Abstract
1. Evidence was obtained that the impulse/quantum (I/Q) ratio of the central response mechanism of retinal ganglion cells in the cat is controlled by the steady effective retinal flux of the background.
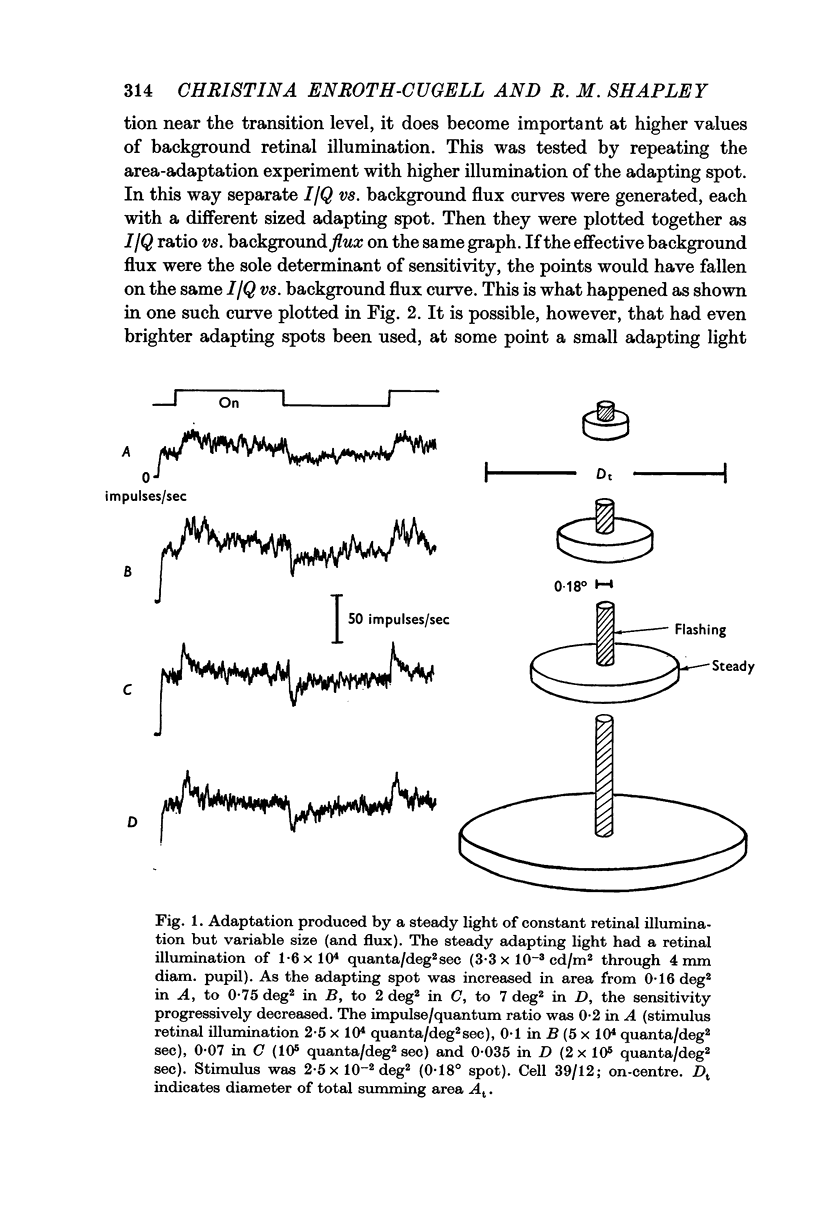
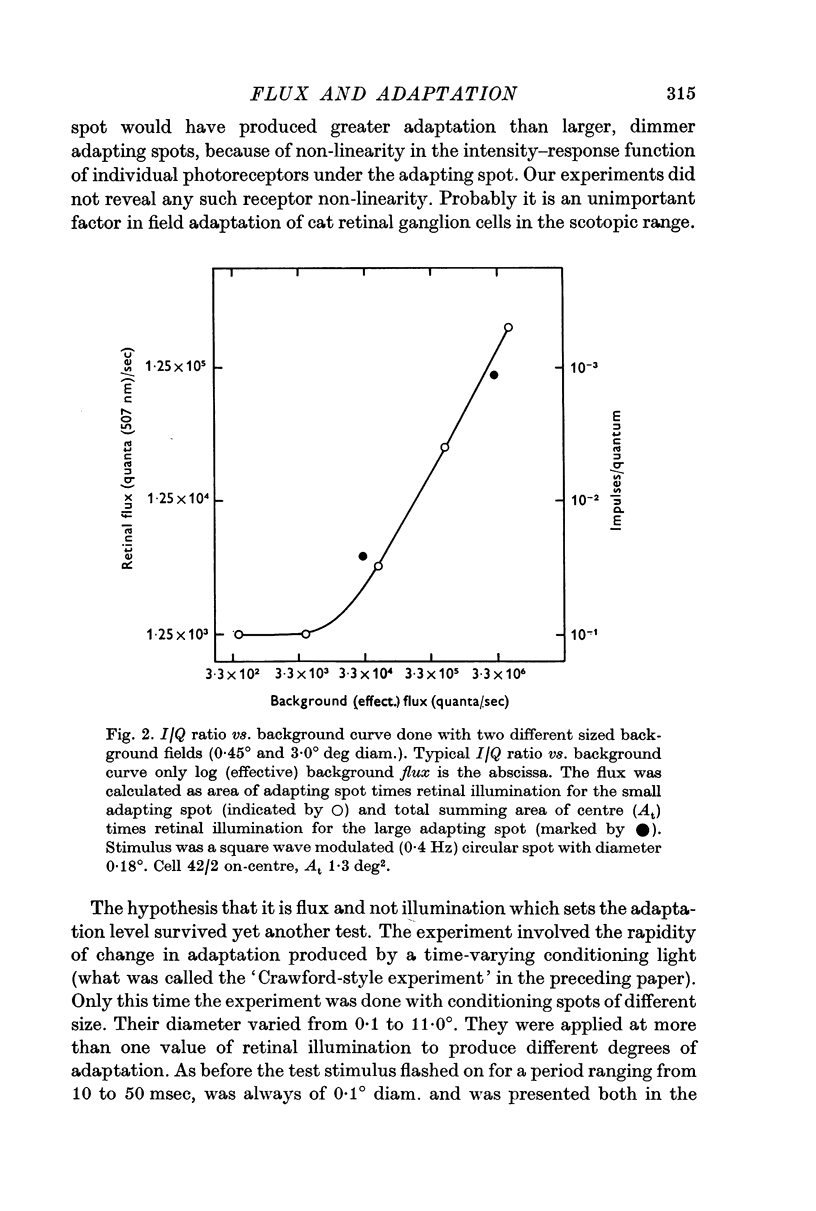
2. One experiment revealed that the I/Q ratio was decreased as the area of an adapting spot, of constant illumination, was increased. The curve relating the I/Q ratio to background flux was the same regardless of the size of the adapting spot.
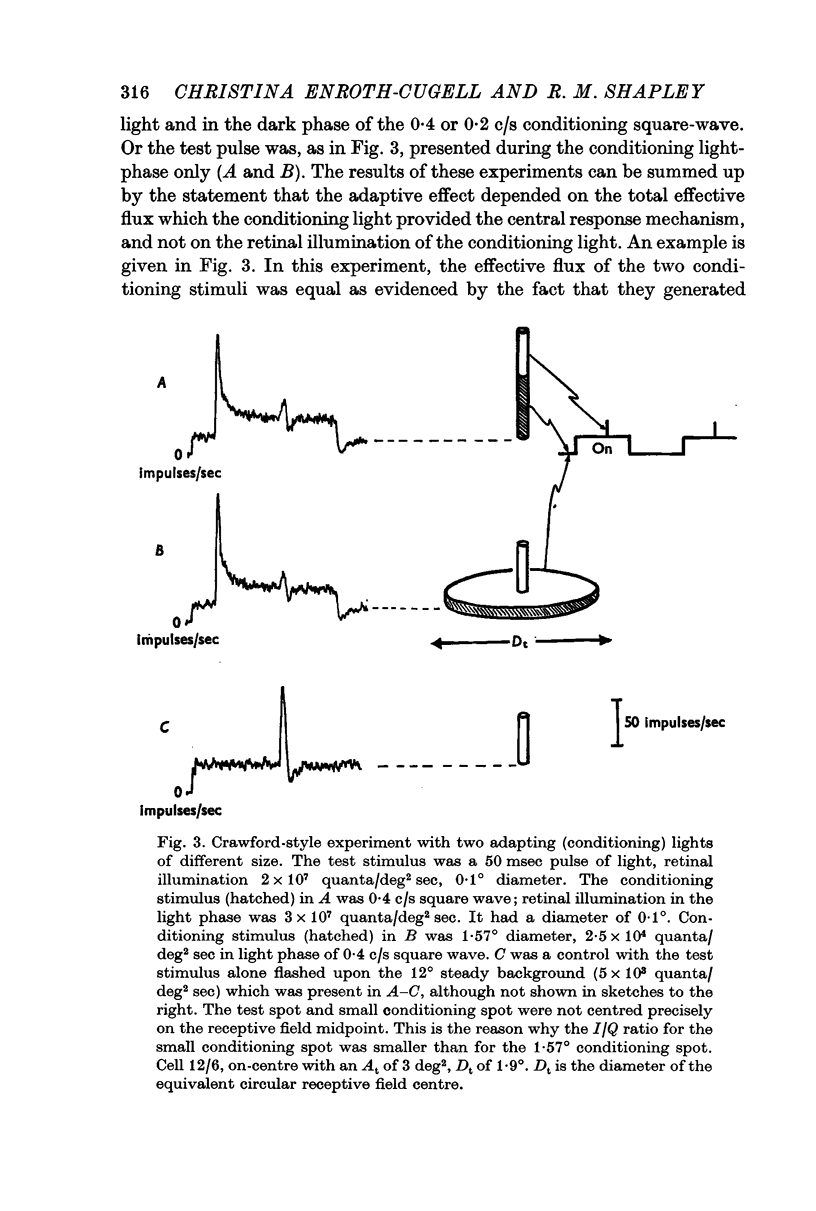
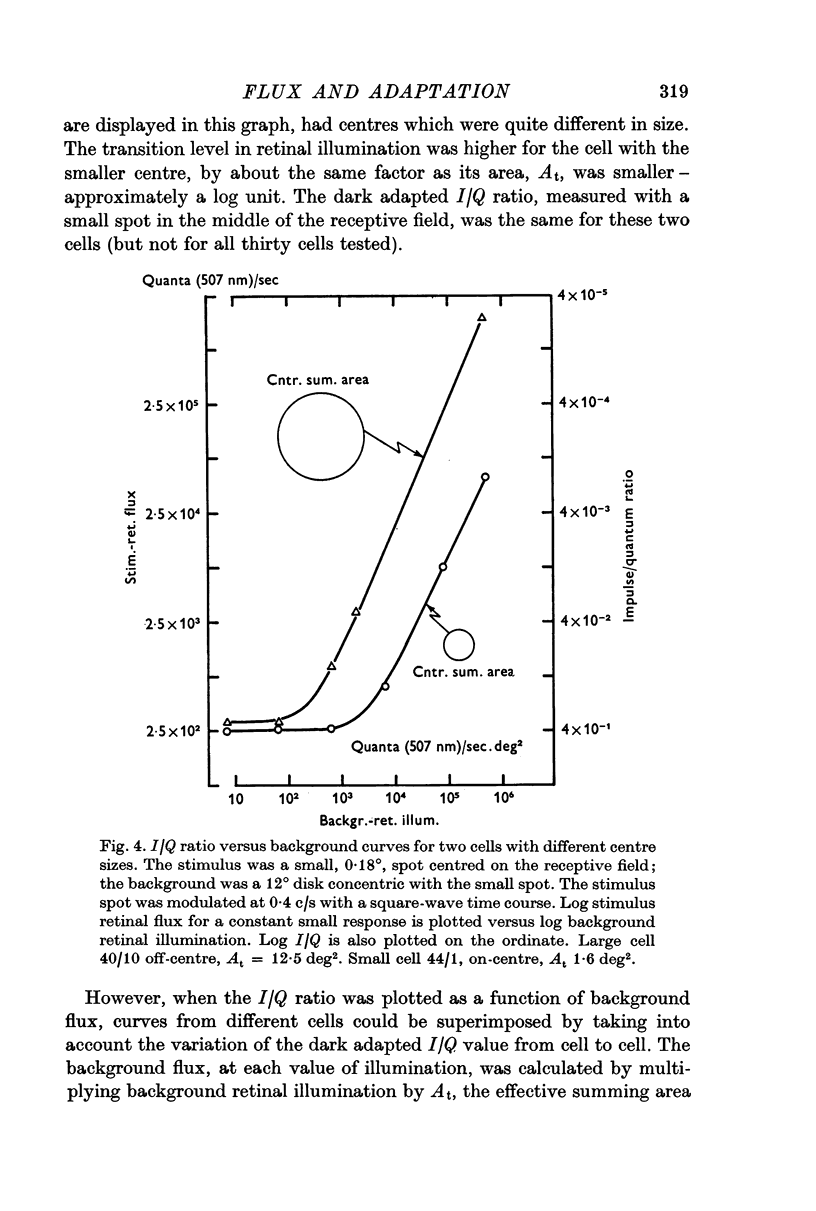
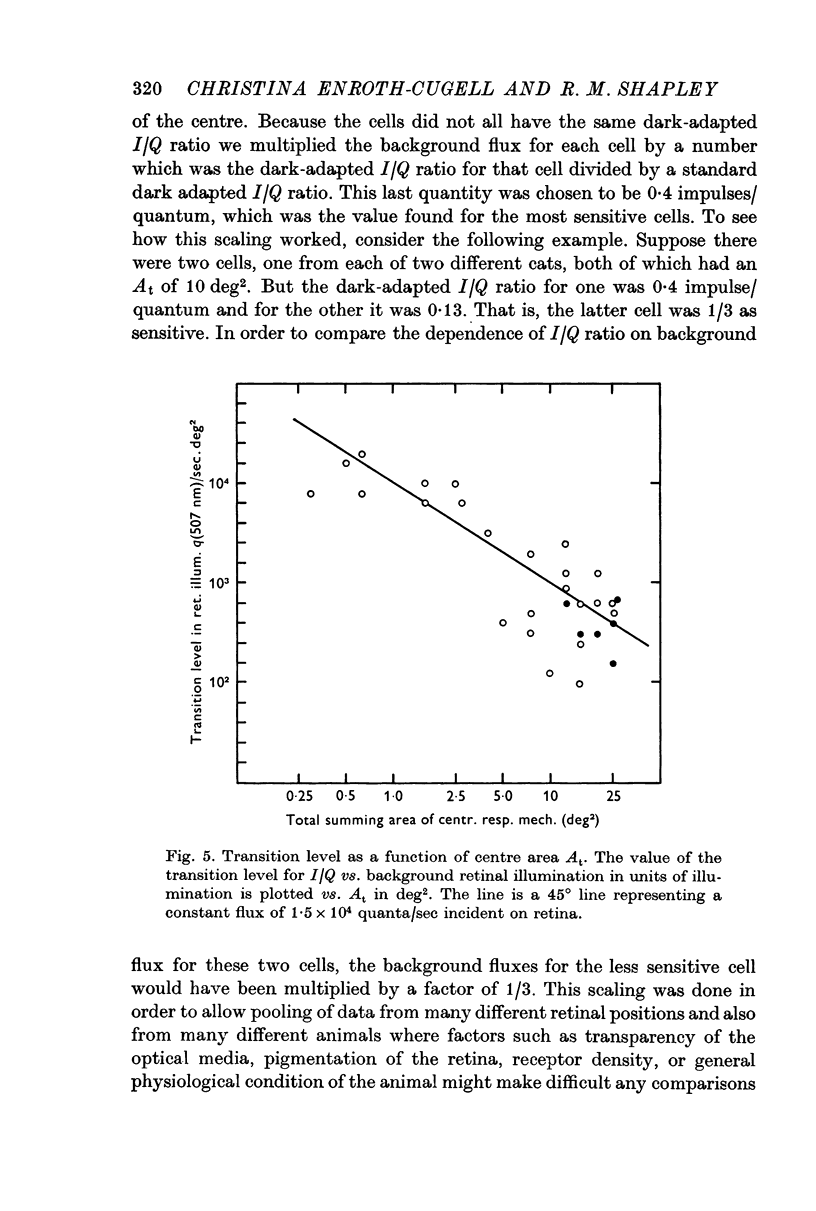
3. The effective central summing area of many retinal ganglion cells was determined. For the same cells, the transition level (Enroth-Cugell & Shapley, 1973) of the impulse/quantum curve was also measured. Diffuse illumination at the transition level was inversely proportional to the effective summing area, when variation in dark-adapted sensitivity between cells was taken into account.
4. Therefore, retinal ganglion cells with large central summing areas are more light-adapted by any given diffuse background than cells with small centres.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A., Berntson G. G. Light adaptation and excitation: lateral spread of signals within the frog retina. Vision Res. 1972 Jun;12(6):1095–1111. doi: 10.1016/0042-6989(72)90100-9. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-Cugell C. Quantitative aspects of gain and latency in the cat retina. J Physiol. 1970 Jan;206(1):73–91. doi: 10.1113/jphysiol.1970.sp008998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Enroth-cugell C. Quantitative aspects of sensitivity and summation in the cat retina. J Physiol. 1968 Sep;198(1):17–38. doi: 10.1113/jphysiol.1968.sp008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Levick W. R., Sanderson K. J. Properties of sustained and transient ganglion cells in the cat retina. J Physiol. 1973 Feb;228(3):649–680. doi: 10.1113/jphysiol.1973.sp010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. S-potentials in the skate retina. Intracellular recordings during light and dark adaptation. J Gen Physiol. 1971 Aug;58(2):163–189. doi: 10.1085/jgp.58.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easter S. S., Jr Adaptation in the goldfish retina. J Physiol. 1968 Mar;195(2):273–281. doi: 10.1113/jphysiol.1968.sp008458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Pinto L. H. Properties of the surround response mechanism of cat retinal ganglion cells and centre-surround interaction. J Physiol. 1972 Jan;220(2):403–439. doi: 10.1113/jphysiol.1972.sp009714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Shapley R. M. Adaptation and dynamics of cat retinal ganglion cells. J Physiol. 1973 Sep;233(2):271–309. doi: 10.1113/jphysiol.1973.sp010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B., May H. U. Invarianzen in der Katzenretina: Gesetzmässige Beziehungen zwischen Empfindlichkeit, Grösse und Lage receptiver Felder von Ganglienzellen. Exp Brain Res. 1970;11(5):448–464. doi: 10.1007/BF00233968. [DOI] [PubMed] [Google Scholar]
- Maffei L. Inhibitory and facilitatory spatial interactions in retinal receptive fields. Vision Res. 1968 Sep;8(9):1187–1194. doi: 10.1016/0042-6989(68)90026-6. [DOI] [PubMed] [Google Scholar]
- Schellart N. A., Spekreijse H. Dynamic characteristics of retinal ganglion cell responses in goldfish. J Gen Physiol. 1972 Jan;59(1):1–21. doi: 10.1085/jgp.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H. Incremental responses to light recorded from pigment epithelial cells and horizontal cells of the cat retina. J Physiol. 1971 Aug;217(1):93–110. doi: 10.1113/jphysiol.1971.sp009561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESEL T. N. Receptive fields of ganglion cells in the cat's retina. J Physiol. 1960 Oct;153:583–594. doi: 10.1113/jphysiol.1960.sp006557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Adaptation in a vertebrate retina: intracellular recording in Necturus. J Neurophysiol. 1971 Mar;34(2):228–241. doi: 10.1152/jn.1971.34.2.228. [DOI] [PubMed] [Google Scholar]
- van Nes F. L., Koenderink J. J., Nas H., Bouman M. A. Spatiotemporal modulation transfer in the human eye. J Opt Soc Am. 1967 Sep;57(9):1082–1088. doi: 10.1364/josa.57.001082. [DOI] [PubMed] [Google Scholar]


