Abstract
1. The experiments were carried out on pretrigeminal cats.
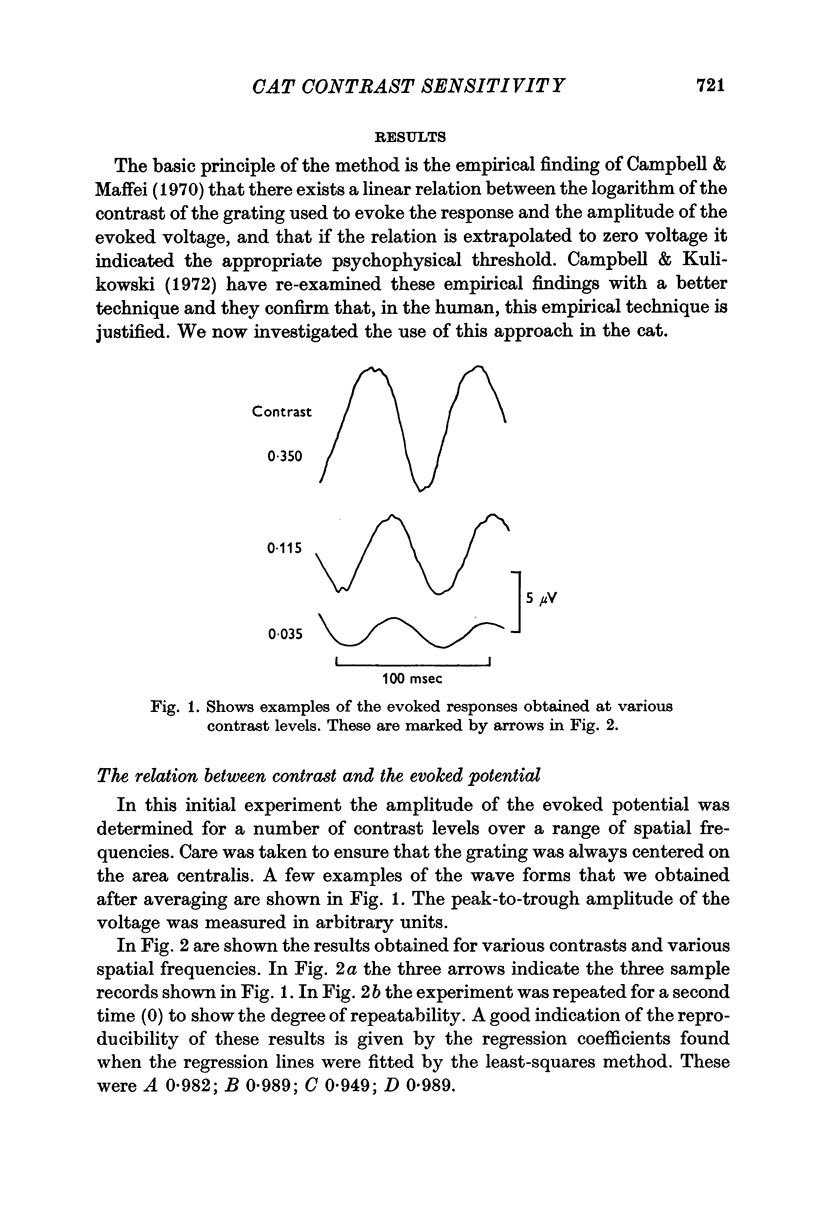
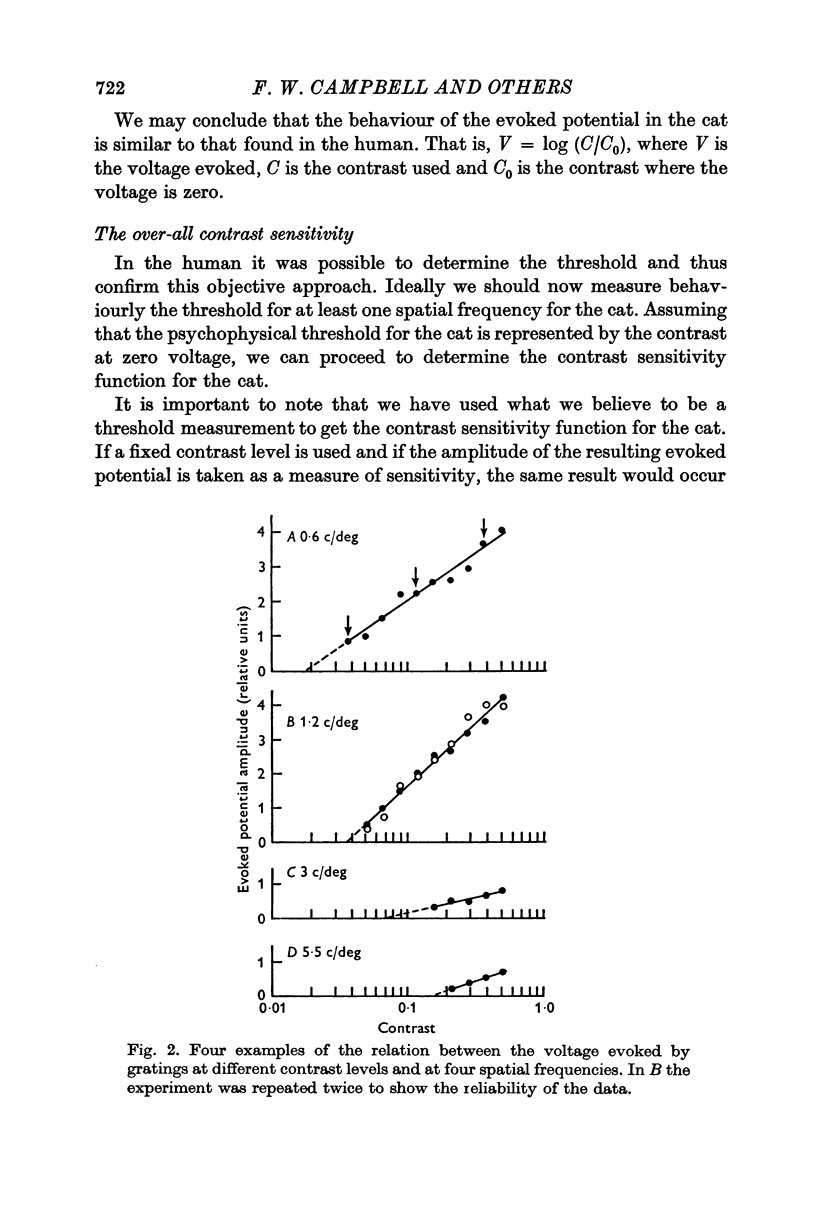
2. By recording potentials evoked from the visual cortex by a grating stimulus, it was established that there was a linear relation between the voltage generated and the logarithm of the contrast of the grating.
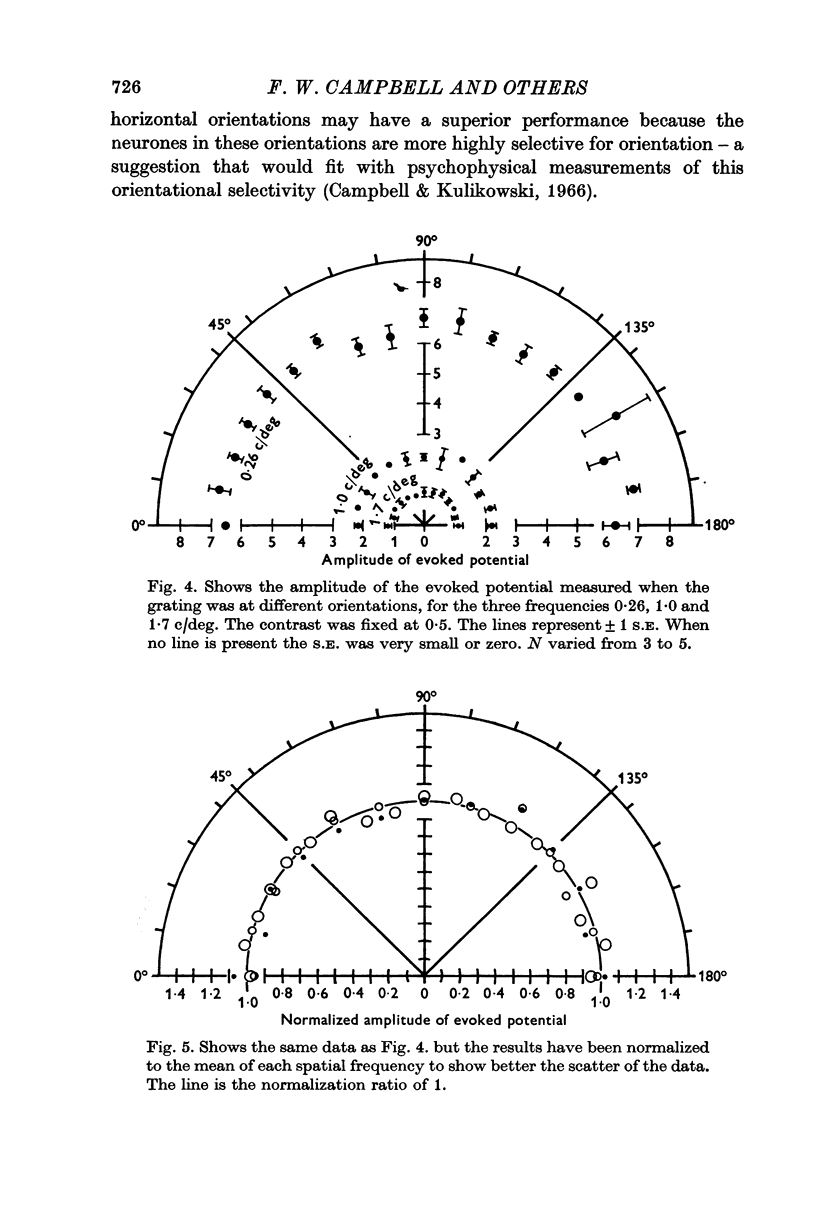
3. The voltage evoked by the grating was independent of the orientation of the grating.
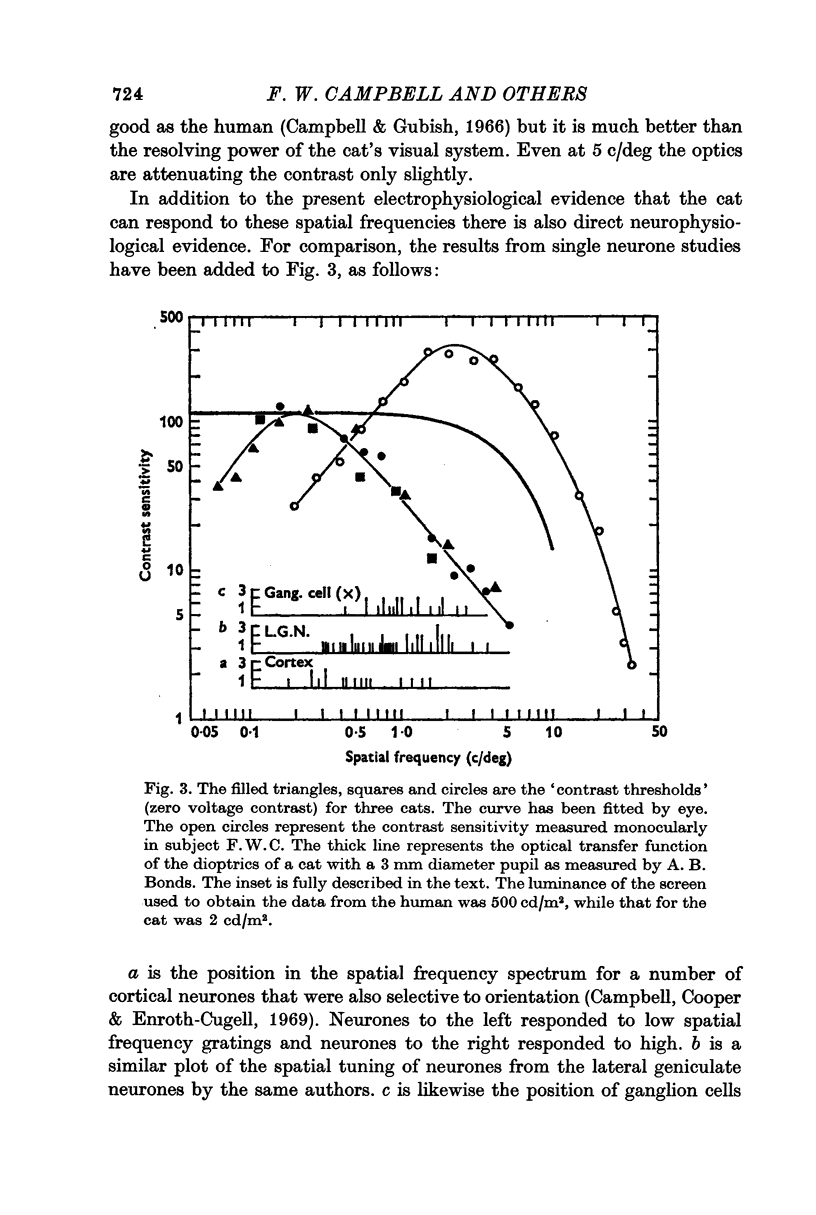
4. It has previously been shown in man that, if the contrast is determined by an extrapolation to the point at which a zero voltage occurs, this value corresponds to the psychophysical threshold. On the assumption that the threshold of the cat also occurs at zero voltage, thresholds for a number of spatial frequencies and orientations were determined.
5. When the threshold sensitivity function for the cat is compared with man it is found to be displaced to lower spatial frequencies by a factor of about ten. This means that while the cat cannot see such high spatial frequencies as man, it can see lower frequencies better than man.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley M. A., Watkins D. W. Visual acuity of the cat estimated from evoked cerebral potentials. Nat New Biol. 1971 Nov 17;234(46):91–92. doi: 10.1038/newbio234091a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Cooper G. F. Development of the brain depends on the visual environment. Nature. 1970 Oct 31;228(5270):477–478. doi: 10.1038/228477a0. [DOI] [PubMed] [Google Scholar]
- Bonds A. B., Enroth-Cugell C., Pinto L. H. Image quality of the cat eye measured during retinal ganglion cell experiments. J Physiol. 1972 Jan;220(2):383–401. doi: 10.1113/jphysiol.1972.sp009713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Carpenter R. H., Levinson J. Z. Visibility of aperiodic patterns compared with that of sinusoidal gratings. J Physiol. 1969 Oct;204(2):283–298. doi: 10.1113/jphysiol.1969.sp008913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Cooper G. F., Enroth-Cugell C. The spatial selectivity of the visual cells of the cat. J Physiol. 1969 Jul;203(1):223–235. doi: 10.1113/jphysiol.1969.sp008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Green D. G. Optical and retinal factors affecting visual resolution. J Physiol. 1965 Dec;181(3):576–593. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Gubisch R. W. Optical quality of the human eye. J Physiol. 1966 Oct;186(3):558–578. doi: 10.1113/jphysiol.1966.sp008056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Kulikowski J. J., Levinson J. The effect of orientation on the visual resolution of gratings. J Physiol. 1966 Nov;187(2):427–436. doi: 10.1113/jphysiol.1966.sp008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Kulikowski J. J. Orientational selectivity of the human visual system. J Physiol. 1966 Nov;187(2):437–445. doi: 10.1113/jphysiol.1966.sp008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Kulikowski J. J. The visual evoked potential as a function of contrast of a grating pattern. J Physiol. 1972 Apr;222(2):345–356. doi: 10.1113/jphysiol.1972.sp009801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F. W., Maffei L. Electrophysiological evidence for the existence of orientation and size detectors in the human visual system. J Physiol. 1970 May;207(3):635–652. doi: 10.1113/jphysiol.1970.sp009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. D., Mitchell D. E., Millodot M. A neural effect of partial visual deprivation in humans. Science. 1972 Mar 24;175(4028):1384–1386. doi: 10.1126/science.175.4028.1384. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields of single neurones in the cat's striate cortex. J Physiol. 1959 Oct;148:574–591. doi: 10.1113/jphysiol.1959.sp006308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Modification of the distribution of receptive field orientation in cats by selective visual exposure during development. Exp Brain Res. 1971 Jun 29;12(5):509–527. doi: 10.1007/BF00234246. [DOI] [PubMed] [Google Scholar]
- Hirsch H. V., Spinelli D. N. Visual experience modifies distribution of horizontally and vertically oriented receptive fields in cats. Science. 1970 May 15;168(3933):869–871. doi: 10.1126/science.168.3933.869. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L., Campbell F. W. Neurophysiological localization of the vertical and horizontal visual coordinates in man. Science. 1970 Jan 23;167(3917):386–387. doi: 10.1126/science.167.3917.386. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D., Nikara T., Bishop P. O. Responses to moving slits by single units in cat striate cortex. Exp Brain Res. 1968;6(4):373–390. doi: 10.1007/BF00233185. [DOI] [PubMed] [Google Scholar]
- VAKKUR G. J., BISHOP P. O., KOZAK W. VISUAL OPTICS IN THE CAT, INCLUDING POSTERIOR NODAL DISTANCE AND RETINAL LANDMARKS. Vision Res. 1963 Nov;61:289–314. doi: 10.1016/0042-6989(63)90004-x. [DOI] [PubMed] [Google Scholar]



