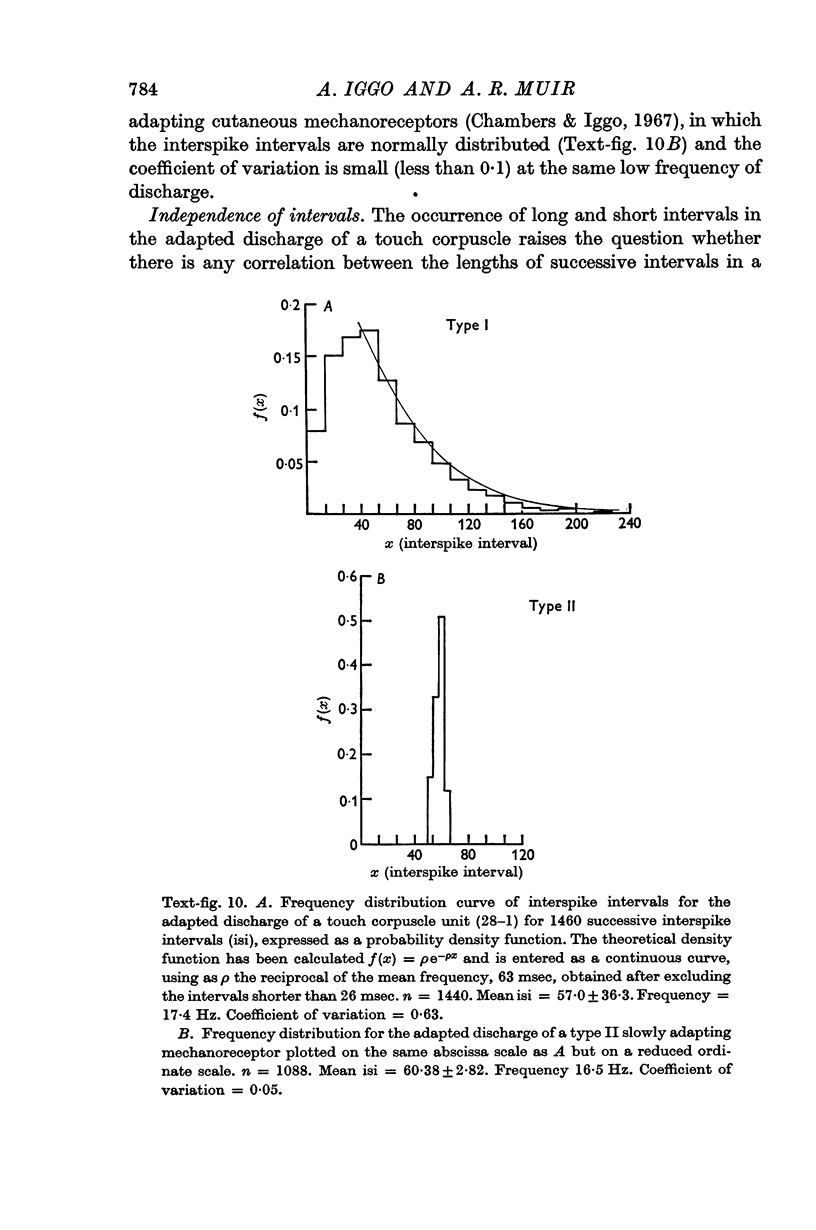
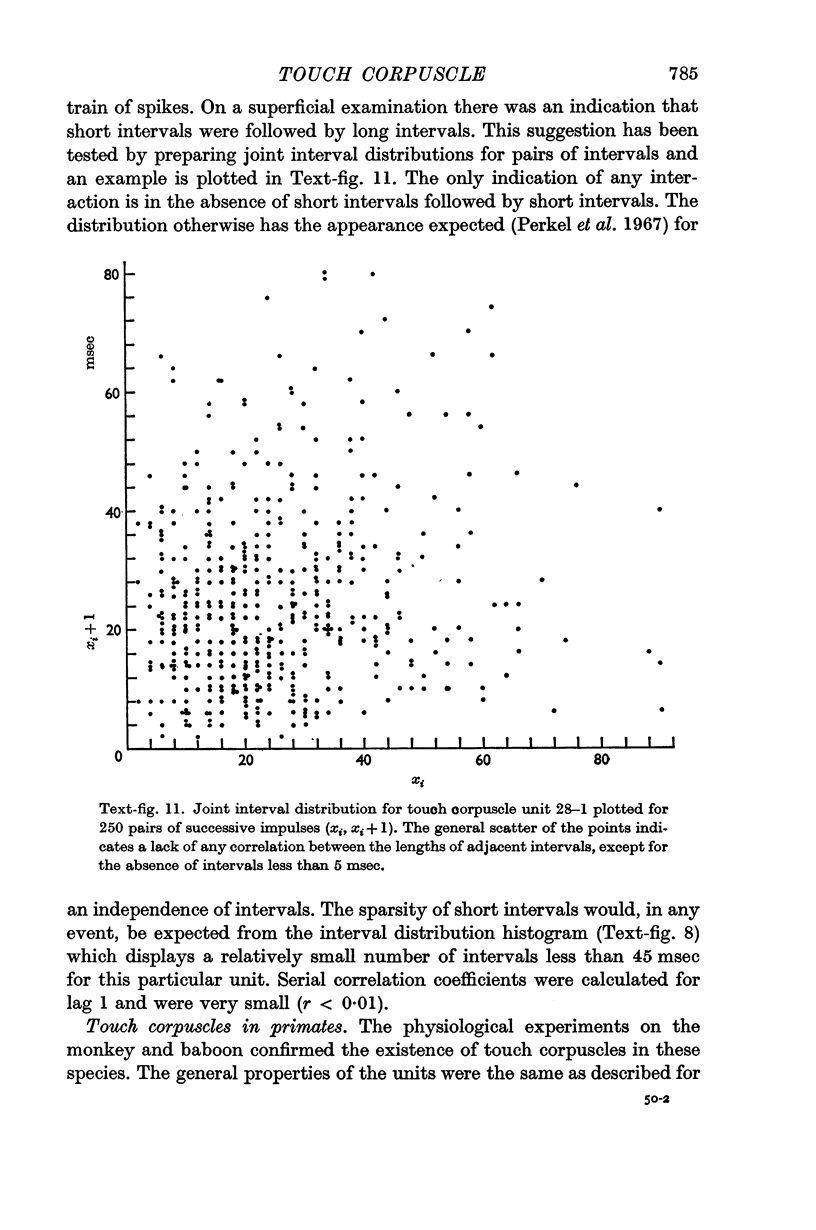
Abstract
1. Slowly adapting cutaneous mechanoreceptors, in the cat and primates, have been studied by histological and neurophysiological methods.
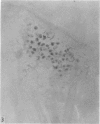
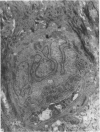
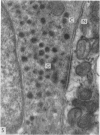
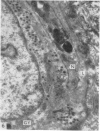
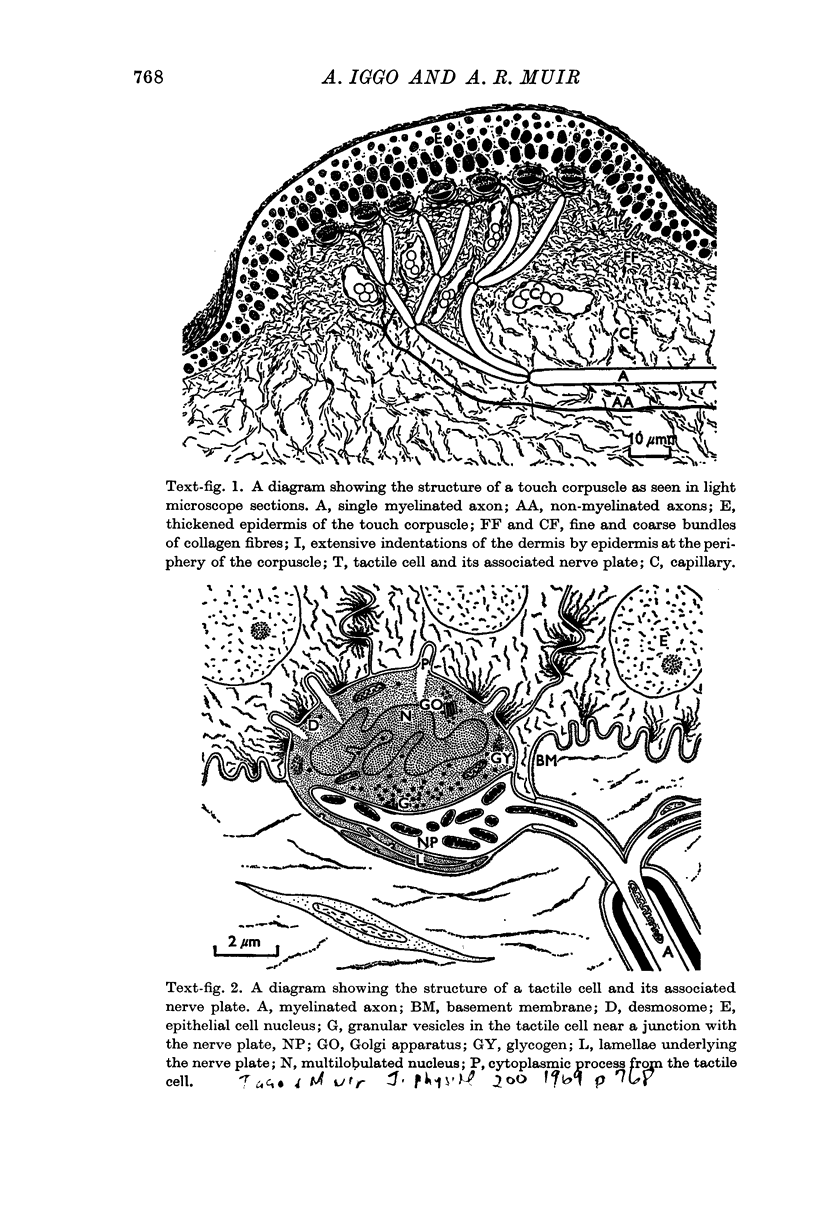
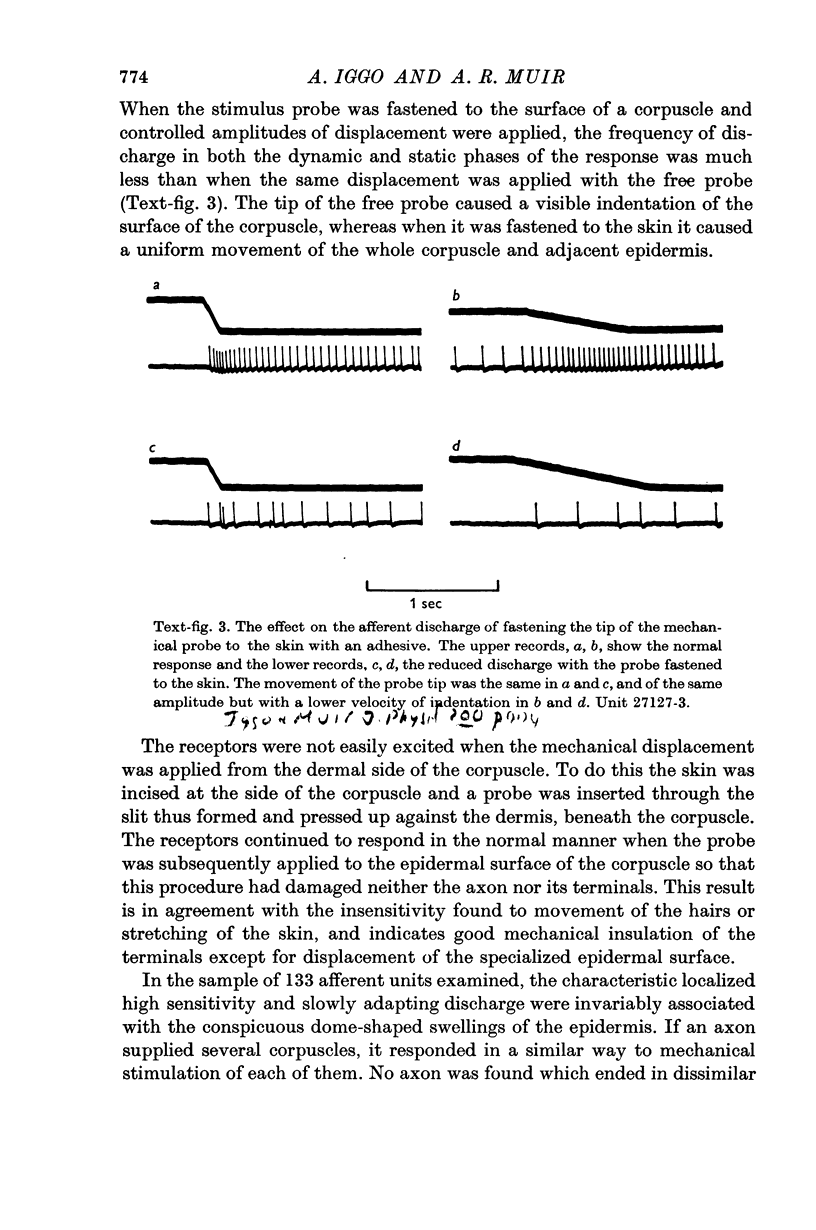
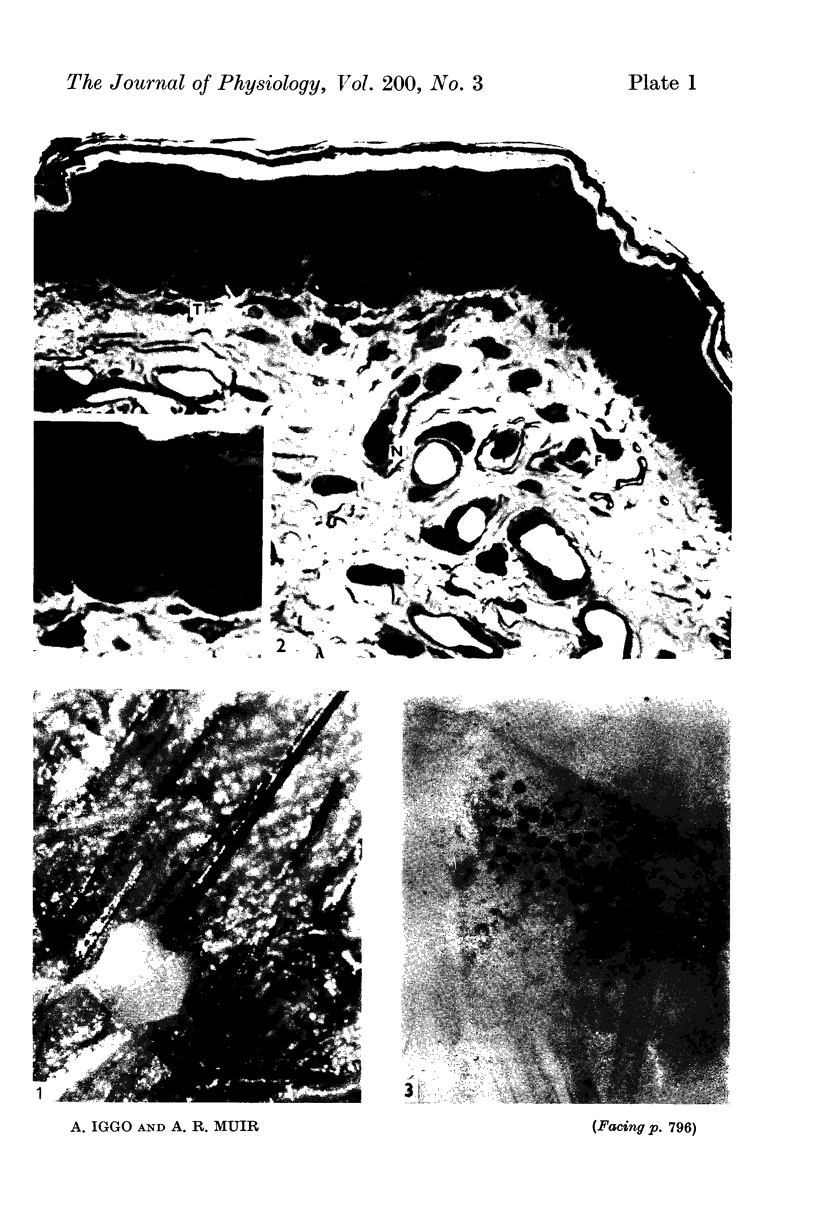
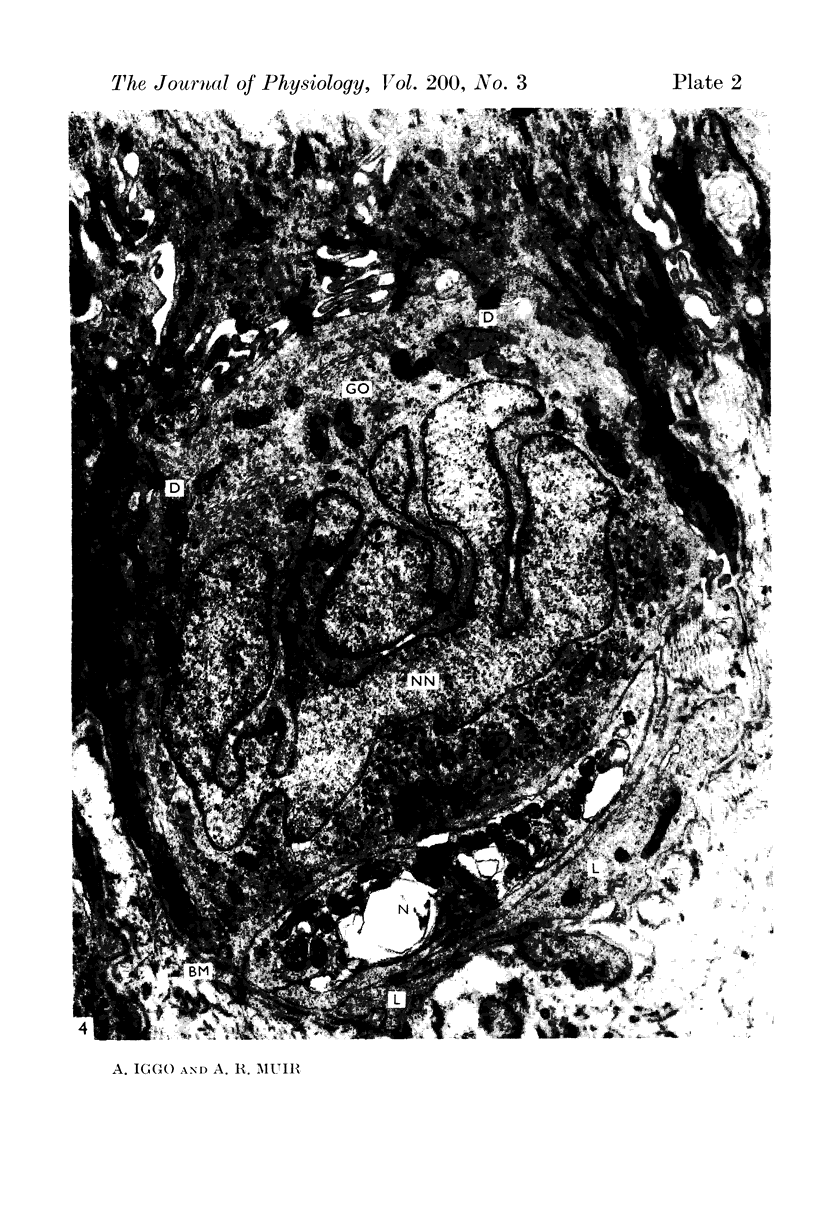
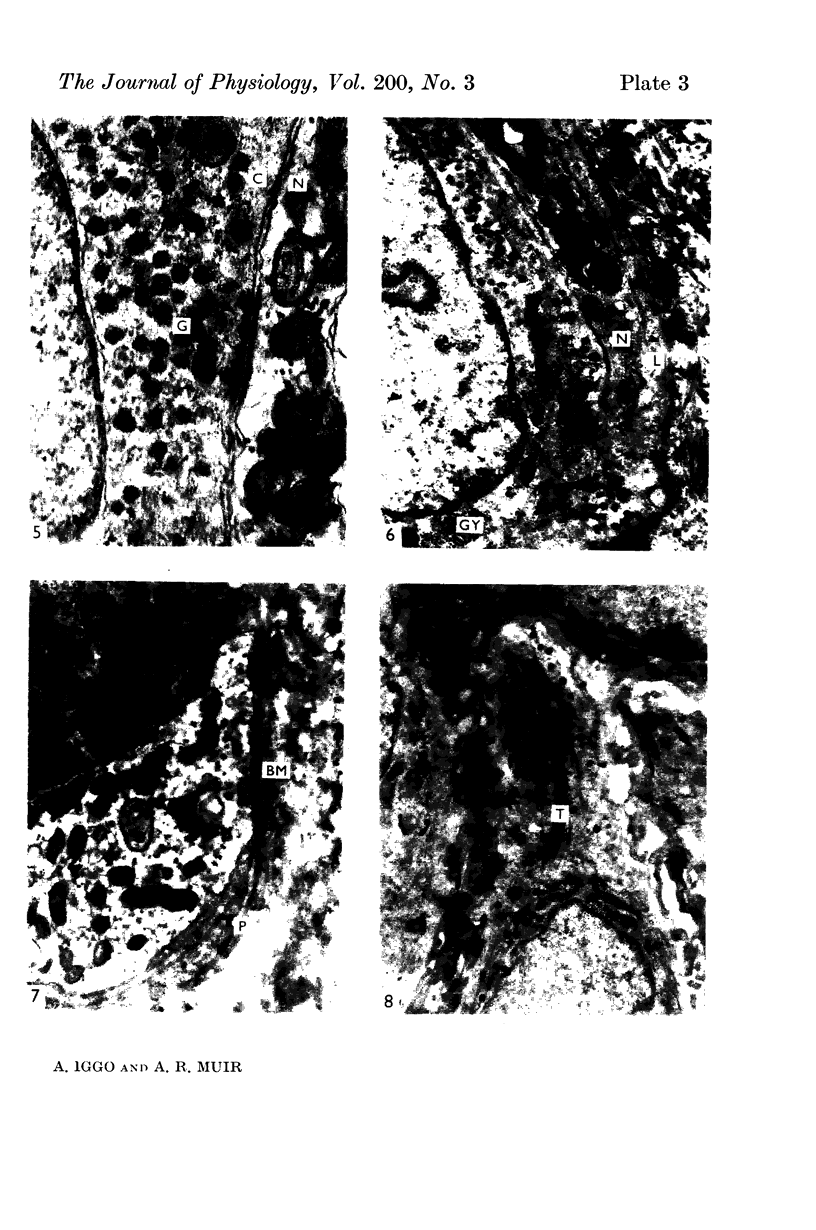
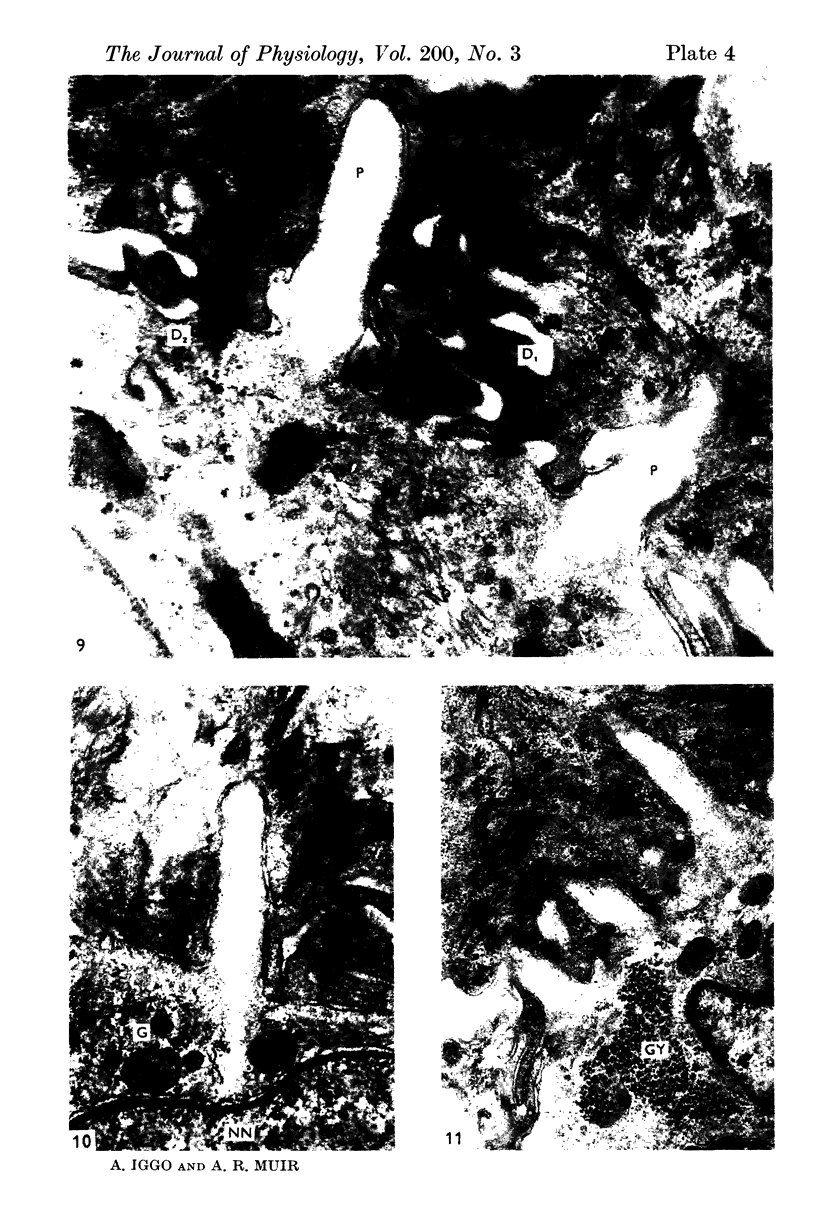
2. Each touch corpuscle is a dome-shaped elevation of the epidermis, whose deepest layer contains up to fifty specialized tactile cells.
3. Nerve plates, enclosed by the tactile cell (Merkel cells), are connected to a single myelinated axon in the dense collagenous core of the corpuscle.
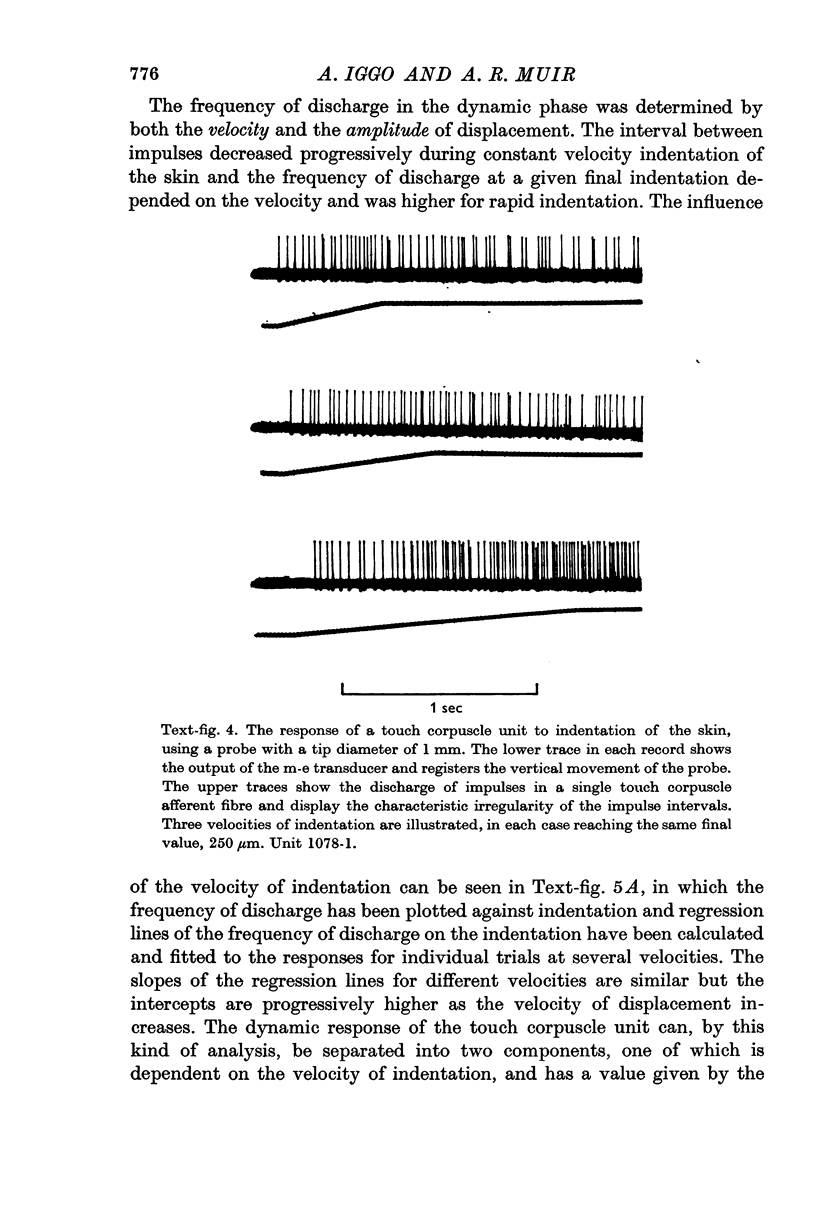
4. The corpuscle generated > 1000 impulses/sec when excited by vertical surface pressure. The response was highly localized and showed a low mechanical threshold, the frequency being dependent upon the velocity and amplitude of the displacement. There was a period of rapid adaptation before a sustained response which might continue for > 30 min.
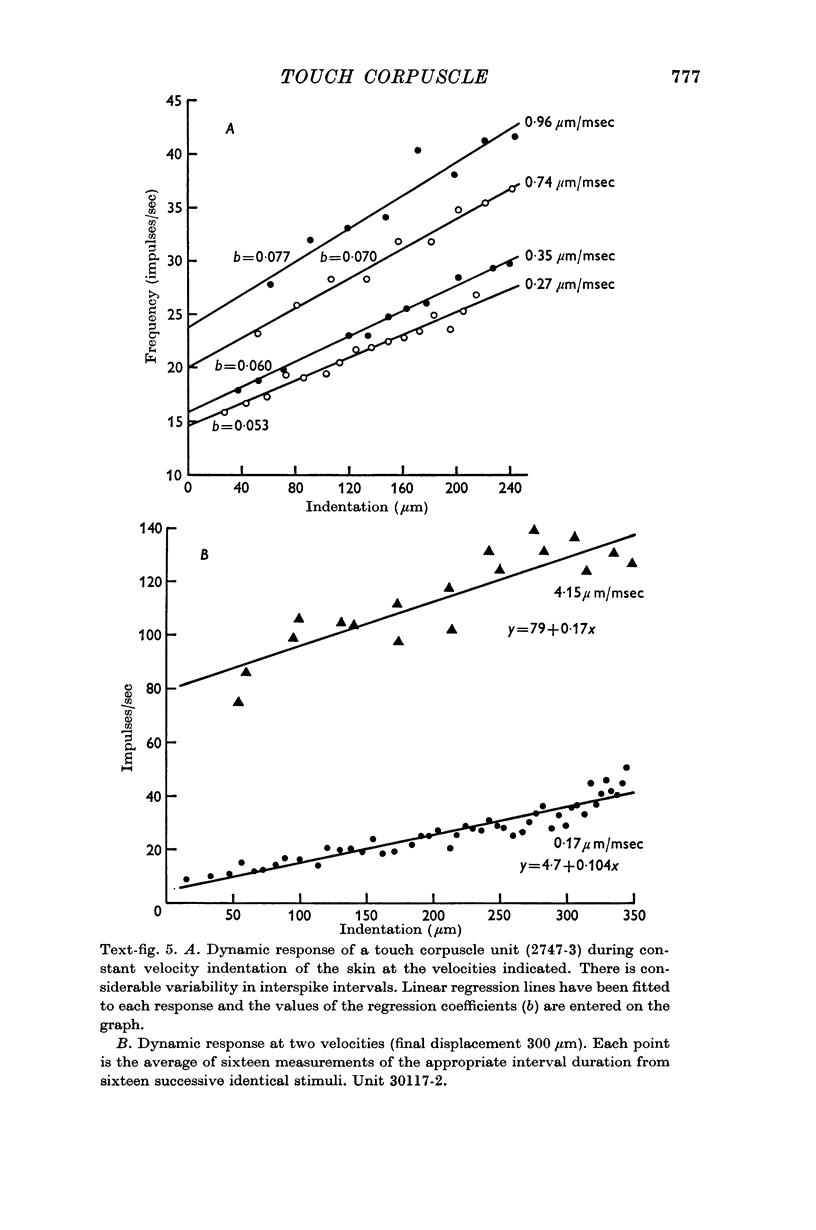
5. A quantitative analysis of the responses to excitation by displacements of differing amplitude, velocity and duration is included.
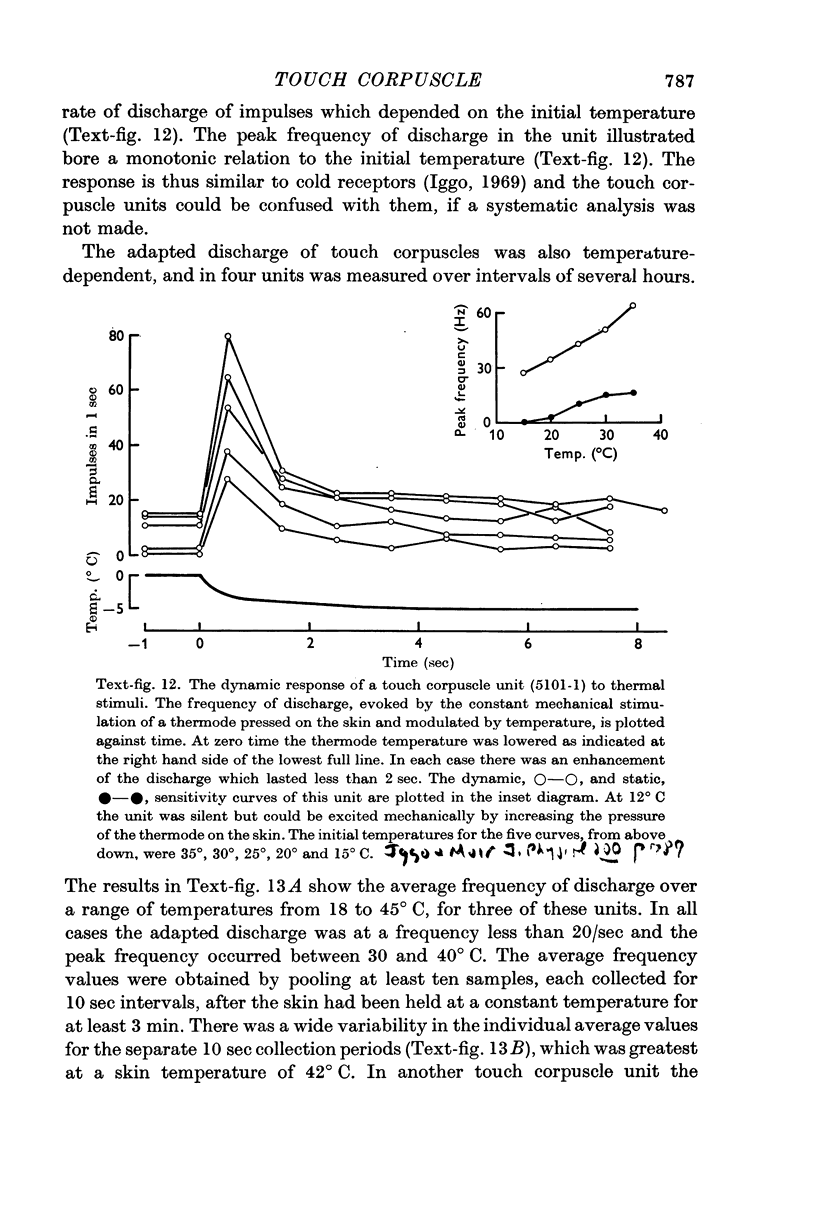
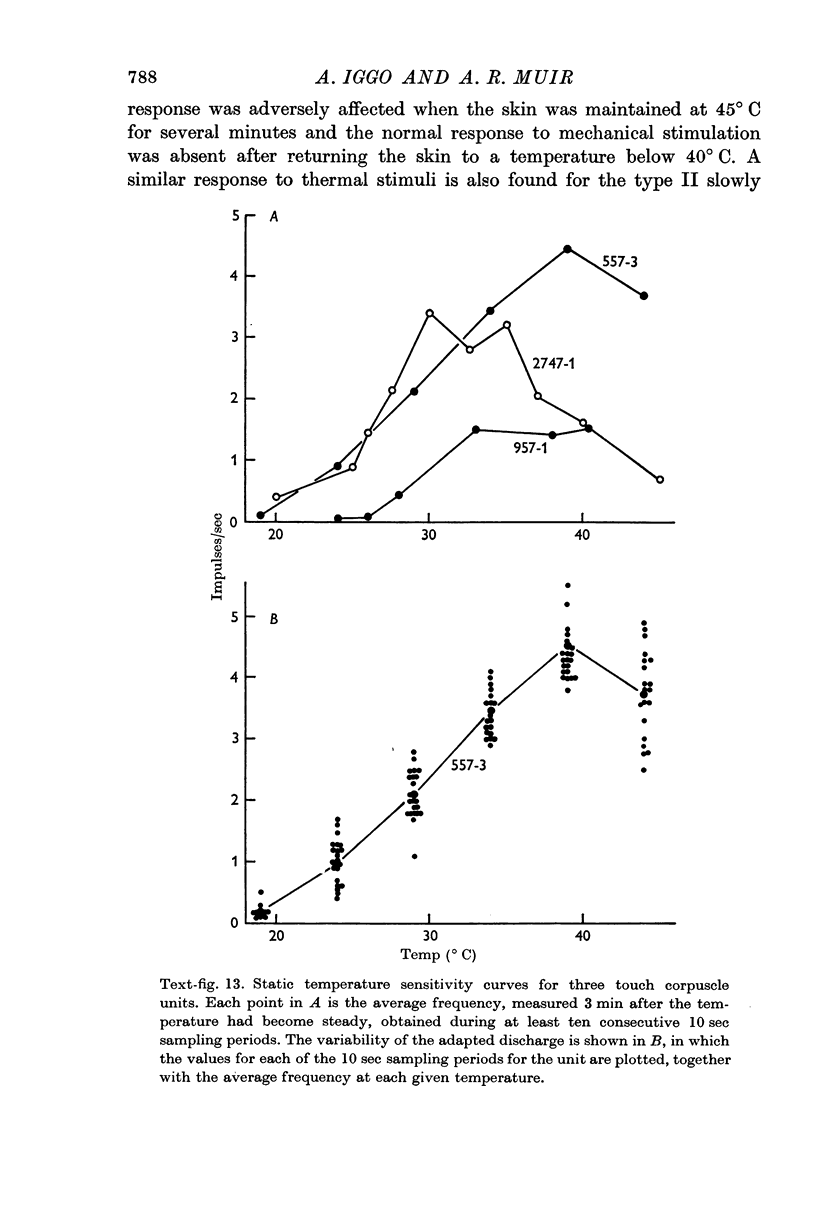
6. The discharge of touch corpuscle units evoked by a mechanical stimulus was temperature-sensitive, and was enhanced by a fall in skin temperature.
Full text
PDF










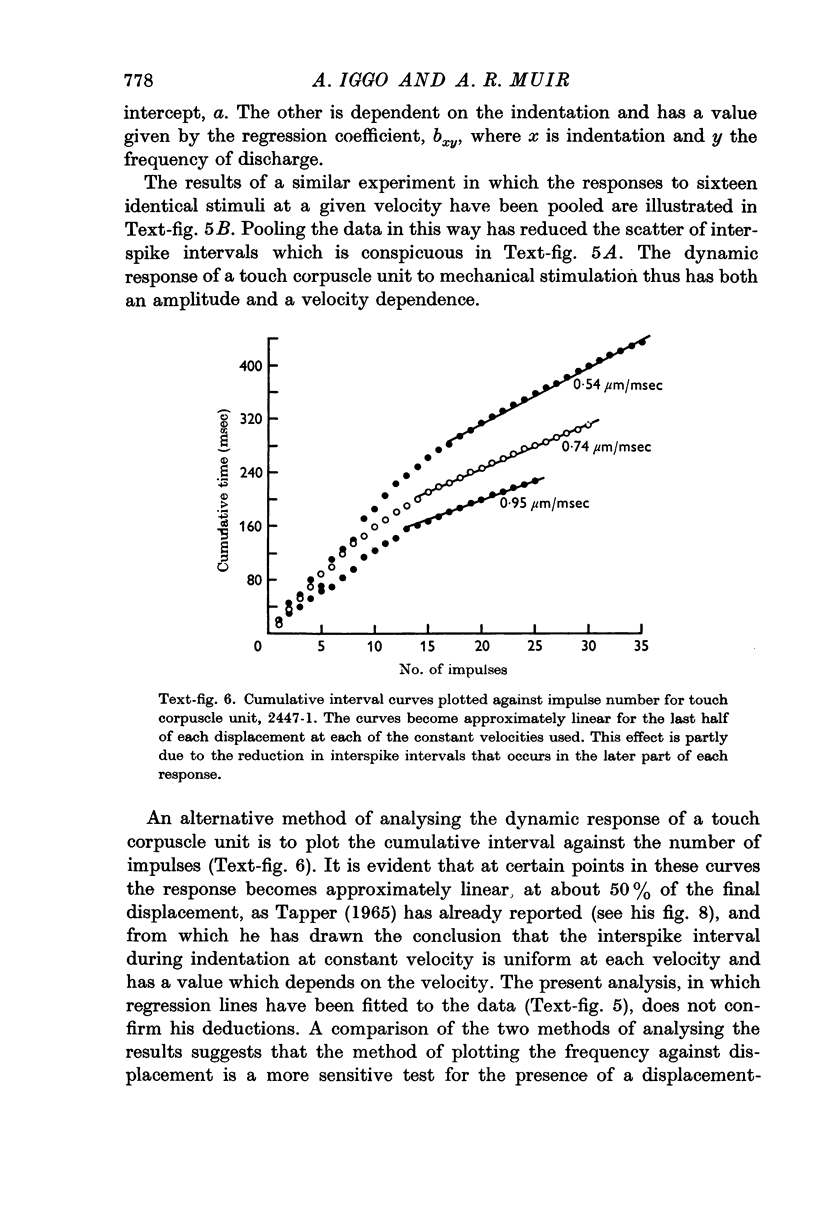

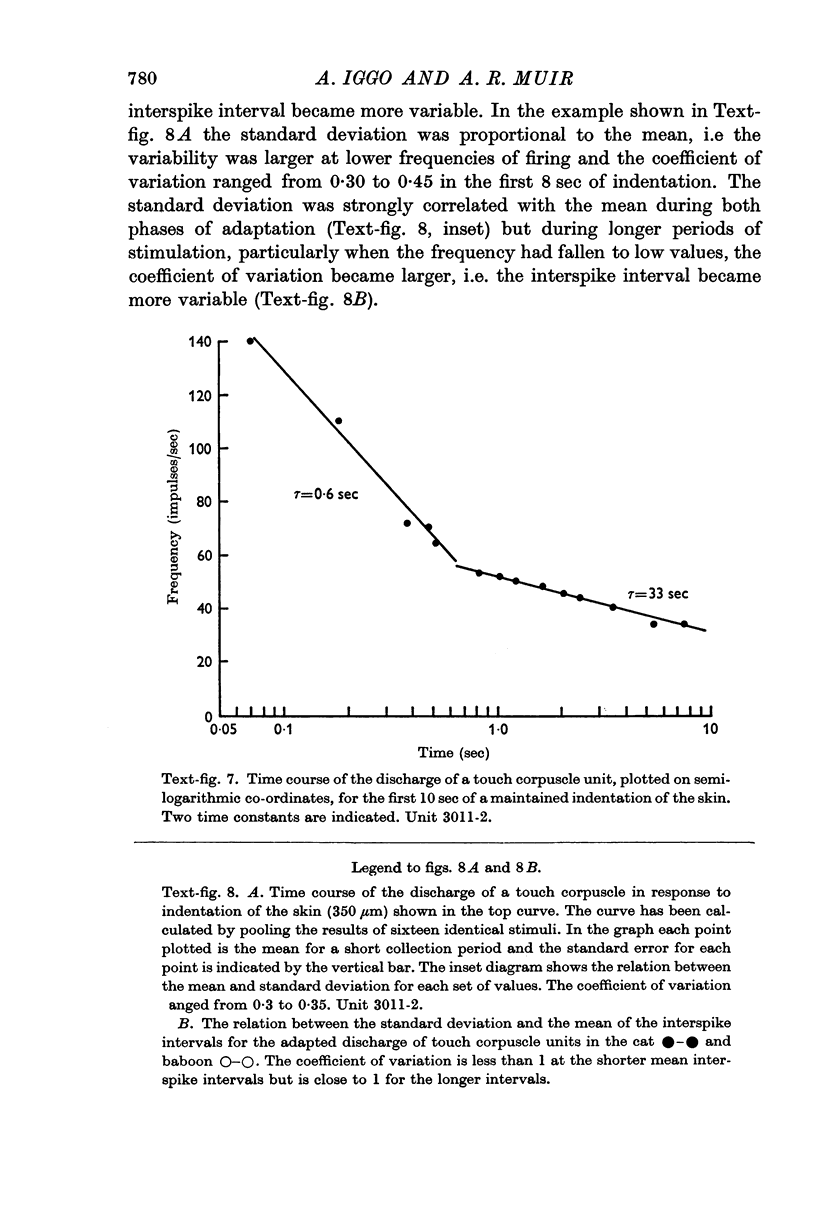
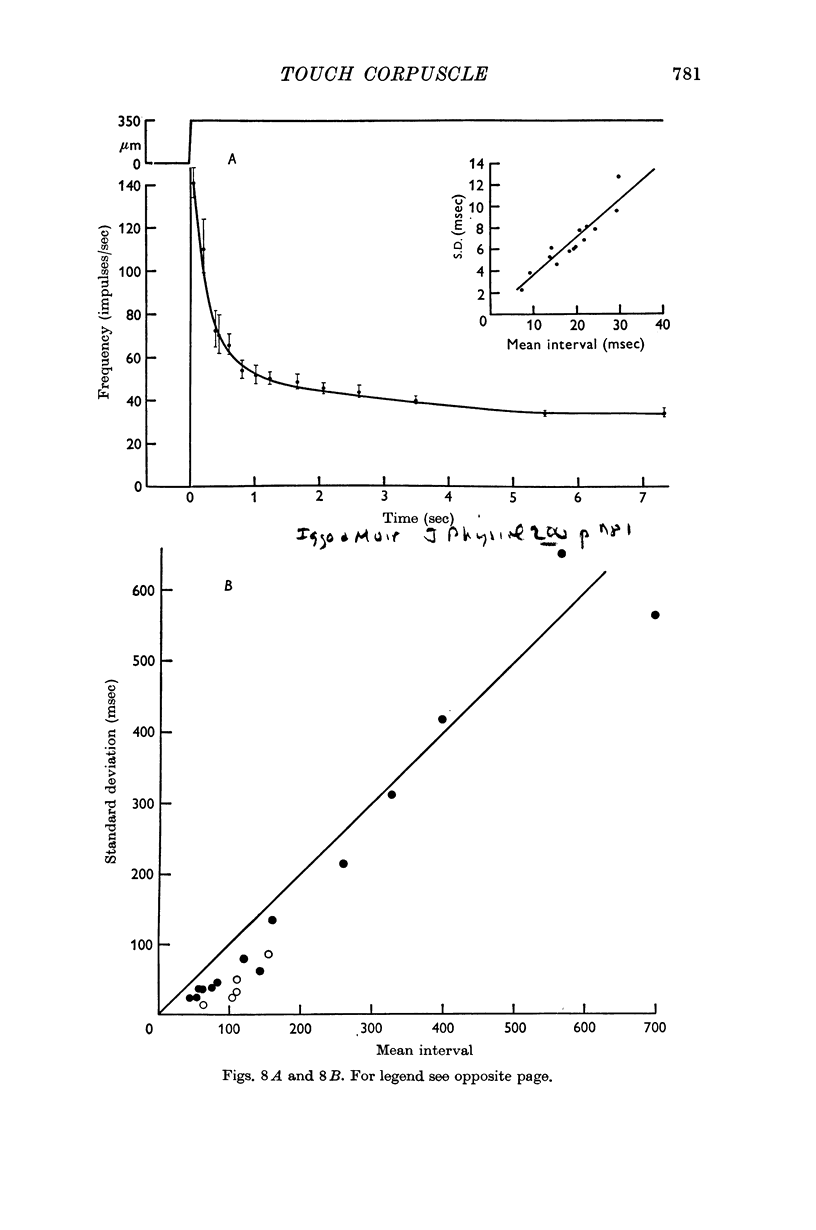
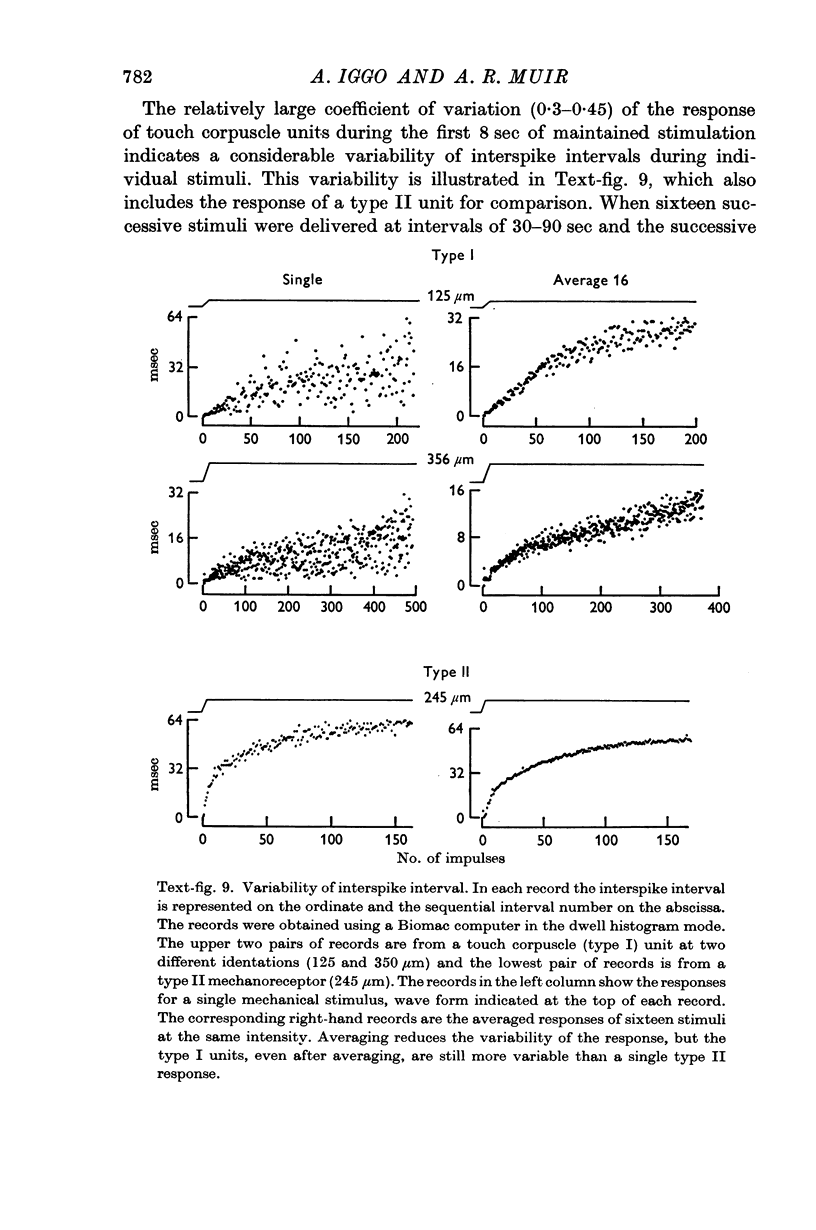










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D. The impulses produced by sensory nerve endings: Part I. J Physiol. 1926 Mar 18;61(1):49–72. doi: 10.1113/jphysiol.1926.sp002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian E. D., Zotterman Y. The impulses produced by sensory nerve-endings: Part II. The response of a Single End-Organ. J Physiol. 1926 Apr 23;61(2):151–171. doi: 10.1113/jphysiol.1926.sp002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCOE T. J., TAYLOR A. THE DISCHARGE PATTERN RECORDED IN CHEMORECEPTOR AFFERENT FIBRES FROM THE CAT CAROTID BODY WITH NORMAL CIRCULATION AND DURING PERFUSION. J Physiol. 1963 Sep;168:332–344. doi: 10.1113/jphysiol.1963.sp007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUCHARD E. F., LEYDEN A. F., POMERANTZ E. Pharmaceutical aspects of reserpine. J Am Pharm Assoc Am Pharm Assoc. 1956 Dec;45(12):771–775. [PubMed] [Google Scholar]
- BULLER A. J., NICHOLLS J. G., STROM G. Spontaneous fluctuations of excitability in the muscle spindle of the frog. J Physiol. 1953 Nov 28;122(2):409–418. doi: 10.1113/jphysiol.1953.sp005011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupland R. E., Hopwood D. The mechanism of the differential staining reaction for adrenaline-and noreadrenaline-storing granules in tissues fixed in glutaraldehyde. J Anat. 1966 Apr;100(Pt 2):227–243. [PMC free article] [PubMed] [Google Scholar]
- EDWARDS C., OTTOSON D. The site of impulse initiation in a nerve cell of a crustacean stretch receptor. J Physiol. 1958 Aug 29;143(1):138–148. doi: 10.1113/jphysiol.1958.sp006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EYZAGUIRRE C., KUFFLER S. W. Processes of excitation in the dendrites and in the soma of single isolated sensory nerve cells of the lobster and crayfish. J Gen Physiol. 1955 Sep 20;39(1):87–119. doi: 10.1085/jgp.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FJALLBRANT N., IGGO A. The effect of histamine, 5-hydroxytryptamine and acetylcholine on cutaneous afferent fibres. J Physiol. 1961 May;156:578–590. doi: 10.1113/jphysiol.1961.sp006694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald O. Discharges from the sensory organs of the cat's vibrissae and the modification in their activity by ions. J Physiol. 1940 May 14;98(2):163–178. doi: 10.1113/jphysiol.1940.sp003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENSEL H., BOMAN K. K. Afferent impulses in cutaneous sensory nerves in human subjects. J Neurophysiol. 1960 Sep;23:564–578. doi: 10.1152/jn.1960.23.5.564. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. An analysis of fibre diameter and receptor characteristics of myelinated cutaneous afferent fibres in cat. J Physiol. 1960 Aug;153:99–112. doi: 10.1113/jphysiol.1960.sp006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. Properties of cutaneous touch receptors in cat. J Physiol. 1960 Aug;153:88–98. doi: 10.1113/jphysiol.1960.sp006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO S., WINCHESTER R. J. The fine structure of the gastric mucosa in the bat. J Cell Biol. 1963 Mar;16:541–577. doi: 10.1083/jcb.16.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Cutaneous thermoreceptors in primates and sub-primates. J Physiol. 1969 Feb;200(2):403–430. doi: 10.1113/jphysiol.1969.sp008701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAMURA T. Uber die menschliche Haarscheibe, unter besonderer Berücksichtigung ihrer Innervation und subepidermalen perineuralen Pigmenthülle. Hautarzt. 1954 Mar;5(3):106–110. [PubMed] [Google Scholar]
- LOEWENSTEIN W. R. On the 'specificity' of a sensory receptor. J Neurophysiol. 1961 Mar;24:150–158. doi: 10.1152/jn.1961.24.2.150. [DOI] [PubMed] [Google Scholar]
- Lindblom Y., Tapper D. N. Integration of impulse activity in a peripheral sensory unit. Exp Neurol. 1966 May;15(1):63–69. doi: 10.1016/0014-4886(66)90034-3. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- MANN S. J., STRAILE W. E. TYLOTRICH (HAIR) FOLLICLE: ASSOCIATION WITH A SLOWLY ADAPTING TACTILE RECEPTOR IN THE CAT. Science. 1965 Feb 26;147(3661):1043–1045. doi: 10.1126/science.147.3661.1043. [DOI] [PubMed] [Google Scholar]
- MARUHASHI J., MIZUGUCHI K., TASAKI I. Action currents in single afferent nerve fibres elicited by stimulation of the skin of the toad and the cat. J Physiol. 1952 Jun;117(2):129–151. doi: 10.1113/jphysiol.1952.sp004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER M. R., RALSTON H. J., 3rd, KASAHARA M. The pattern of cutaneous innervation of the human hand. Am J Anat. 1958 Mar;102(2):183–217. doi: 10.1002/aja.1001020203. [DOI] [PubMed] [Google Scholar]
- Matthews B. H. Nerve endings in mammalian muscle. J Physiol. 1933 Apr 13;78(1):1–53. doi: 10.1113/jphysiol.1933.sp002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger B. L. The intraepidermal innervation of the snout skin of the opossum. A light and electron microscope study, with observations on the nature of Merkel's Tastzellen. J Cell Biol. 1965 Jul;26(1):79–97. doi: 10.1083/jcb.26.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINKUS H. PINKUS'S HAARSCHEIBE AND TACTILE RECEPTORS IN CATS. Science. 1964 May 15;144(3620):891–891. [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J. 1967 Jul;7(4):391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAILE W. E. Atypical guard-hair follicles in the skin of the rabbit. Nature. 1958 Jun 7;181(4623):1604–1605. doi: 10.1038/1811604a0. [DOI] [PubMed] [Google Scholar]
- STRAILE W. E. The morphology of tylotrich follicles in the skin of the rabbit. Am J Anat. 1961 Jul;109:1–13. doi: 10.1002/aja.1001090102. [DOI] [PubMed] [Google Scholar]
- Siminoff R. Functional organization of hairy skin in response to sensory stimuli. Exp Neurol. 1965 Dec;13(4):331–350. doi: 10.1016/0014-4886(65)90123-8. [DOI] [PubMed] [Google Scholar]
- Smith K. R., Jr, Creech B. J. Effects of pharmacological agents on the physiological responses of hair discs. Exp Neurol. 1967 Dec;19(4):477–482. doi: 10.1016/0014-4886(67)90167-7. [DOI] [PubMed] [Google Scholar]
- Smith K. R., Jr The structure and function of the Haarscheibe. J Comp Neurol. 1967 Dec;131(4):459–474. doi: 10.1002/cne.901310406. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Tapper D. N. Stimulus-response relationships in the cutaneous slowly-adapting mechanoreceptor in hairy skin of the cat. Exp Neurol. 1965 Dec;13(4):364–385. doi: 10.1016/0014-4886(65)90125-1. [DOI] [PubMed] [Google Scholar]
- WEDDELL G., MILLER S. Cutaneous sensibility. Annu Rev Physiol. 1962;24:199–222. doi: 10.1146/annurev.ph.24.030162.001215. [DOI] [PubMed] [Google Scholar]
- WERNER G., MOUNTCASTLE V. B. NEURAL ACTIVITY IN MECHANORECEPTIVE CUTANEOUS AFFERENTS: STIMULUS-RESPONSE RELATIONS, WEBER FUNCTIONS, AND INFORMATION TRANSMISSION. J Neurophysiol. 1965 Mar;28:359–397. doi: 10.1152/jn.1965.28.2.359. [DOI] [PubMed] [Google Scholar]
- WINKELMANN R. K. The innervation of a hair follicle. Ann N Y Acad Sci. 1959 Nov 20;83:400–407. doi: 10.1111/j.1749-6632.1960.tb40915.x. [DOI] [PubMed] [Google Scholar]
- WITT I., HENSEL H. Afferente Impulse aus der Extremitätenhaut der Katze bei thermischer und mechanischer Reizung. Pflugers Arch. 1959;268(6):582–596. doi: 10.1007/BF00362294. [DOI] [PubMed] [Google Scholar]
- Zotterman Y. Touch, pain and tickling: an electro-physiological investigation on cutaneous sensory nerves. J Physiol. 1939 Feb 14;95(1):1–28. doi: 10.1113/jphysiol.1939.sp003707. [DOI] [PMC free article] [PubMed] [Google Scholar]