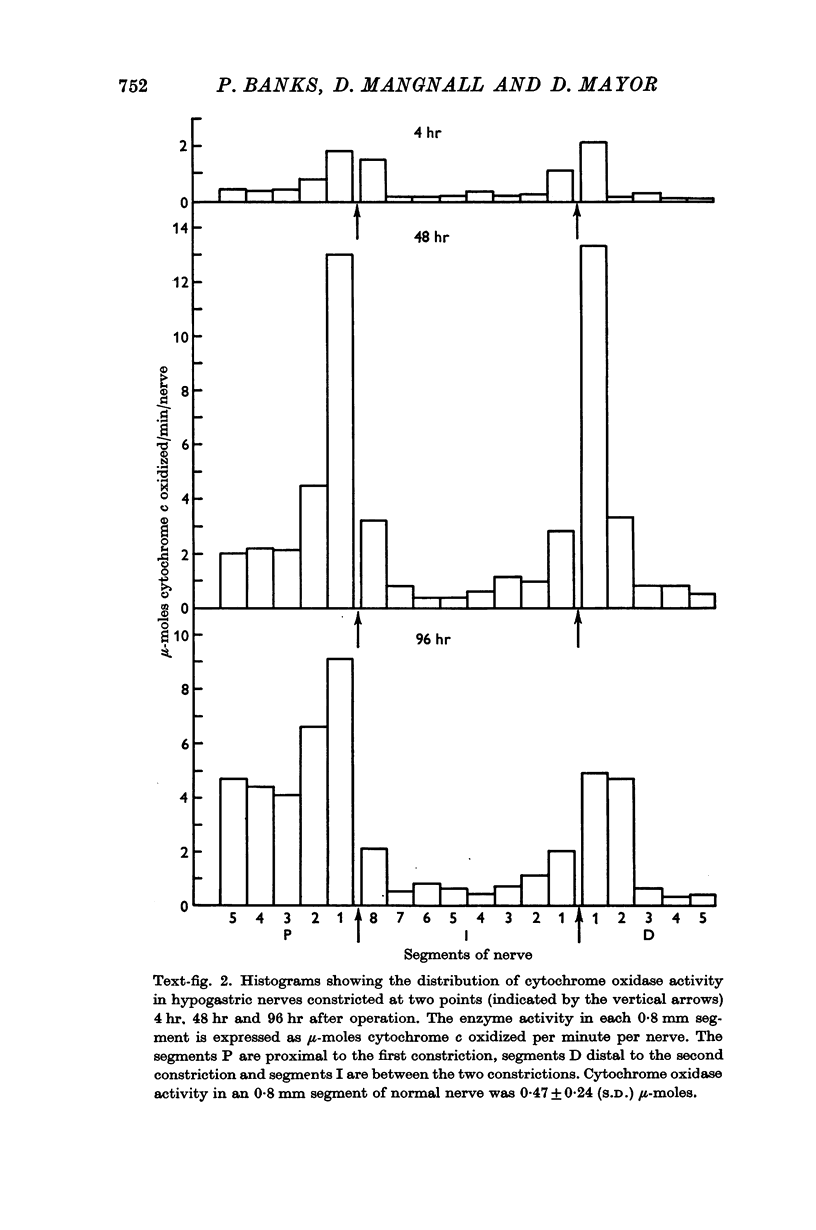
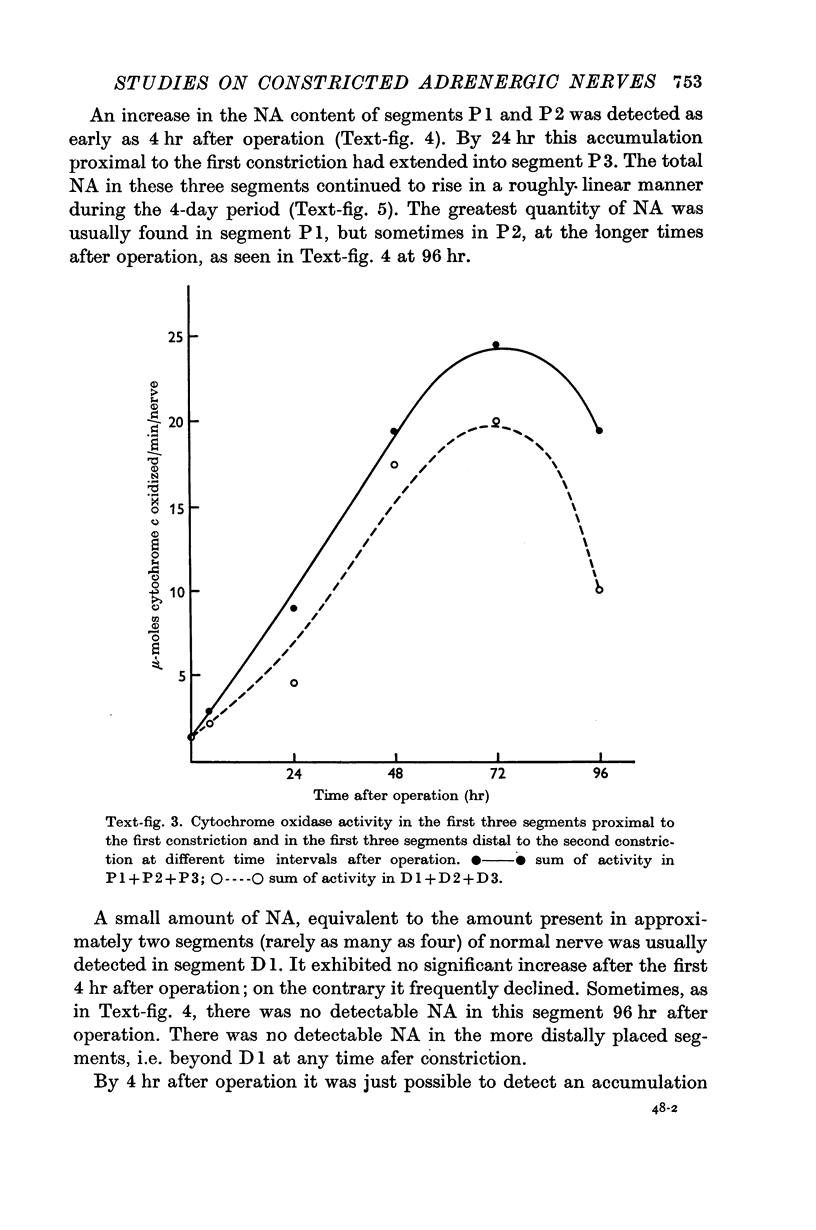
3. Mitochondria and cytochrome oxidase accumulated on both sides of the two constrictions, indicating a bi-directional movement of mitochondria in the damaged axons. The possibility that some of the increase in the cytochrome oxidase could be related to an increase in the number of mitochondria in cells other than neurones is considered.
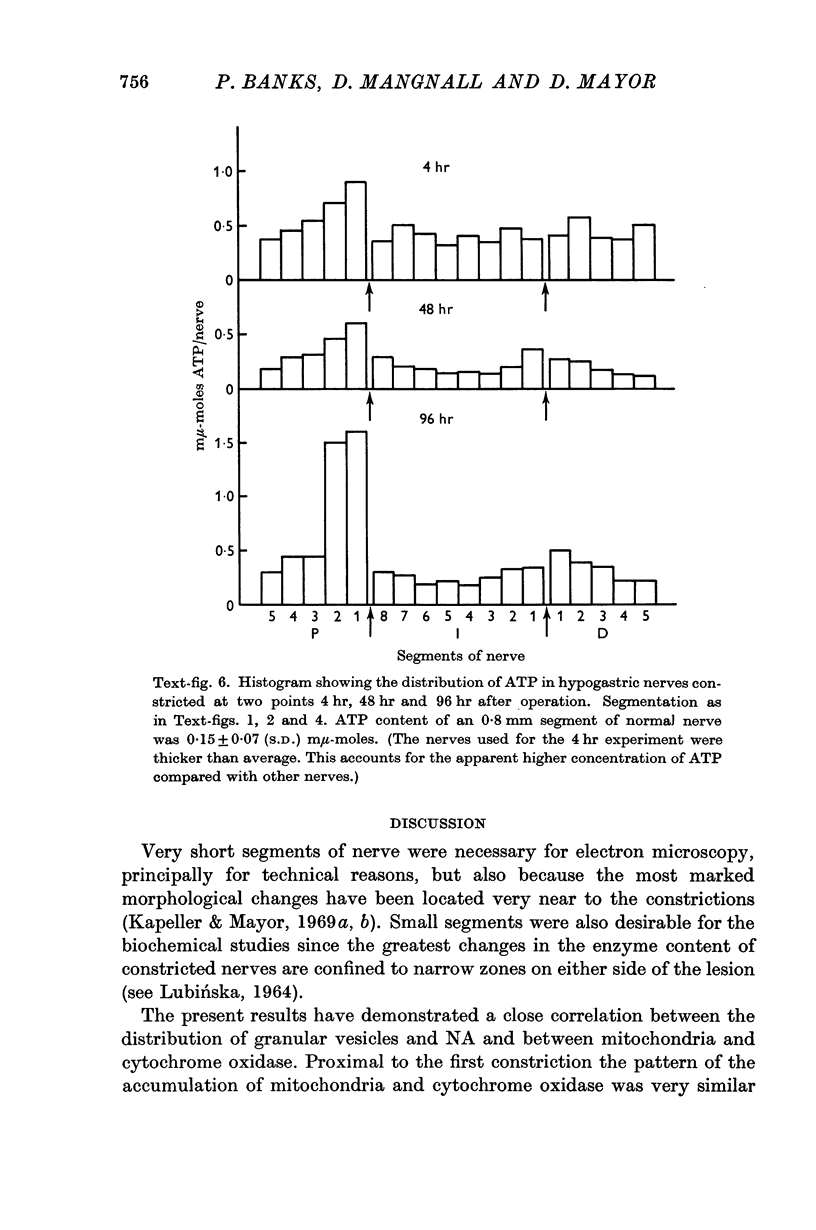
4. The adenosine triphosphate content increased on both sides of the two constrictions. This increase developed more slowly and was less marked than that of the other two substances.
5. It was concluded that (a) there was a close correlation between the behaviour of noradrenaline and granular vesicles and between cytochrome oxidase and mitochondria, (b) the dense cored vesicles and the mitochondria moved independently of one another and at different rates after constriction of non-myelinated axons, (c) while some of the changes may be attributed to an obstruction to the free movement of axoplasm others may be due to an active reaction to axonal injury, and (d) localized intraaxonal synthesis of noradrenaline and cytochrome oxidase did not occur between the two constrictions.
Full text
PDF













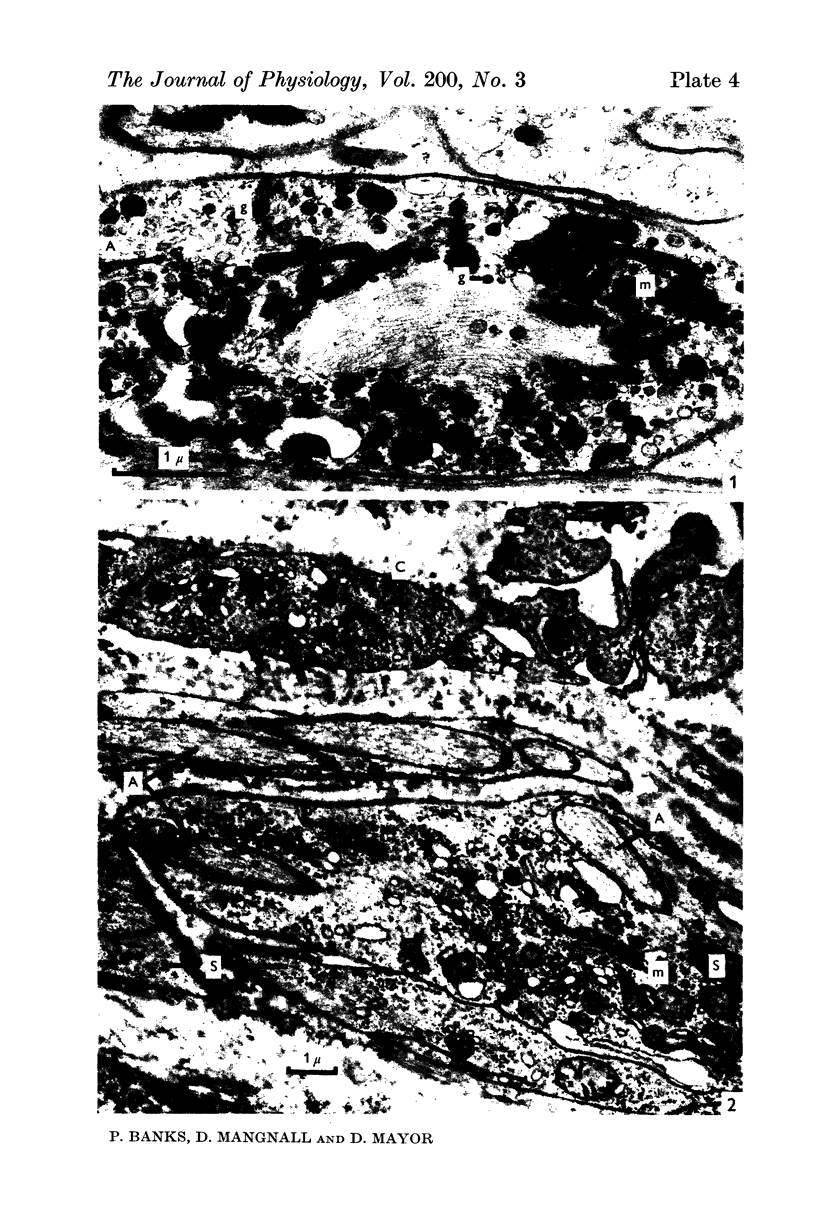
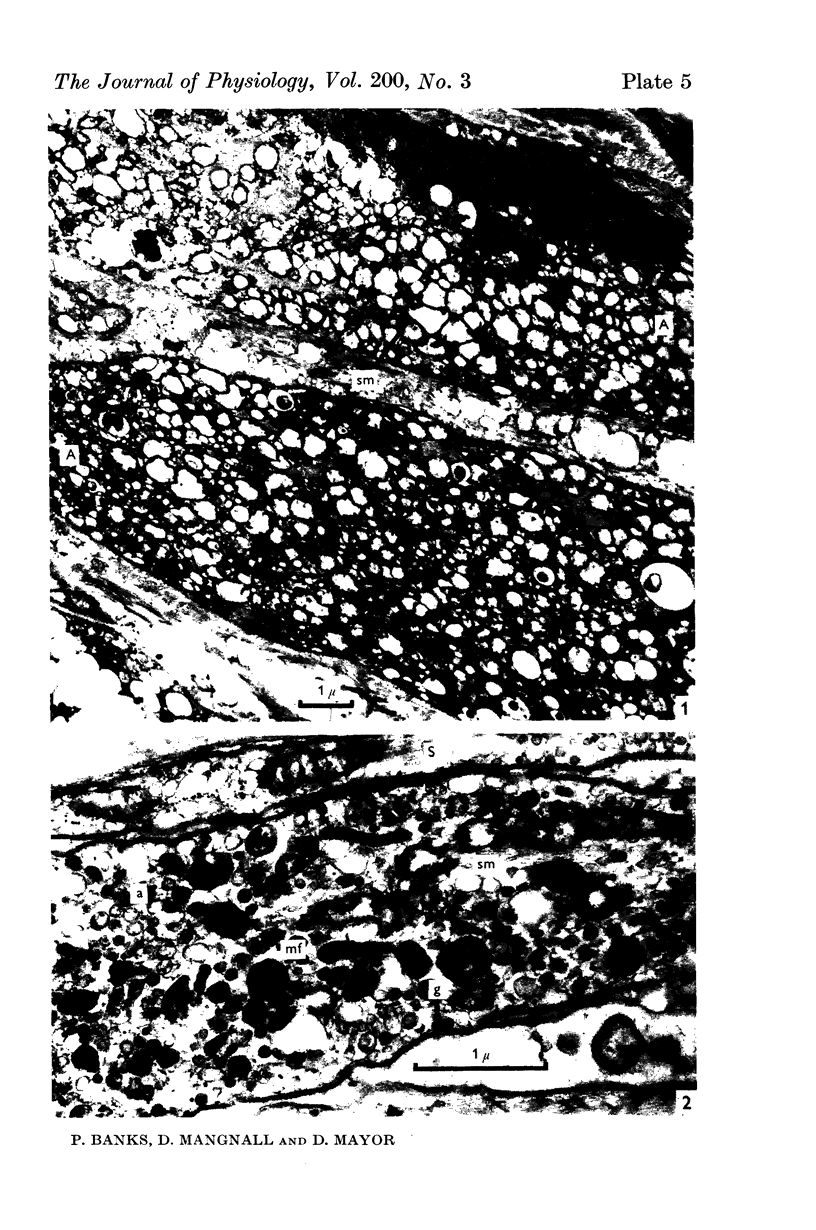

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blümcke S., Niedorf H. R. Fluoreszenzmikroskopische und elektronenmikroskopische Untersuchungen an regenerierenden adrenergischen Nervenfasern. Z Zellforsch Mikrosk Anat. 1965 Dec 10;68(5):724–732. [PubMed] [Google Scholar]
- DROZ B., LEBLOND C. P. AXONAL MIGRATION OF PROTEINS IN THE CENTRAL NERVOUS SYSTEM AND PERIPHERAL NERVES AS SHOWN BY RADIOAUTOGRAPHY. J Comp Neurol. 1963 Dec;121:325–346. doi: 10.1002/cne.901210304. [DOI] [PubMed] [Google Scholar]
- Dahlström A., Häggendal J. Studies on the transport and life-span of amine storage granules in a peripheral adrenergic neuron system. Acta Physiol Scand. 1966 Jul-Aug;67(3):278–288. doi: 10.1111/j.1748-1716.1966.tb03313.x. [DOI] [PubMed] [Google Scholar]
- HAEGGENDAL J. AN IMPROVED METHOD FOR FLUORIMETRIC DETERMINATION OF SMALL AMOUNTS OF ADRENALINE AND NORADRENALINE IN PLASMA AND TISSUES. Acta Physiol Scand. 1963 Nov;59:242–254. doi: 10.1111/j.1748-1716.1963.tb02739.x. [DOI] [PubMed] [Google Scholar]
- Hamberger B., Norberg K. A. Studies on some systems of adrenergic synaptic terminals in the abdominal ganglia of the cat. Acta Physiol Scand. 1965 Nov;65(3):235–242. doi: 10.1111/j.1748-1716.1965.tb04266.x. [DOI] [PubMed] [Google Scholar]
- Kapeller K., Mayor D. The accumulation of noradrenaline in constricted sympathetic nerves as studied by fluorescence and electron microscopy. Proc R Soc Lond B Biol Sci. 1967 Mar 28;167(1008):282–292. doi: 10.1098/rspb.1967.0027. [DOI] [PubMed] [Google Scholar]
- Korr I. M., Wilkinson P. N., Chornock F. W. Axonal delivery of neuroplasmic components to muscle cells. Science. 1967 Jan 20;155(3760):342–345. doi: 10.1126/science.155.3760.342. [DOI] [PubMed] [Google Scholar]
- Laduron P., Belpaire F. Transport of noradrenaline and dopamine-beta-hydroxylase in sympathetic nerves. Life Sci. 1968 Jan 1;7(1):1–7. doi: 10.1016/0024-3205(68)90355-x. [DOI] [PubMed] [Google Scholar]
- MIANI N. PROXIMO-DISTAL MOVEMENT OF PHOSPHOLIPID IN THE AXOPLASM OF THE INTACT AND REGENERATING NEURONS. Prog Brain Res. 1964;13:115–126. doi: 10.1016/s0079-6123(08)60141-7. [DOI] [PubMed] [Google Scholar]
- Mayor D., Kapeller K. Fluorescence microscopy and electron microscopy of adrenergic nerves after constriction at two points. J R Microsc Soc. 1967;87(2):277–294. [PubMed] [Google Scholar]
- Polakis E. S., Bartley W., Meek G. A. Changes in the structure and enzyme activity of Saccharomyces cerevisiae in response to changes in the environment. Biochem J. 1964 Feb;90(2):369–374. doi: 10.1042/bj0900369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjärne L. Storage particles in noradrenergic tissues. Pharmacol Rev. 1966 Mar;18(1):425–432. [PubMed] [Google Scholar]
- Taylor A. C., Weiss P. Demonstration of axonal flow by the movement of tritium-labeled protein in mature optic nerve fibers. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1521–1527. doi: 10.1073/pnas.54.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANOV S., VOGT M. CATECHOLAMINE-CONTAINING STRUCTURES IN THE HYPOGASTRIC NERVES OF THE DOG. J Physiol. 1963 Oct;168:939–944. doi: 10.1113/jphysiol.1963.sp007232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBSTER H. D. Transient, focal accumulation of axonal mitochondria during the early stages of wallerian degeneration. J Cell Biol. 1962 Feb;12:361–383. doi: 10.1083/jcb.12.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P., Holland Y. Neuronal dynamics and axonal flow, ii. The olfactory nerve as model test object. Proc Natl Acad Sci U S A. 1967 Feb;57(2):258–264. doi: 10.1073/pnas.57.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss P. Neuronal dynamics and axonal flow. 3. Cellulifugal transport of labeled neuroplasm in isolated nerve preparations. Proc Natl Acad Sci U S A. 1967 May;57(5):1239–1245. doi: 10.1073/pnas.57.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]