Abstract
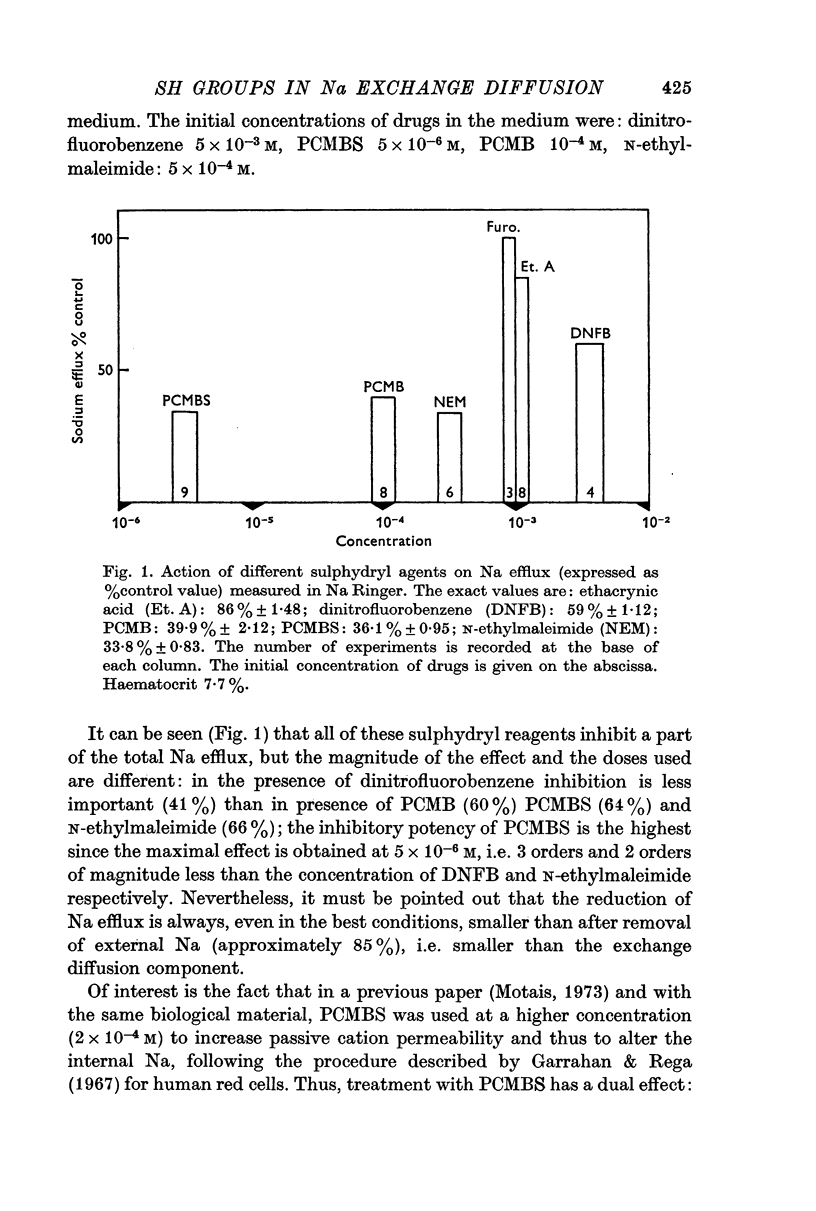
1. In the beef red blood cell, the component of the Na efflux which is insensitive to ouabain but depends on the presence of external Na, is not affected by furosemide but is reduced by several agents: ethacrynic acid, dinitrofluorobenzene, p-chloromercuribenzene (PCMB), N-ethylmaleimide and p-chloromercuribenzene sulphonate (PCMBS). Some of these agents increased a parallel passive permeability which could mask the reduction of efflux.
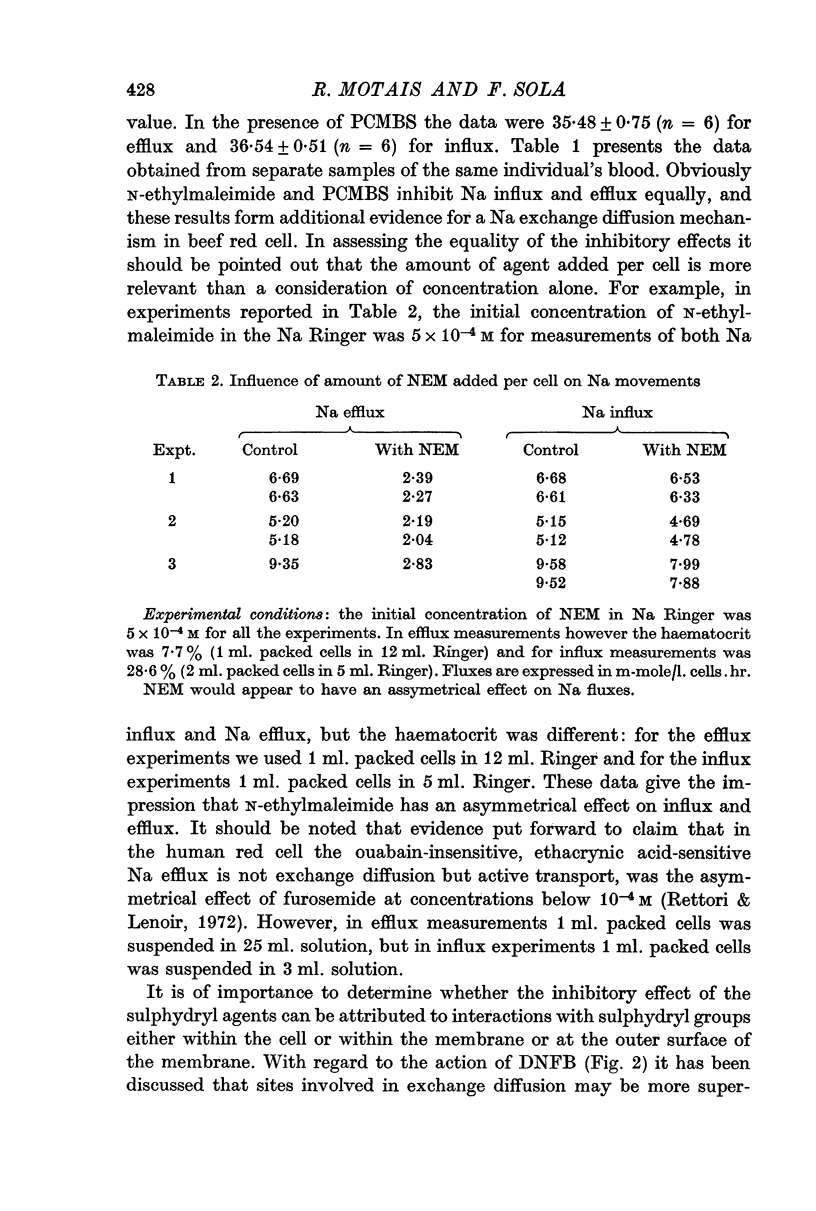
2. N-ethylmaleimide and PCMBS, which in our experimental conditions (initial concentration 5 × 10-4 M and 5 × 10-6 M respectively, haematocrit 7·7%) do not increase the leak, inhibit Na influx and efflux markedly and equally. This provides further evidence of the existence of a typical ouabain-insensitive Na exchange diffusion in beef red blood cell.
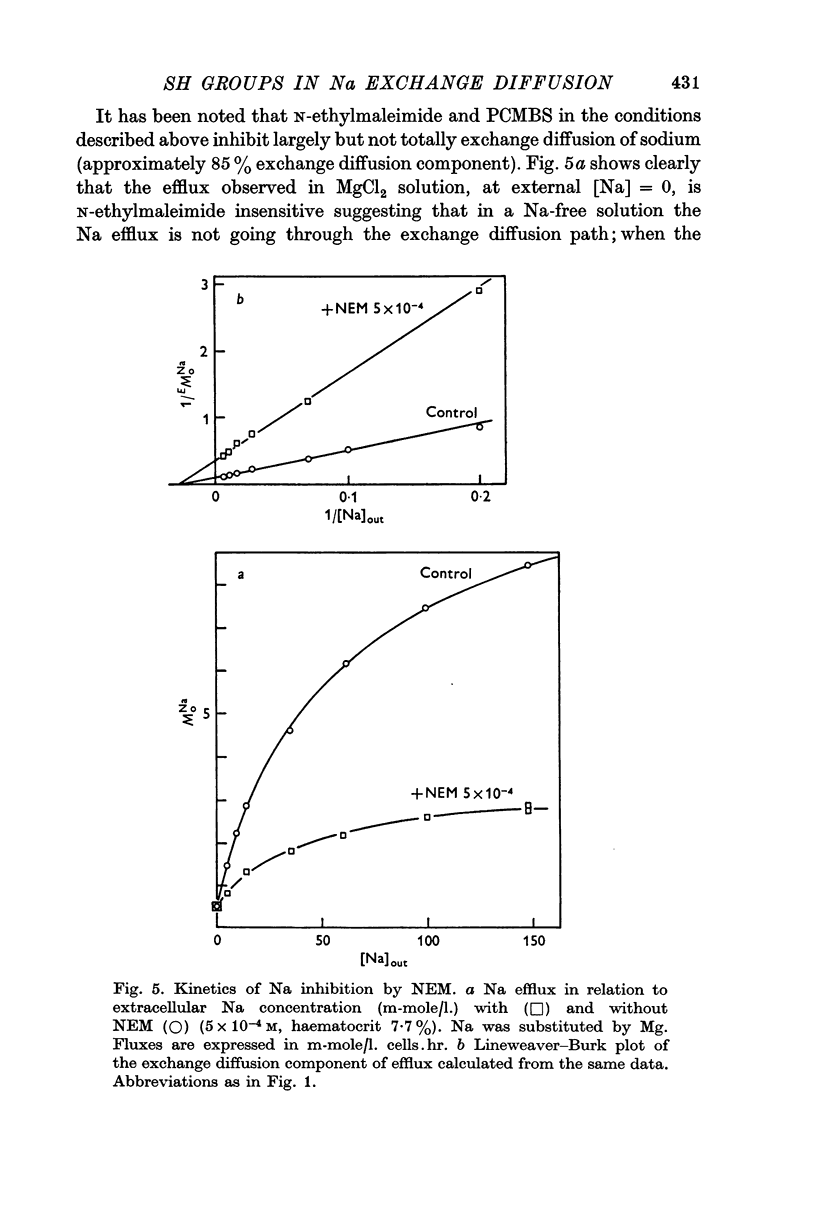
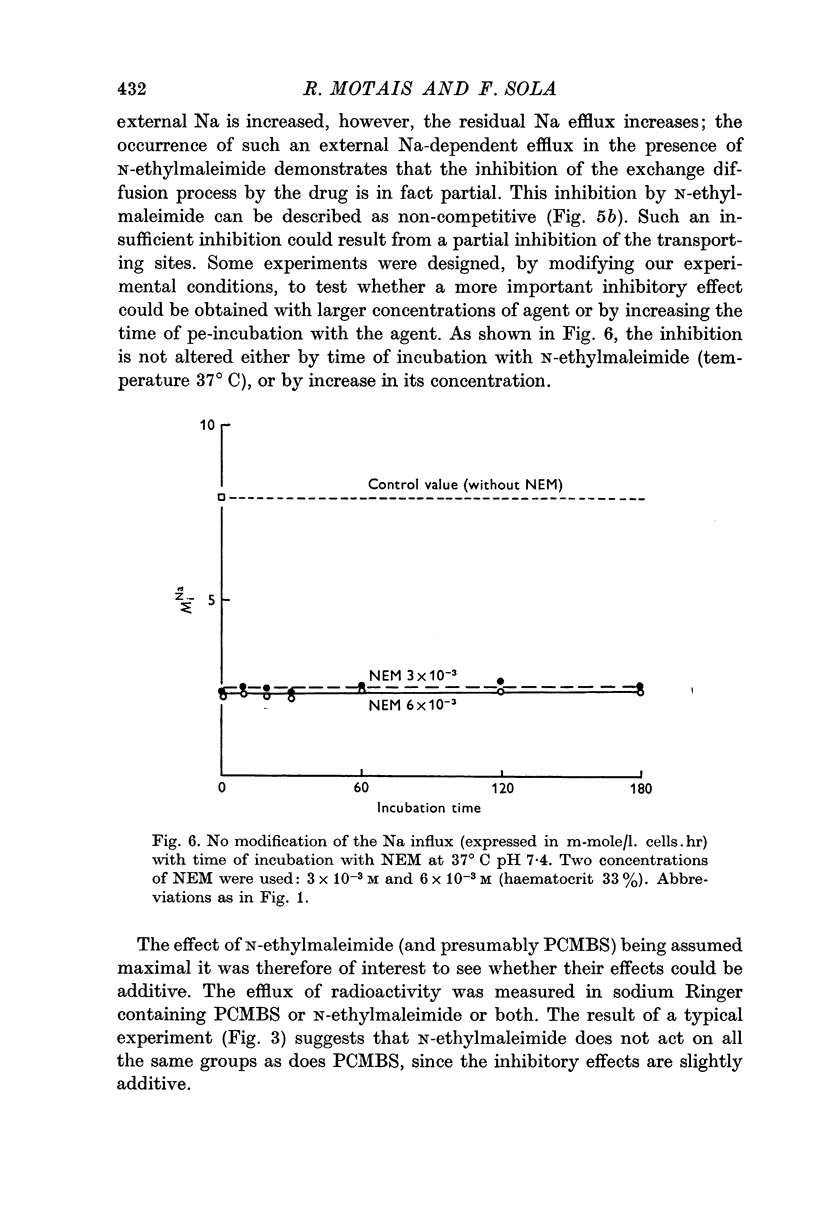
3. Inhibition of the exchange diffusion mechanism by N-ethylmaleimide or PCMBS is not total and their inhibitory effects are slightly additive. Various arguments suggest that their effects on exchange diffusion can be attributed to a reaction with sulphydryl groups.
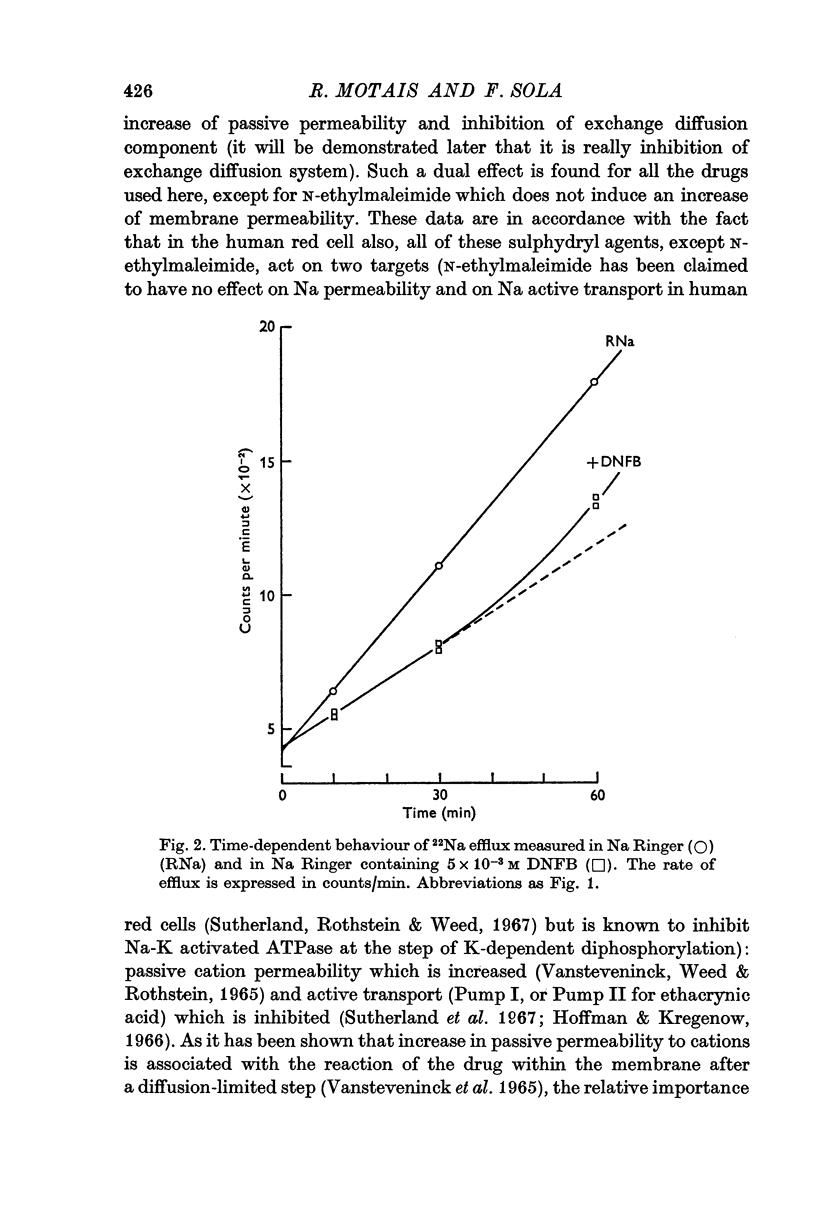
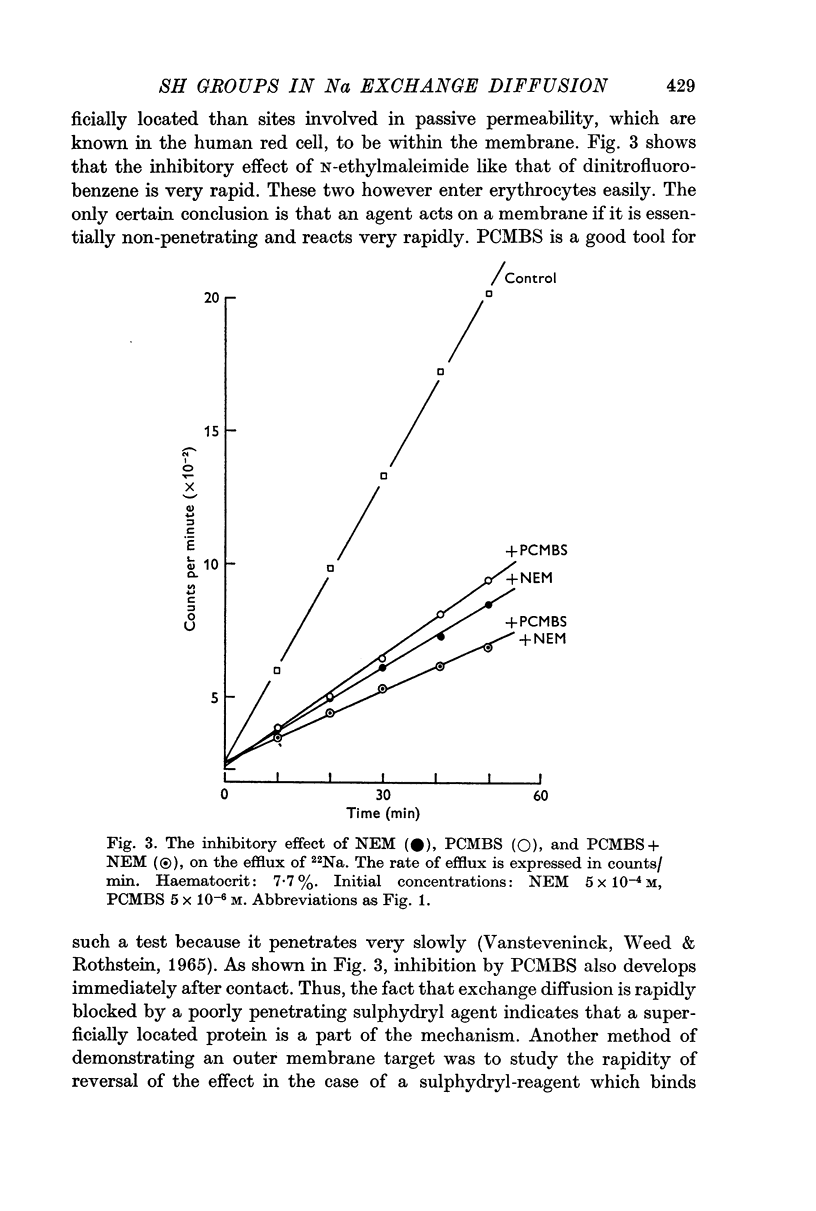
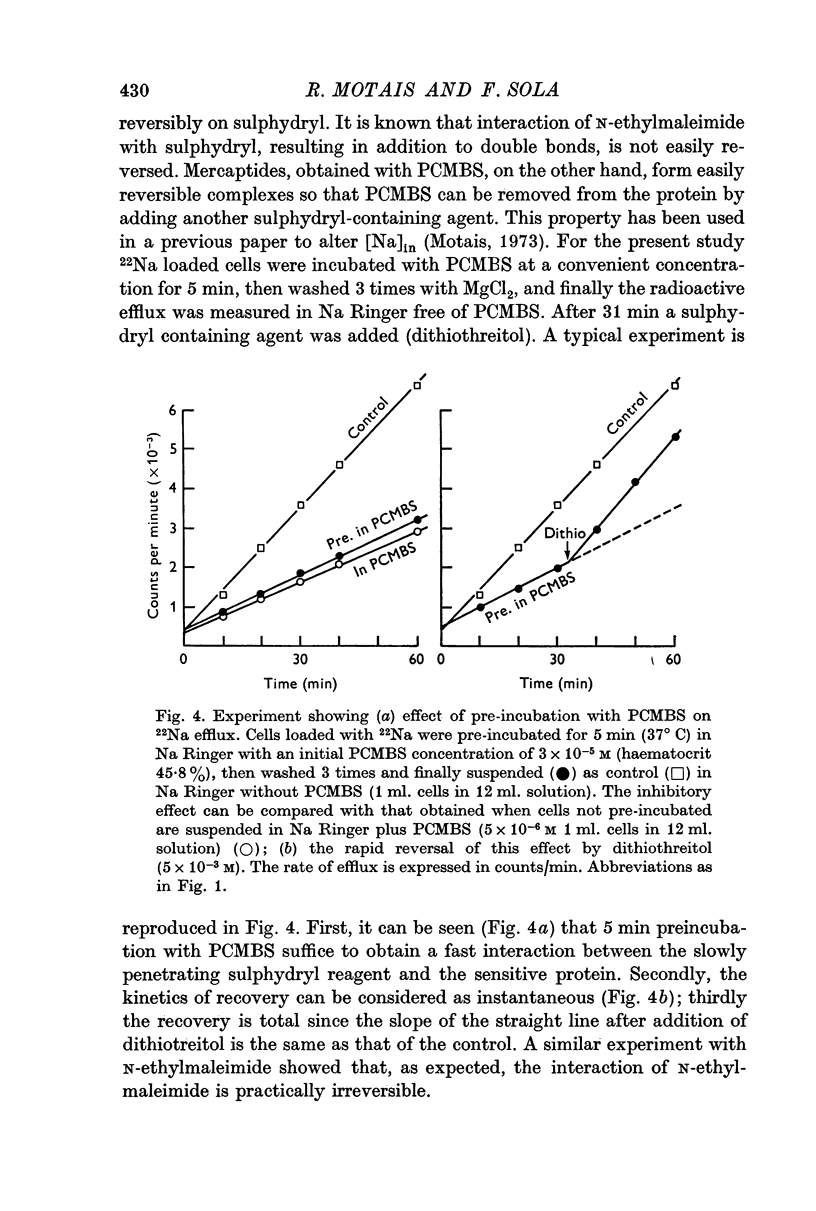
4. These sulphydryl groups are rapidly titrable by a poorly penetrating agent such as PCMBS, and the inhibitory effect is rapidly reversible. Thus, it is assumed that the sulphydryl groups containing proteins are superficially located on the outer border of the membrane.
5. After inhibition, there is no change in half saturation constant for the complexing reaction for transfer, suggesting that the inhibited sites are no longer functioning but that the uninhibited sites are in every way normal.
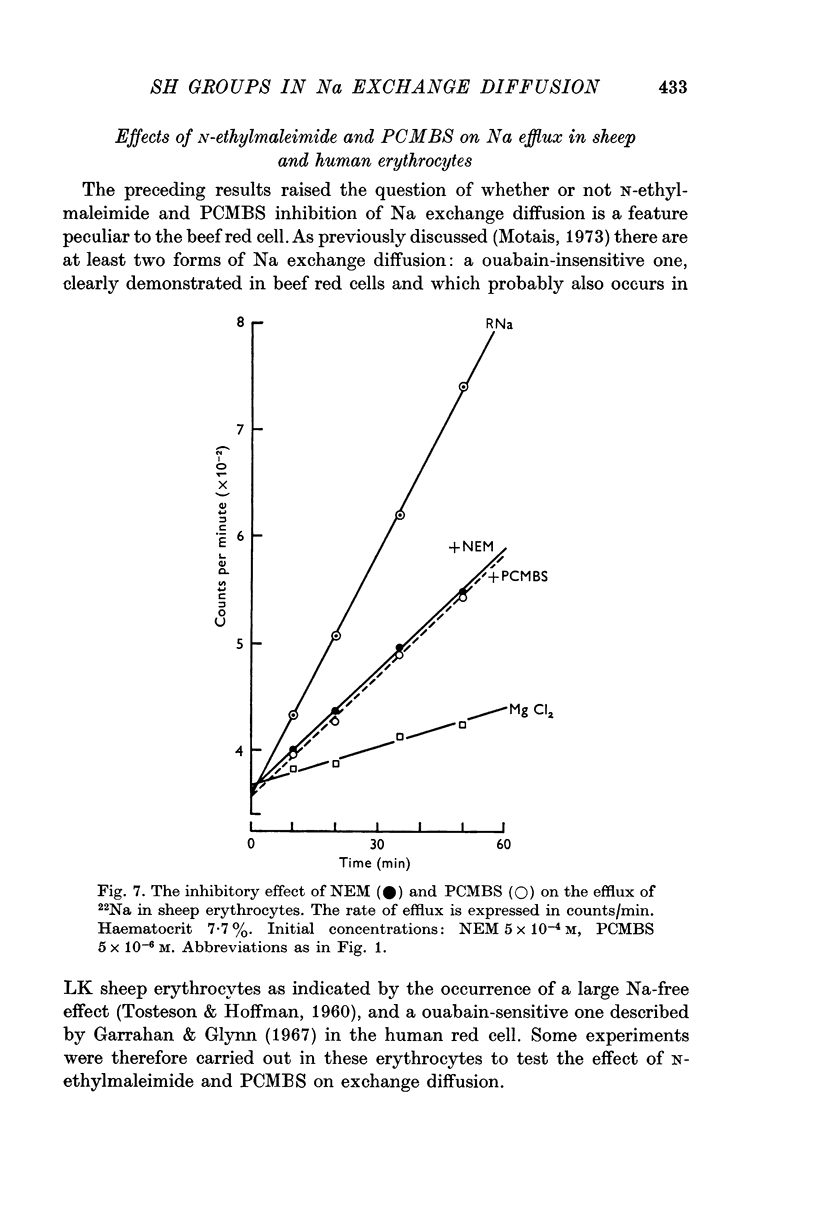
6. N-ethylmaleimide and PCMBS act similarly in sheep red blood cells.
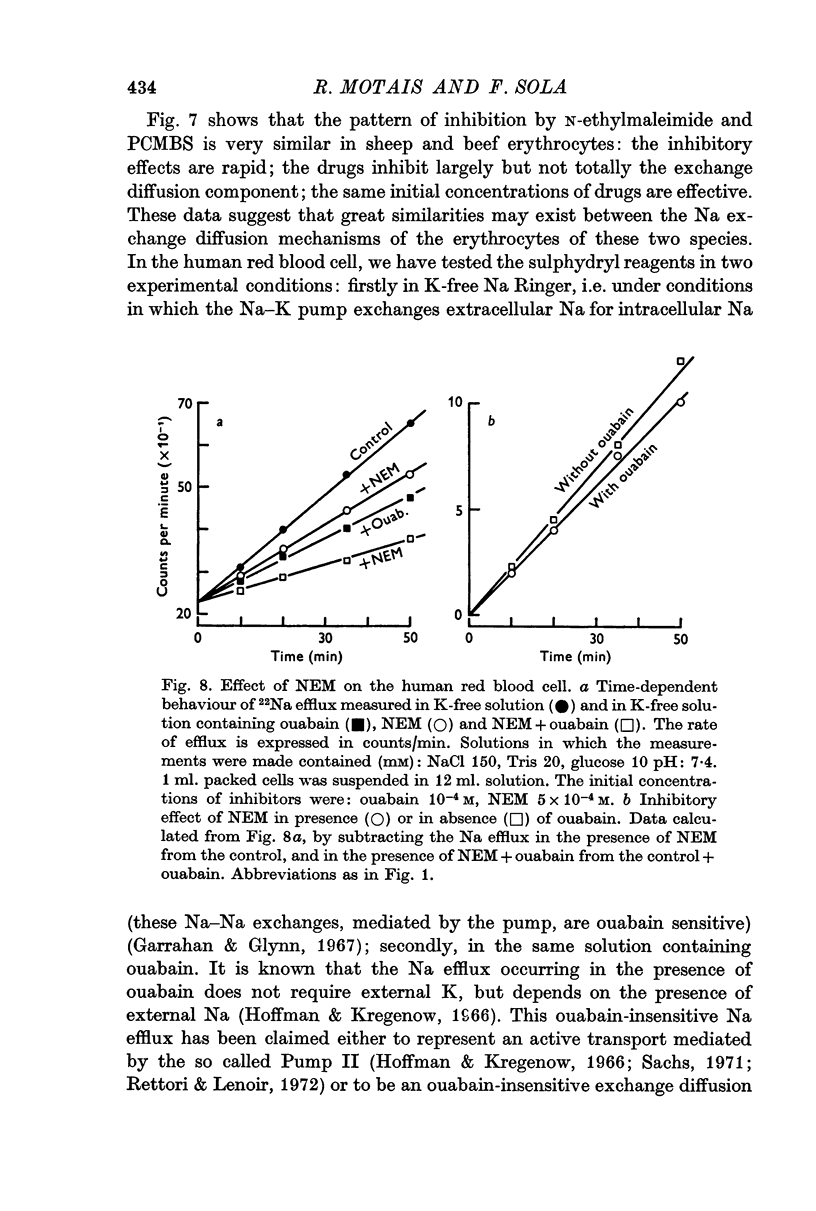
7. PCMBS does not affect sodium movement in human erythrocytes, but N-ethylmaleimide inhibits markedly the ouabain-insensitive Na efflux.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAWSON A. C., WIDDAS W. F. INHIBITION OF THE GLUCOSE PERMEABILITY OF HUMAN ERYTHROCYTES BY N-ETHYL MALEIMIDE. J Physiol. 1963 Oct;168:644–659. doi: 10.1113/jphysiol.1963.sp007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M. J. The effects of transport inhibitors on sodium outflux and influx in red blood cells: evidence for exchange diffusion. J Clin Invest. 1970 Oct;49(10):1804–1814. doi: 10.1172/JCI106398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The behaviour of the sodium pump in red cells in the absence of external potassium. J Physiol. 1967 Sep;192(1):159–174. doi: 10.1113/jphysiol.1967.sp008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Rega A. F. Cation loading of red blood cells. J Physiol. 1967 Nov;193(2):459–466. doi: 10.1113/jphysiol.1967.sp008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. F., Kregenow F. M. The characterization of new energy dependent cation transport processes in red blood cells. Ann N Y Acad Sci. 1966 Jul 14;137(2):566–576. doi: 10.1111/j.1749-6632.1966.tb50182.x. [DOI] [PubMed] [Google Scholar]
- Lubowitz H. Exchange diffusion in human red blood cells. Proc Soc Exp Biol Med. 1972 May;140(1):153–156. doi: 10.3181/00379727-140-36414. [DOI] [PubMed] [Google Scholar]
- Motais R. Sodium movements in high-sodium beef red cells: properties of a ouabain-insensitive exchange diffusion. J Physiol. 1973 Sep;233(2):395–422. doi: 10.1113/jphysiol.1973.sp010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rega A. F., Rothstein A., Weed R. I. Erythrocyte membrane sulfhydryl groups and the active transport of cations. J Cell Physiol. 1967 Aug;70(1):45–52. doi: 10.1002/jcp.1040700107. [DOI] [PubMed] [Google Scholar]
- Rettori O., Lenoir J. P. Ouabain-insensitive active socium transport in erythrocytes: effect of external cation. Am J Physiol. 1972 Apr;222(4):880–884. doi: 10.1152/ajplegacy.1972.222.4.880. [DOI] [PubMed] [Google Scholar]
- Sachs J. R. Ouabain-insensitive sodium movements in the human red blood cell. J Gen Physiol. 1971 Mar;57(3):259–282. doi: 10.1085/jgp.57.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R. M., Rothstein A., Weed R. I. Erythrocyte membrane sulfhydryl groups and cation permeability. J Cell Physiol. 1967 Apr;69(2):185–198. doi: 10.1002/jcp.1040690209. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANSTEVENINCK J., WEED R. I., ROTHSTEIN A. LOCALIZATION OF ERYTHROCYTE MEMBRANE SULFHYDRYL GROUPS ESSENTIAL FOR GLUCOSE TRANSPORT. J Gen Physiol. 1965 Mar;48:617–632. doi: 10.1085/jgp.48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEED R., EBER J., ROTHSTEIN A. Interaction of mercury with human erythrocytes. J Gen Physiol. 1962 Jan;45:395–410. doi: 10.1085/jgp.45.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]


