Abstract
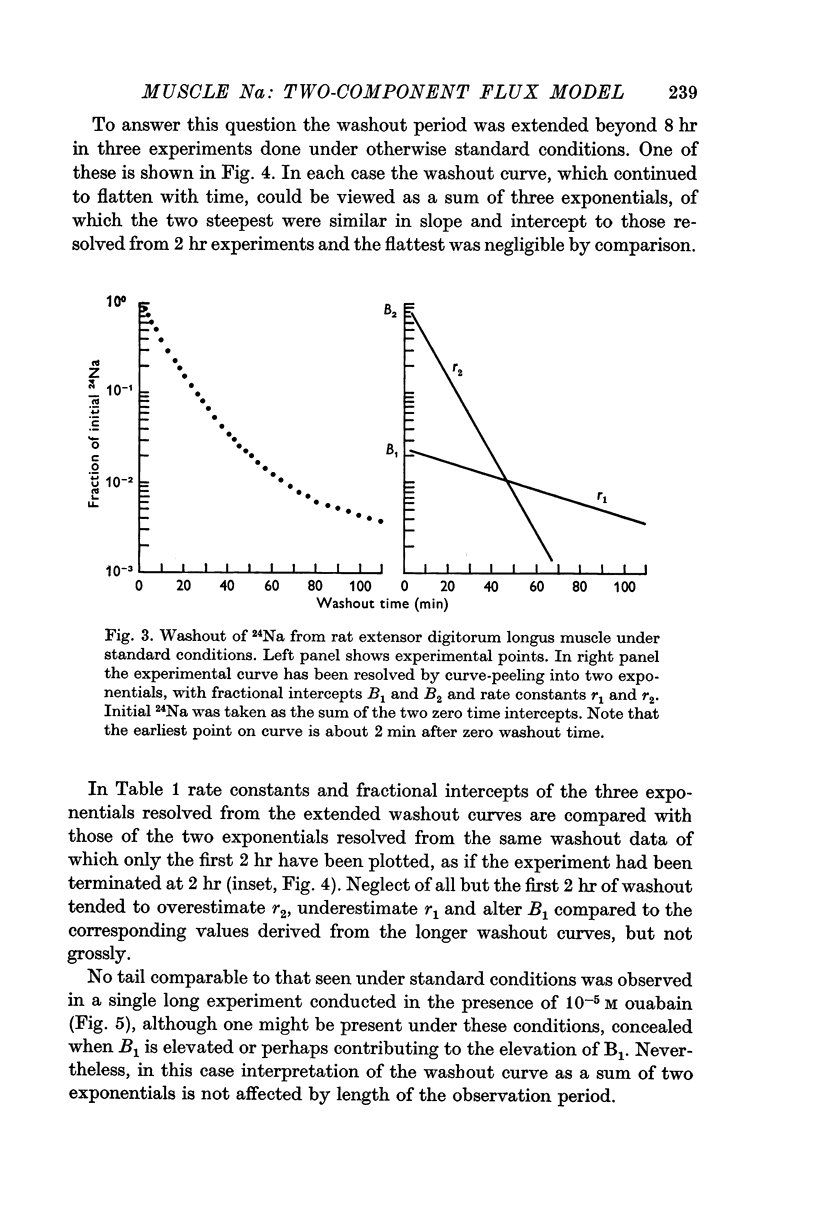
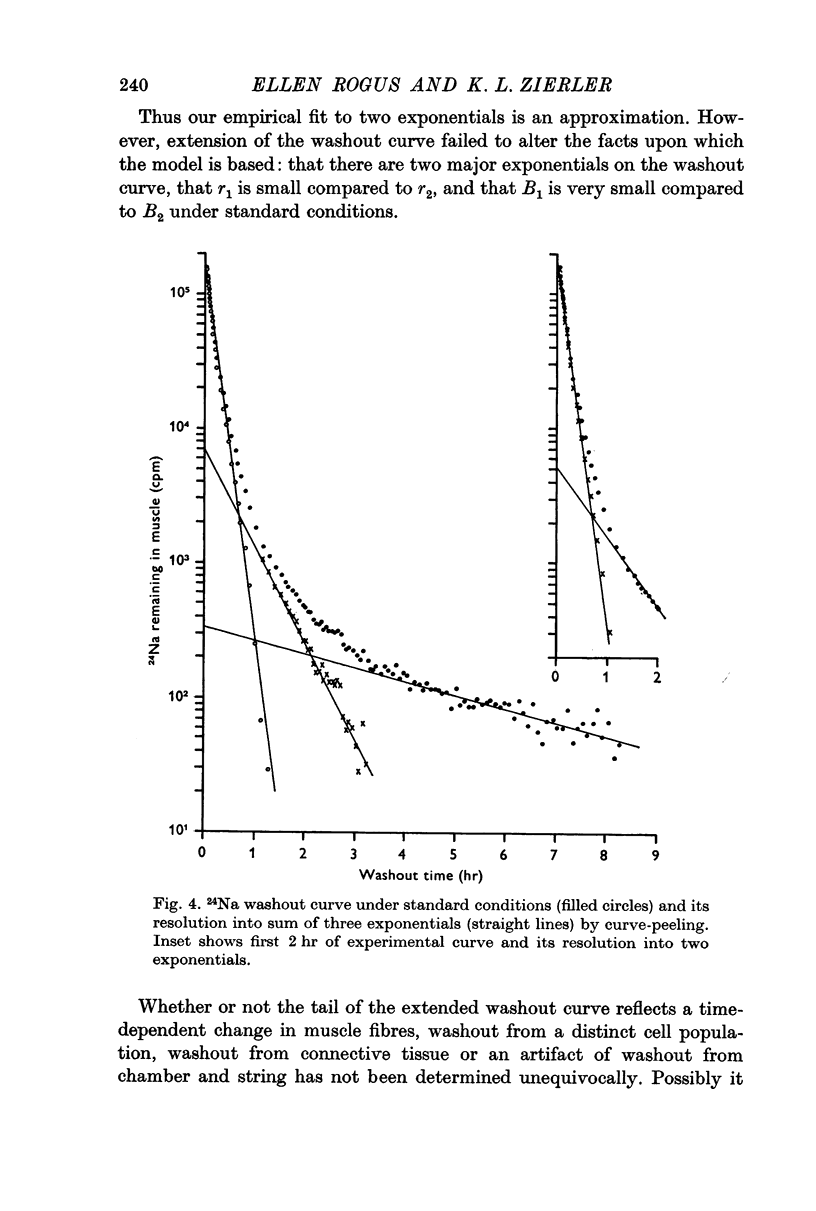
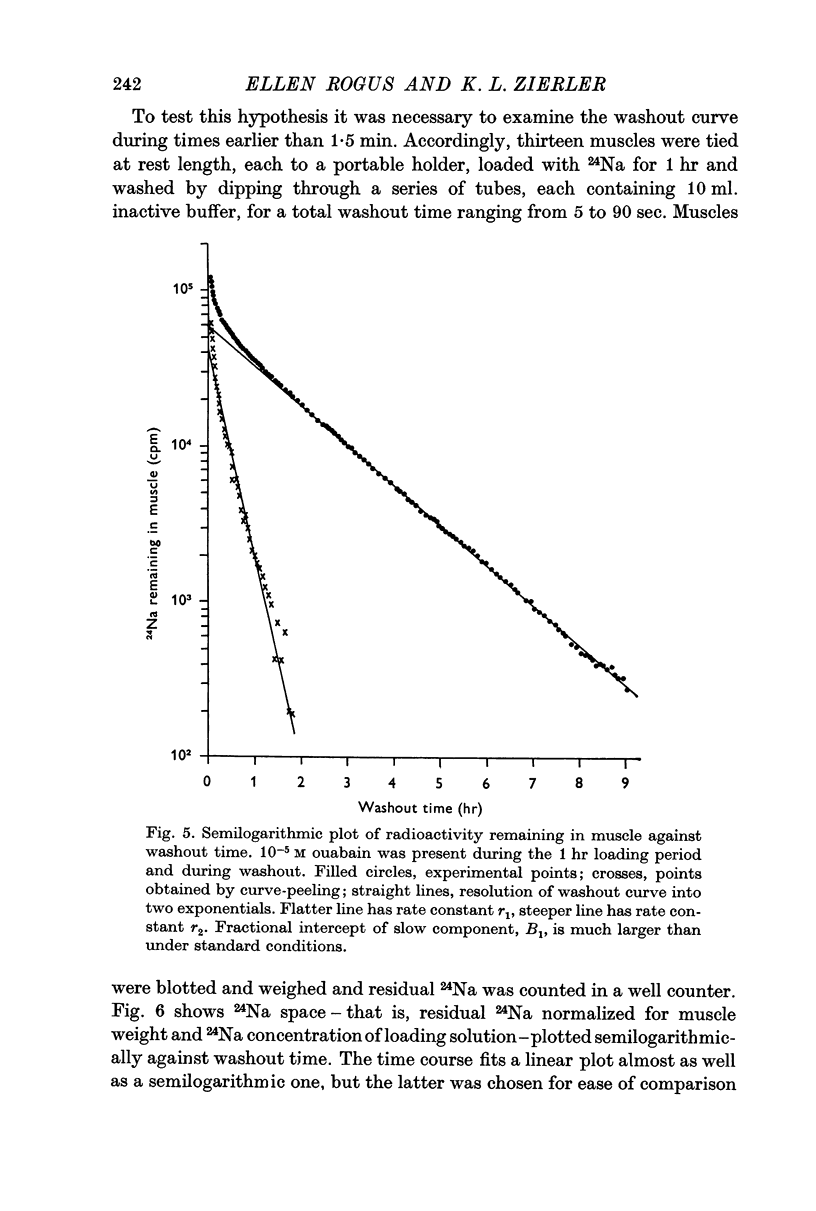
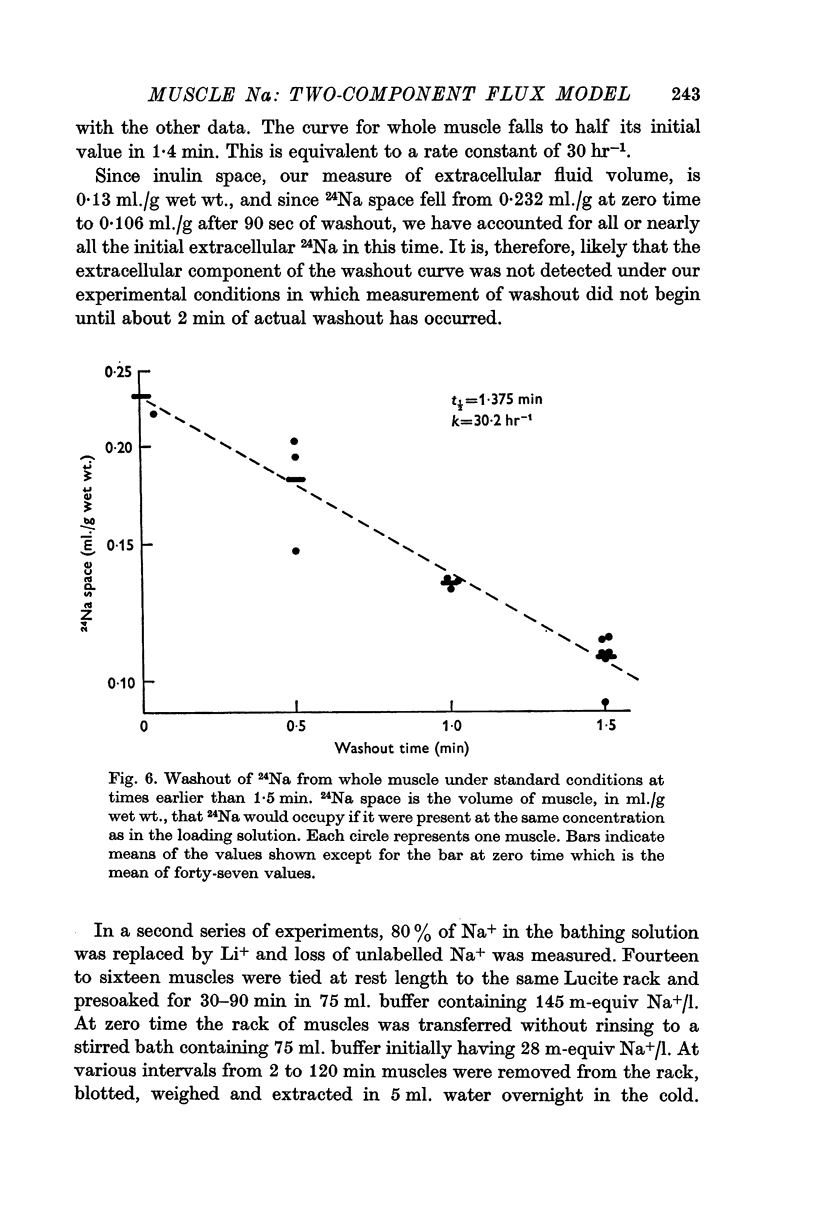
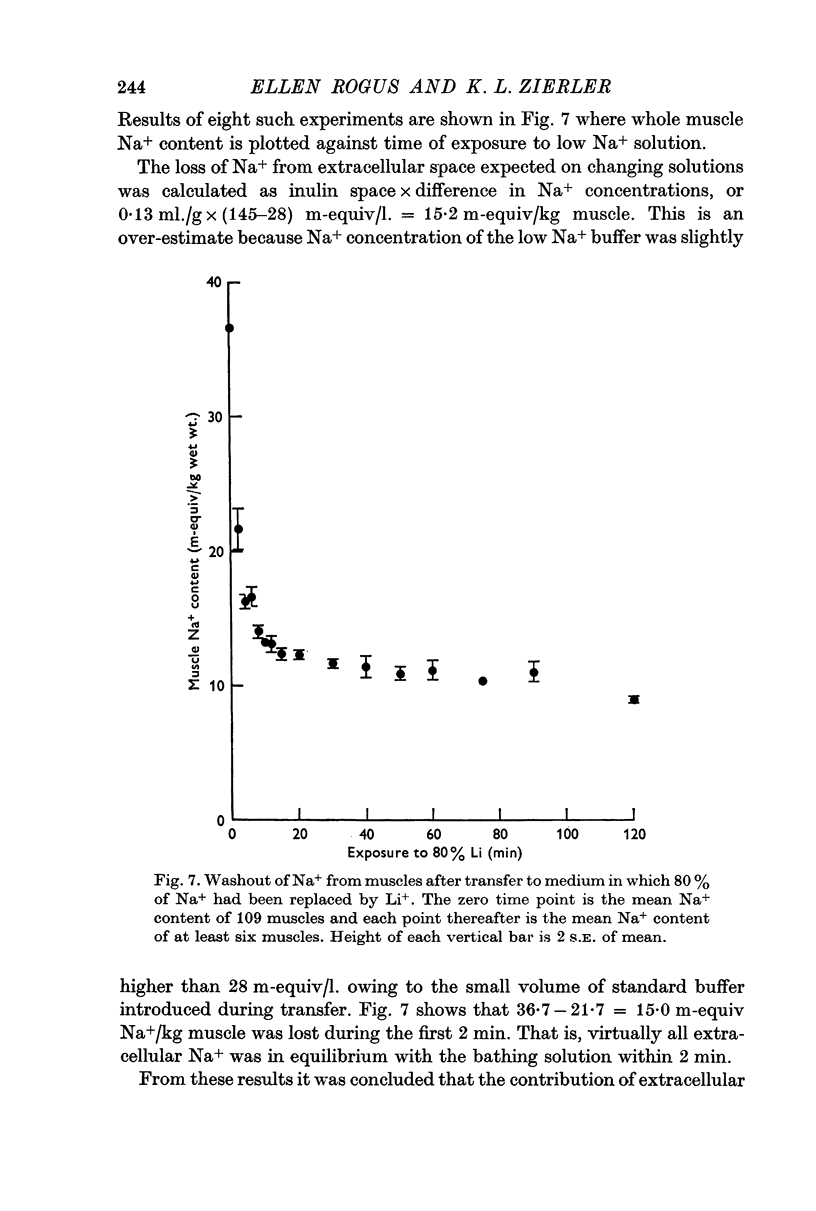
1. During the first 2 hr washout of 24Na from rat extensor digitorum longus muscle fits a sum of two exponentials, neither of which represents loss of extracellular tracer. This implies a model with two intracellular components.
2. Results of suitably designed experiments indicate that the two components are bidirectionally connected to each other as well as to extracellular space. These results are incompatible with a model in which every fibre is homogeneous with respect to Na concentration and flux, but in which there is a distribution of these properties among fibres.
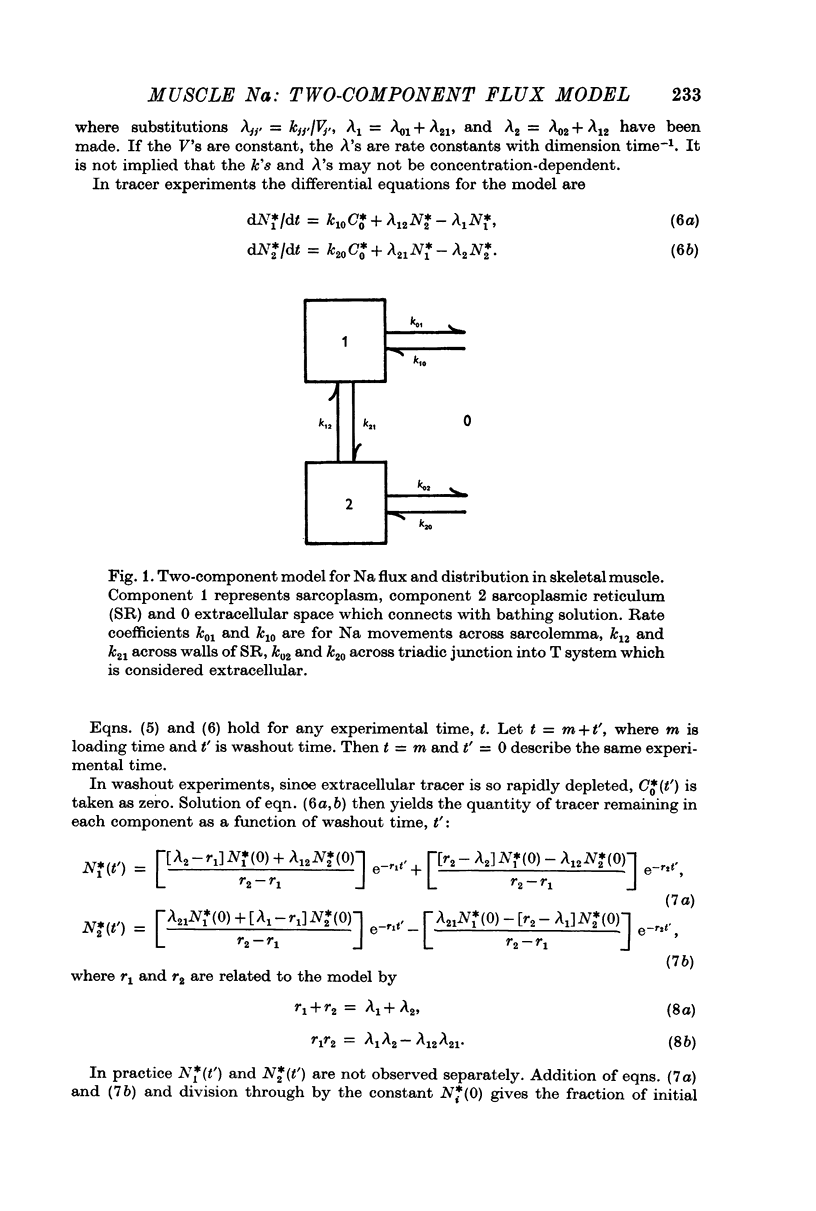
3. Results are consistent with identification of the more slowly exchanging component as sarcoplasm and the more rapidly exchanging component as sarcoplasmic reticulum (SR).
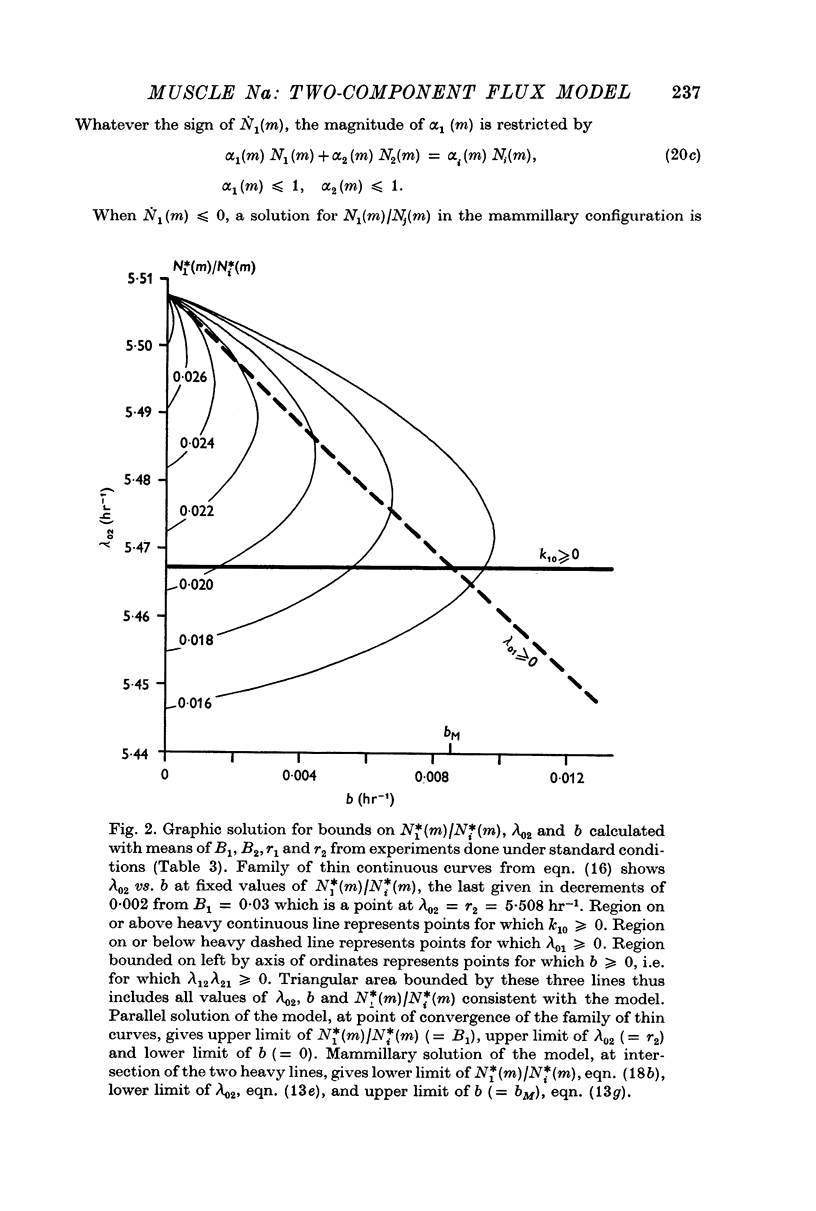
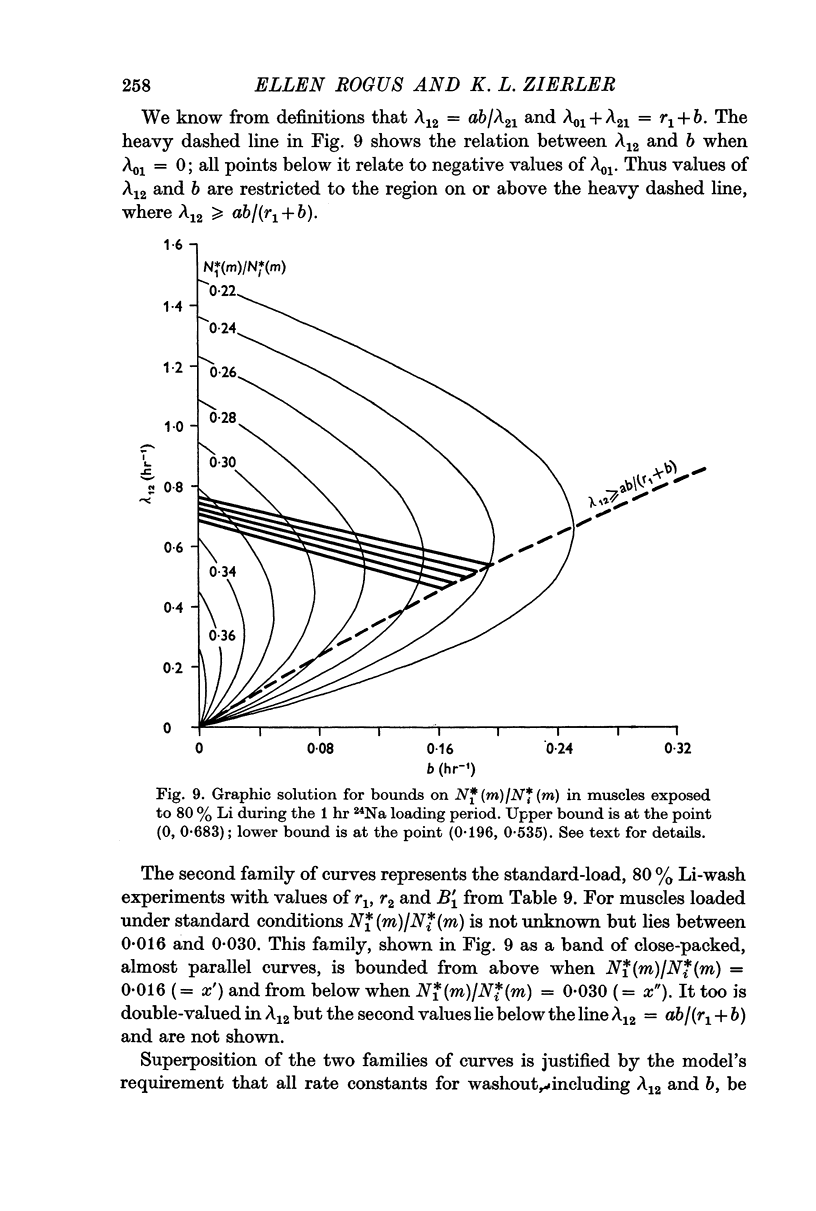
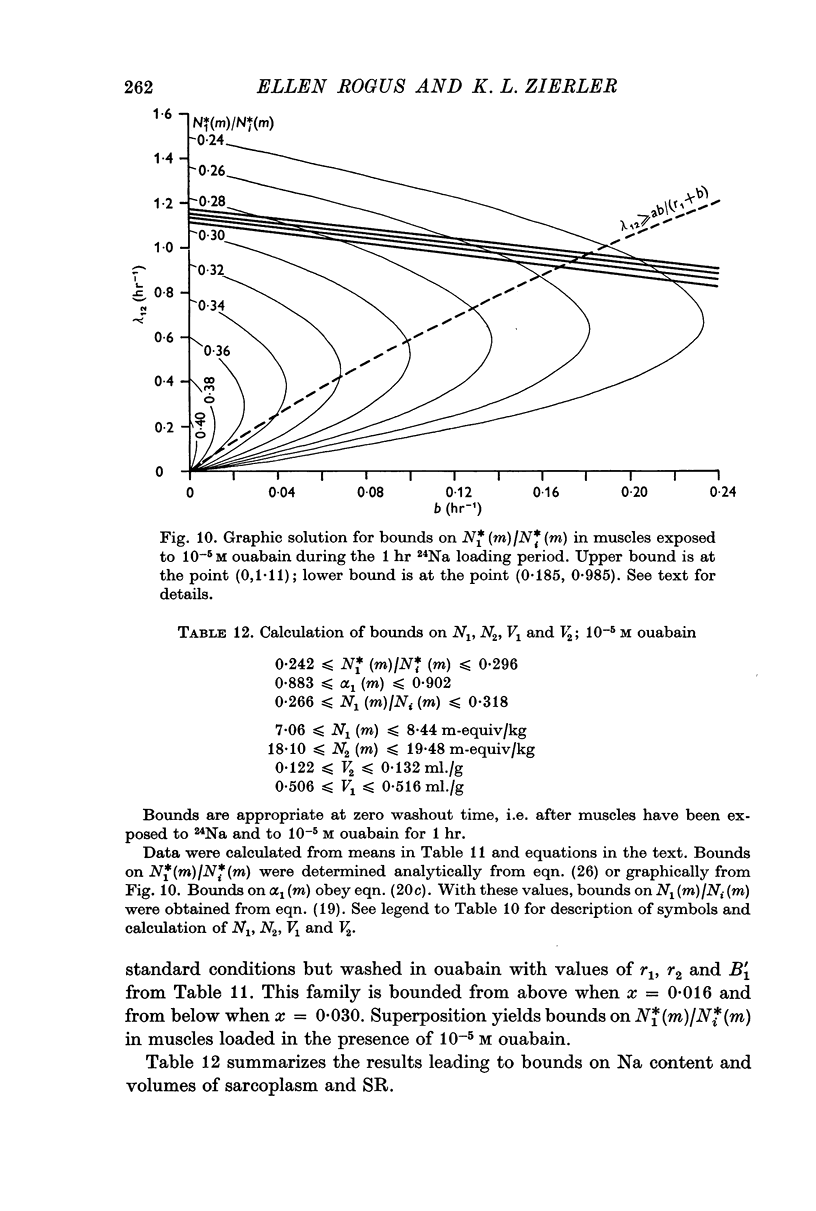
4. Parameters of the general model include six transport coefficients, two volumes, and contents of two Na pools. The number of equations is inadequate to yield unique solutions by which the values of these parameters can be calculated. However, we derive inequalities that place upper and lower limits on the parameters.
5. If the model is correct, the rate constant for Na efflux from SR to extracellular space is at least five times greater than that across sarcolemma. Under standard conditions flux (per muscle weight) from SR is at least 100 times greater than that from sarcoplasm.
6. Under standard conditions, only 2-4% of intracellular Na, or 0·5-0·9 m-equiv/kg wet wt., is in sarcoplasm, and the rest is in SR.
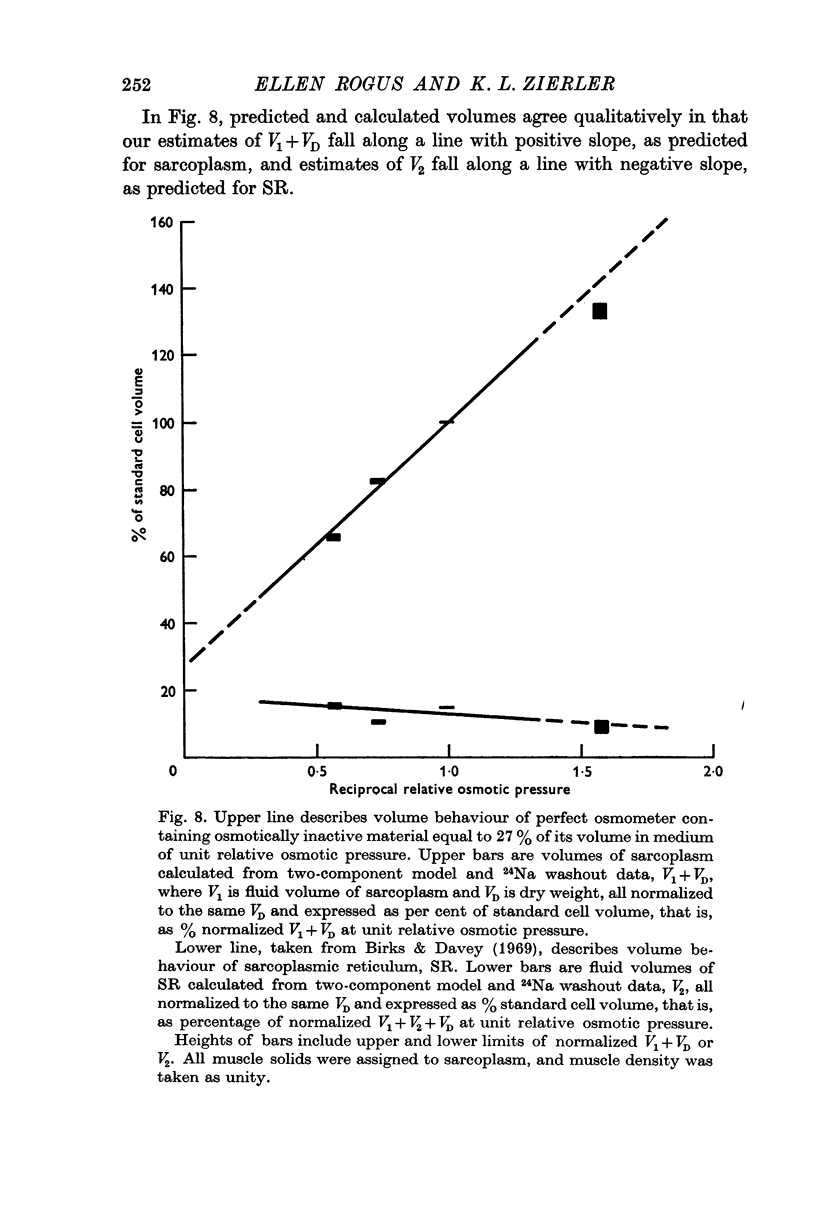
7. Bounds on fluid volumes of sarcoplasm and SR under standard conditions are calculated with the assumption that Na concentration in SR is the same as in extracellular space. According to the calculations, fluid volume of sarcoplasm is 0·54 ml./g wet wt. Fluid volume of SR is about 0·124 ml./g wet wt., or 14·3% of fibre volume, in agreement with Peachey's estimate (1965) of volume of SR in frog muscle.
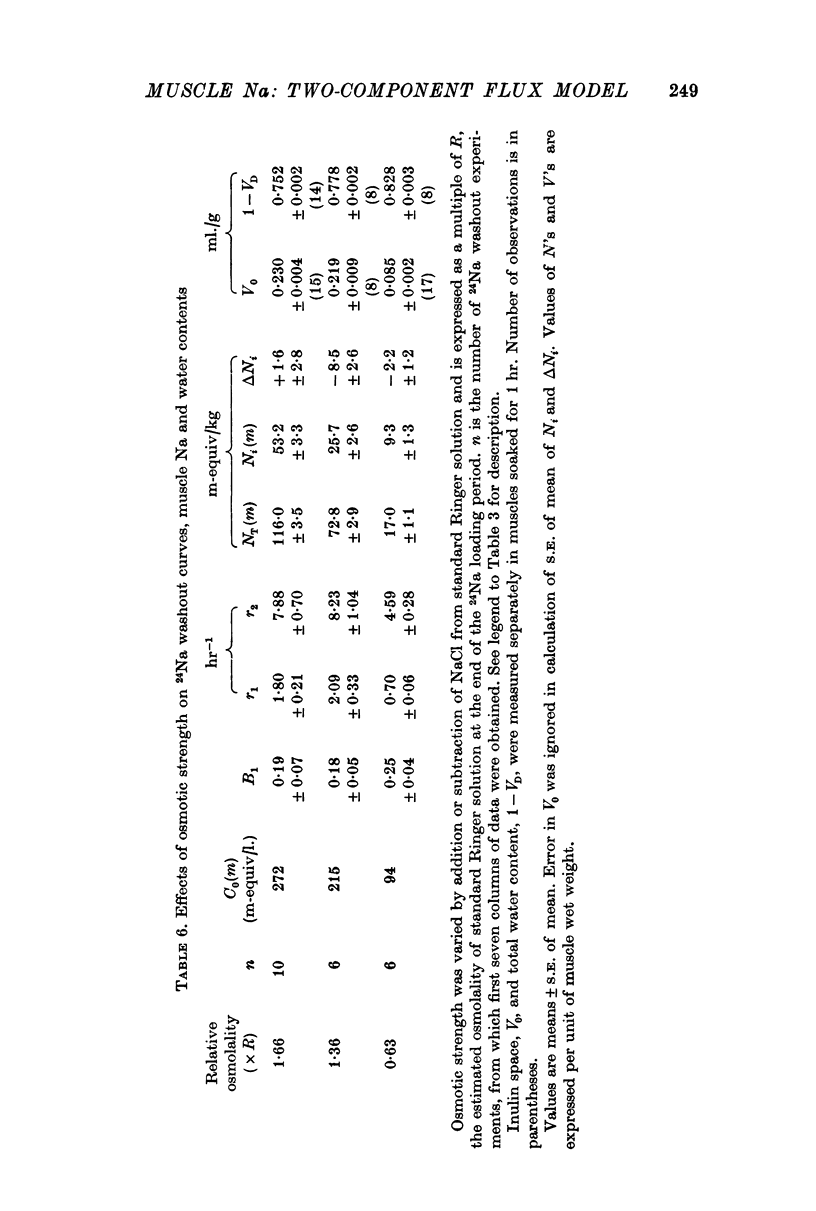
8. Three tests are applied to the model, with the following results: (a) volume of sarcoplasm increases in hypotonic solution and decreases in hypertonic solutions, as predicted for an osmometer. Volume of SR tends to change in the opposite direction, in agreement with results of Birks & Davey (1969) from electron microscopy on frog muscle; (b) the major effect of partial substitution of external Na by Li is a reduction in Na content of SR, with no significant change in that of sarcoplasm or in volume of either component; (c) the major effect of 10-5 M ouabain is an increase in Na content of sarcoplasm, with no demonstrable change in that of SR or in volume of either component.
9. These results support the model, particularly our identification of the slowly exchanging component as sarcoplasm, identification of the rapidly exchanging component as SR, and the assumption that Na concentration in SR is close to that in extracellular fluid.
Full text
PDF
































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaugé L. A., Ortiz O. Lithium-stimulated sodium efflux in frog skeletal muscle. Biochim Biophys Acta. 1970 Dec 1;219(2):479–483. doi: 10.1016/0005-2736(70)90226-9. [DOI] [PubMed] [Google Scholar]
- Beaugé L. A., Sjodin R. A. The dual effect of lithium ions on sodium efflux in skeletal muscle. J Gen Physiol. 1968 Sep;52(3):408–423. doi: 10.1085/jgp.52.3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks R. I., Davey D. F. Osmotic responses demonstrating the extracellular character of the sarcoplasmic reticulum. J Physiol. 1969 May;202(1):171–188. doi: 10.1113/jphysiol.1969.sp008802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAREY M. J., CONWAY E. J. Comparison of various media for immersing frog sartorii at room temperature, and evidence for the regional distribution of fibre Na+. J Physiol. 1954 Aug 27;125(2):232–250. doi: 10.1113/jphysiol.1954.sp005154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J. Nature and significance of concentration relations of potassium and sodium ions in skeletal muscle. Physiol Rev. 1957 Jan;37(1):84–132. doi: 10.1152/physrev.1957.37.1.84. [DOI] [PubMed] [Google Scholar]
- Caldwell P. C. Factors governing movement and distribution of inorganic ions in nerve and muscle. Physiol Rev. 1968 Jan;48(1):1–64. doi: 10.1152/physrev.1968.48.1.1. [DOI] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J. Depolarization of the internal membrane system in the activation of frog skeletal muscle. J Gen Physiol. 1967 May;50(5):1101–1124. doi: 10.1085/jgp.50.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R. Sodium fluxes in diaphragm muscle and the effects of insulin and serum proteins. J Physiol. 1968 Jul;197(2):255–278. doi: 10.1113/jphysiol.1968.sp008558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J. Distribution and movement of muscle chloride. J Physiol. 1963 Apr;166:87–109. doi: 10.1113/jphysiol.1963.sp007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J., STEINBACH H. B. The extraction of ions from muscle by water and sugar solutions with a study of the degree of exchange with tracer of the sodium and potassium in the extracts. J Physiol. 1956 Aug 28;133(2):385–401. doi: 10.1113/jphysiol.1956.sp005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Movements of Na and K in single muscle fibres. J Physiol. 1959 Mar 3;145(2):405–432. doi: 10.1113/jphysiol.1959.sp006150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWICZ P., GERBER C. J. EFFECTS OF EXTERNAL POTASSIUM AND STROPHANTHIDIN ON SODIUM FLUXES IN FROG STRIATED MUSCLE. J Gen Physiol. 1965 Jan;48:489–514. doi: 10.1085/jgp.48.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E. G. Potassium exchange and afterpotentials in frog sartorius muscles treated with glycerol. J Gen Physiol. 1970 Dec;56(6):692–715. doi: 10.1085/jgp.56.6.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic fluxes in frog muscle. Proc R Soc Lond B Biol Sci. 1954 May 27;142(908):359–382. doi: 10.1098/rspb.1954.0030. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Steinhardt R. A. The components of the sodium efflux in frog muscle. J Physiol. 1968 Oct;198(3):581–599. doi: 10.1113/jphysiol.1968.sp008627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Rogus E., Price T., Zierler K. L. Sodium plus potassium-activated, ouabain-inhibited adenosine triphosphatase from a fraction of rat skeletal muscle, and lack of insulin effect on it. J Gen Physiol. 1969 Aug;54(2):188–202. doi: 10.1085/jgp.54.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H. Transport of ions across cellular membranes. Physiol Rev. 1949 Apr;29(2):127–155. doi: 10.1152/physrev.1949.29.2.127. [DOI] [PubMed] [Google Scholar]
- Zierler K. L., Rogus E., Hazlewood C. F. Effect of insulin on potassium flux and water and electrolyte content of muscles from normal and from hypophysectomized rats. J Gen Physiol. 1966 Jan;49(3):433–456. doi: 10.1085/jgp.49.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]


