Abstract
OBJECTIVE: This retrospective study examined whether changes in ventricular volume correspond with changes in adjustable valve pressure settings in a cohort of patients who received shunts to treat idiopathic normal pressure hydrocephalus. We also examined whether these pressure—volume curves and other patient variables would co-occur with a positive clinical response to shunting.
METHODS: We selected 51 patients diagnosed with idiopathic normal pressure hydrocephalus who had undergone implantation of a Codman Hakim programmable valve (Medos S.A., Le Locle, Switzerland). Clinical data were gathered from the patients’ records and clinical notes by an investigator blinded to patients’ ventricular volumes. Ventricular volume was measured using 3D Slicer, an image analysis and interactive visualization software package developed and maintained at the Surgical Planning Laboratory at Brigham and Women’s Hospital.
RESULTS: Eighty-six percent of patients with gait disturbance at presentation showed improvement of this symptom, 70% experienced improvement in incontinence, and 69% experienced improvement in dementia. For the group showing 100% clinical improvement, the correlation coefficient of average changes in valve pressure over time (ΔP/ΔT) and average changes in ventricular volume over time (ΔV/ΔT) were high at 0.843 (P < 0.05). For the group experiencing no or only partial improvement, the correlation coefficient was 0.257 (P = 0.32), indicating no correlation between average ΔV/ΔT and average ΔP/ΔT for each patient.
CONCLUSION: This was a carefully analyzed modeling study of idiopathic normal pressure hydrocephalus treatment made possible only by adjustable valve technology. With careful volumetric analysis, we found that changes in ventricular volume correlated with adjustments in valve pressure settings for those patients who improved clinically after shunting. This suggests that positive clinical responders retained parenchymal elasticity, emphasizing the importance of dynamic changes in this cohort.
Keywords: Hydrocephalus, Idiopathic normal pressure hydrocephalus, Programmable shunt, Ventricular volume, Ventriculoperitoneal shunt
Salomon Hakim in 1964 described the syndrome of idiopathic normal pressure hydrocephalus (INPH) as a treatable medical condition physiologically separate from the more common forms of communicating and noncommunicating hydrocephalus that occur as a result of known pathological mechanisms (10). INPH is characterized clinically by gait difficulty, incontinence, and dementia and typically occurs in the sixth or seventh decade (4, 5, 16). INPH is increasingly important for the neurosurgical community because large numbers of people are surviving into old age; maintaining mobility, continence, and cognitive function are central to quality of life for this population. Additionally, evolving shunt valve technology is decreasing the potential complications of shunt placement for INPH, making this procedure appropriate for even greater numbers of patients. INPH is still one of the few treatable causes of dementia and gait disturbance.
One of the major challenges in understanding INPH is the relationship between ventricular volume and clinical response. Previous studies have found no correlation between decreases in ventricular size as measured on follow-up computed tomographic (CT) scans and clinical response to shunting (5, 6, 14, 17, 19, 21, 23, 26). These previous studies had only two-dimensional measures, namely the Evans ratio, with which to follow any changes in ventricular size after shunting; such linear measures have been shown to be inadequately sensitive to the minimal changes in ventricular size typically sought for the treatment of INPH. The dynamic relationship between valve pressure setting and ventricular volume in the setting of INPH also is poorly understood.
Anderson et al. (1) recently used a three-dimensional analysis to measure ventricular volume changes after shunting for INPH. They observed a decrease in ventricular volume after shunting in 10 of 11 patients. In their study, a traditional two-dimensional analysis by an independent radiologist found decreases in ventricular size in only 2 of the 11 patients. Anderson et al., however, were unable to examine any relationship between decreasing ventricular volume and clinical response, because all of the patients included in the study showed a positive clinical response. They also were unable to comment on ventricular volume changes as they relate to changes in ventricular pressure over time, because the researchers limited their analysis to comparisons of ventricular volume at only two points in the course of treatment: just before surgery for shunt implantation and at one visit during the postoperative period.
The purpose of the current retrospective study was twofold: first, to examine the relationship between ventricular volume and adjustable valve pressure setting in a cohort of patients being treated for INPH during a longer follow-up period of at least 1 year, and second, to assess whether these pressure—volume curves as well as other patient variables may co-occur with a positive clinical response to shunting.
PATIENTS AND METHODS
Patient Selection
Permission was obtained to conduct this study by the Internal Review Board of Brigham and Women’s Hospital (Protocol no. 2002-P-001448/1; BWH). Two hundred twenty-seven patients with hydrocephalus of various causes underwent implantation with Codman Hakim programmable valves (Medos S.A., Le Locle, Switzerland) from November 1997 through December 2002 at Brigham and Women’s Hospital primarily by one neurosurgeon (PMcLB) and were included in the Brigham and Women’s Hydrocephalus Database (mean age ± standard deviation [SD], 62 ± 20 yr; range, 7–100 yr). We selected 51 patients who met the following criteria: 1) diagnosed with INPH and implanted with a Codman Hakim programmable valve, and 2) having at least two follow-up CT scans at Brigham and Women’s Hospital, digitized and retrievable through the hospital’s AGFA IMPAX system (version 4.1; Agfa-Gevaert, Mortsel, Belgium), which stores and displays radiographic images. It was this latter requirement that limited the patient number to 51.
Clinical Data
Clinical data regarding the patients’ presenting symptoms and clinical improvement after shunting were determined by the nurse coordinator for the hydrocephalus clinic (NOB), who was blinded to each patient’s ventricular volume information. These data were gathered from the patients’ records and clinical notes. Because standardized and quantitative measures of clinical improvement were unavailable for this retrospective study, patients were determined to have responded positively to shunting if they demonstrated improvement in the three symptom categories associated with INPH:gait, incontinence, and dementia. Follow-up time at which improvement was determined was approximately 6 months.
Ventricular Volume Measurements
Ventricular volume was measured using 3D Slicer, an image analysis and interactive visualization software package developed and maintained at the Surgical Planning Laboratory at Brigham and Women’s Hospital (8). This application package is a freely available, open-source tool for clinicians and scientists (http://www.slicer.org). Using 3D Slicer, one can import both magnetic resonance and CT images and can reformat those images so that one may select the cross sectional view on which to perform the segmentation; for this study the view was axial. Segmentation is the process of outlining the anatomic features of interest, in this case the ventricular system, in two-dimensional axial slices using the program’s suite of editing tools, including thresholding and free-hand drawing. These segmentations are called labelmaps and are an intermediate step in the process of creating a three-dimensional model of the anatomic feature of interest. 3D Slicer then extracts these labelmaps and represents them as a collection of triangles using the marching cubes algorithm (18). 3D Slicer then performs a decimation algorithm that systematically reduces the number of triangles in such a way as to decrease the time required to render these labelmaps into three-dimensional volumes while minimizing the loss of structural detail (24). Using an accessory feature in 3D Slicer, one can then measure the volume in milliliters of these three-dimensional models with exquisite precision (Fig. 1). Most patients were referred for treatment of their INPH from outside physicians and thus did not have preoperative scans available within the Brigham and Women’s IMPAX system, so we were not able to include preoperative ventricular volume measurements in the study.
FIGURE 1.
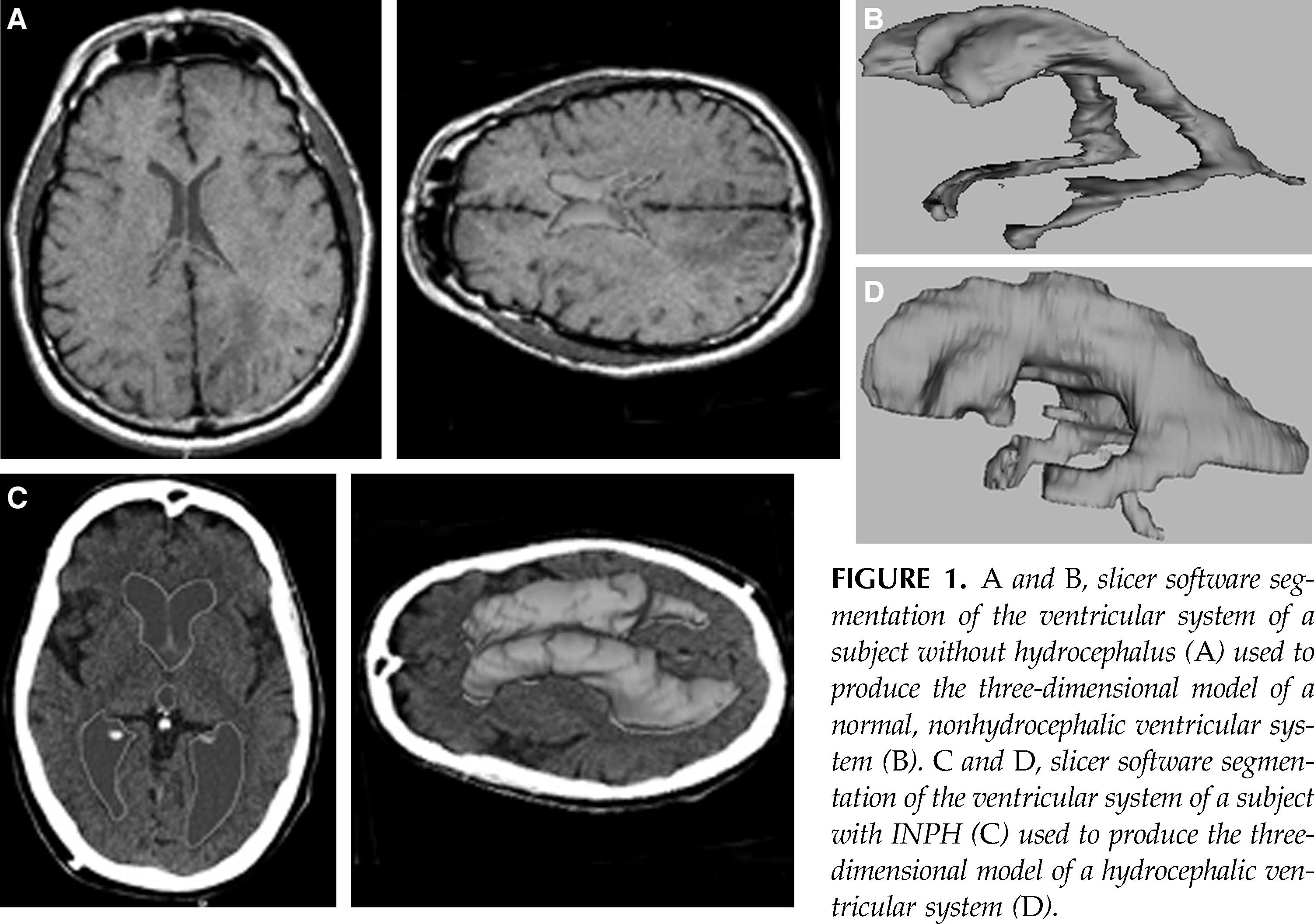
A and B, slicer software segmentation of the ventricular system of a subject without hydrocephalus (A ) used to produce the three-dimensional model of a normal, nonhydrocephalic ventricular system (B). C and D, slicer software segmentation of the ventricular system of a subject with INPH (C) used to produce the three-dimensional model of a hydrocephalic ventricular system (D).
Statistical Analysis
Our database was generated using Microsoft Excel (Microsoft, Seattle, WA). Statistical analyses and graphic representations were produced using both Excel and JMP software (SAS Institute, Cary, NC). Data were analyzed so as to examine 1) the correlation of ventricular volume with adjustable valve pressure setting; 2) the variability of ventricular volume at constant pressure; 3) differences in ventricular volume response to changes in adjustable valve pressure setting for two groups: those who showed positive clinical response to shunting and those who showed no or incomplete response to shunting; and 4) several variables as potential indicators of positive clinical response to shunting in all three symptom categories: gait, incontinence, and dementia. We performed comparisons between valve pressure and ventricular volume by constructing regression analyses and Student’s two-tailed t tests with unequal variances assuming a 95% confidence interval. Variables indicating clinical response were analyzed using a Fisher’s two-tailed t test contingency analysis.
For our analysis of study variables as they relate to clinical outcomes, it was desirable to have a numerical score that took into account all three symptom categories combined. To represent a patient’s clinical response to shunting in all three symptom categories, we developed a single numerical weighted response score that described a patient’s clinical response to shunting by comparing presenting symptoms with symptoms present at follow-up for each patient. Determinations of symptom presentation and clinical response are described above, under "Clinical Data." This weighted factor score represents the percentage of presenting symptoms that improved after surgery in each patient.
RESULTS
The population included in the Brigham and Women’s Hospital hydrocephalus database consisted of 227 patients (119 women, 108 men). Fifty-one patients met the criteria for inclusion in this study, including 28 women and 23 men ranging in age from 52 to 88 years (mean ± SD, 52 ± 8.2 yr). See Table 1 for a summary of patient demographics.
TABLE 1.
Characteristics, adjustable valve, ventricular volume, and clinical outcomes data for all 51 patientsa
| Patient characteristics | |
|---|---|
| Age (yr) | |
| Mean ± SD | 75 ± 8.2 |
| Range | 52-88 |
| Sex (women/men) | 28/23 |
| Presenting symptoms (no. of patients with particular symptom/total for whom there are data) | |
| Gait | 50/51 (98%) |
| Incontinence | 40/51 (78.4%) |
| Dementia | 49/51 (96.1%) |
| Initial adjustable valve setting (mmH2O) | |
| Median | 120 |
| Mean ± SD | 125 ± 33.8 |
| Range | 40-200 |
| Initial ventricular volume (ml) | |
| Mean ± SD | 135.4 ± 106.7 |
| Range | 14.6-731.4 |
| Follow-up time (wk) from first to last volume measurement/CT scan | |
| Mean ± SD | 50.8 ± 43.6 |
| Range | 3-219 |
| No. of valve adjustments | |
| Mean ± SD | 2 ± 1.6 |
| Range | 0-10 |
| Symptom improvement (no. of patients improved/total for whom there are data) | |
| Gait | 44/51 (86.3%) |
| Incontinence | 35/50 (70%) |
| Dementia | 33/48 (68.8%) |
| Weighted factor for clinical improvement in all three symptom categories | |
| Mean ± SD | 73.8 ± 37.4 |
SD, standard deviation; CT, computed tomographic.
At presentation, 98% of patients had gait difficulty, 78% had incontinence, and 96% had dementia. The initial valve setting for Codman Hakim adjustable valves ranged from 40 to 200 mm H2 O (median, 120 mm H2 O; mean ± SD, 125 ± 33.8 mm H2 O). Initial ventricular volume (i.e., the ventricular volume at the time of first postoperative CT scan) ranged from 14.6 to 731.4 ml (mean ± SD, 135.4 ± 106.7 ml). Follow-up time, defined as the number of weeks from initial postoperative CT scan to final CT scan, ranged from 3 weeks to 219 weeks (mean ± SD, 50.8 ± 43.6 wk), and the number of valve adjustments made during follow-up ranged from 0 to 10 (mean ± SD, 2 ± 1.6). As illustrated in Figure 2, 86% of patients with gait disturbance at presentation showed improvement for this symptom, 70% experienced improvement in incontinence, and 69% experienced improvement in dementia.
FIGURE 2.
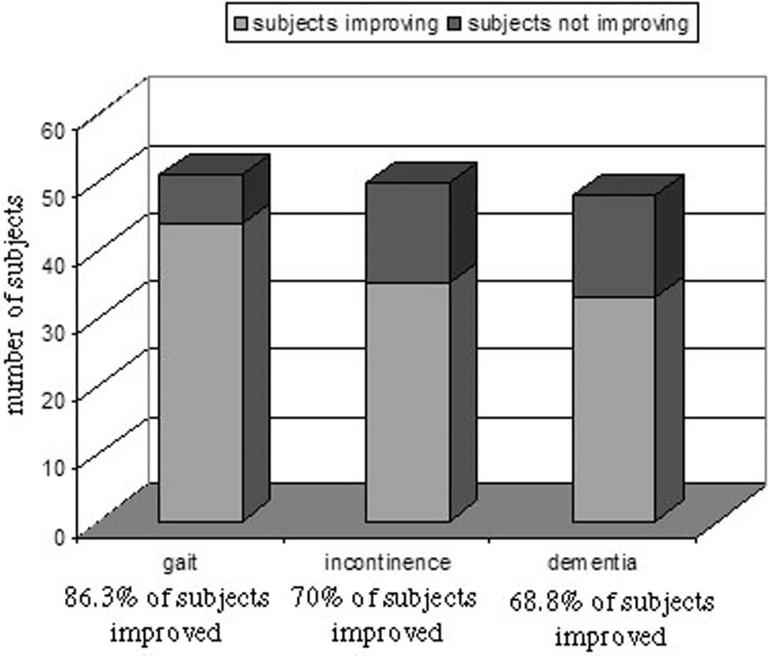
Three-dimensional bar graph illustrating the clinical outcomes for all patients included in the present study in each symptom category: gait, incontinence, and dementia.
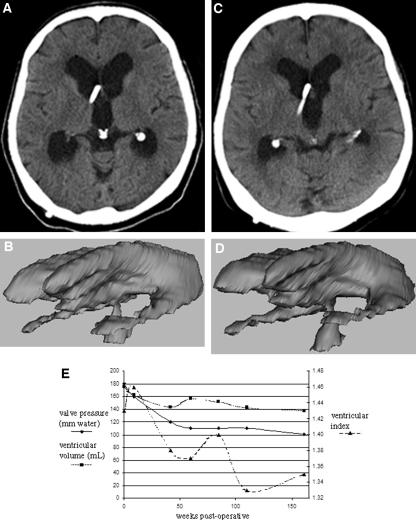
Figure 3 illustrates this relationship between decreasing valve pressure setting and decreasing ventricular volume for one patient, for whom we were able to retrieve and analyze seven postoperative CT scans over a 3-year period from September 1998 through October 2001. The chart in Figure 3E graphs the patient’s valve pressure settings, ventricular volume, and ventricular index as measured using a formula for assessing hydro-cephalus two-dimensionally in INPH patients devised by Tans and Poortvliet (25). One can observe readily that for this patient, both the ventricular volume and the ventricular index decreased as valve pressure setting decreased, but the two-dimensionally derived ventricular index does not conform as closely to changes in valve settings as ventricular volume does.
FIGURE 3.
A , and B , three-dimensional model of the ventricular system measuring 180 ml ( A ), segmented from the initial postoperative CT scan date ( B ). C and D , three-dimensional model with a volume of 100 ml ( C ), and CT scan for the same patient 3 years later ( D ). E , graph illustrating our observation that changes in ventricular volume more closely follow changes in valve pressure than does the ventricular index, suggesting that ventricular volume would be a more accurate means of monitoring hydro-cephalus. Ventricular volume (in milliliters) and valve pressure (in millimeters of H 2 O) are graphed on the left axis, and the ventricular index is graphed on the right axis. Time measured in weeks after initial shunt implantation is illustrated on the x axis.
Correlation of Ventricular Volume with Adjustable Valve Pressure
In looking at pressure volume relationships, we excluded 17 patients because their pressure and volume values differed significantly from those of the rest of the group or because they experienced subdural collections that were assumed to alter the relationship between valve pressure and ventricular volume. The subgroup of patients in whom subdural collections developed after surgery did not differ significantly from the rest of the population either in sex, age, initial valve pressure setting, or rate of valve and pressure changes before the development of the subdural collection. We defined and analyzed eight relationships between ventricular volume (measured in milliliters) and adjustable valve pressure (measured in millimeters of H2 O) for the population of 34 patients who had undergone two or more CT scans available for ventricular volume analysis, excluding those 17 who were determined to be outliers as described above. See Table 2 for a complete list of the pressure-volume relationships analyzed and for the variables used in describing these relationships. The most relevant and significant of these findings are summarized in Table 3.
TABLE 2.
Relationships between adjustable valve pressure setting and ventricular volume subjected to regression analyses, and the variables with their definitionsa
| Relationships between adjustable valve pressure setting and ventricular volume | |
| 1 | Individual values of P and V across all subjects |
| 2 | Individual values of ΔP and corresponding values of ΔV across all subjects |
| 3 | Average individual values of P and V within each subject |
| 4 | Average values for ΔP and ΔV within each subject |
| 5 | Average ΔP/ΔT and ΔV/ΔT for each subject |
| 6 | The differences between Pmax – Pmin and Vmax – Vmin within each subject |
| 7 | The differences between Pfinal – Pinitial and Vfinal – Vinitial within each subject |
| 8 | Corresponding values of ΔP and ΔV within each subject |
| Definition of abbreviations | |
| (P) | Adjustable valve pressure setting |
| (V) | Ventricular volume |
| (ΔP) | Change in adjustable valve pressure setting |
| (ΔV) | Change in ventricular volume |
| (ΔP/ΔT) | Change in adjustable valve pressure setting over time |
| (ΔV/ΔT) | Change in ventricular volume over time |
| (Pmax) | Highest adjustable valve pressure setting |
| (Pmin) | Lowest adjustable valve pressure setting |
| (Vmax) | Largest ventricular volume |
| (Vmin) | Smallest ventricular volume |
| (Pfinal) | Last value for adjustable valve pressure setting available for each subject |
| (Pinitial) | First value for adjustable valve setting as determined at time of shunt surgery |
| (Vfinal) | Last value for ventricular volume available for each subject |
| (Vinitial) | First ventricular volume measure available at first computed tomographic scan after surgery |
TABLE 3.
Relationships of interest between adjustable valve pressure and ventricular volume for the total population of 34 patientsa
| Relationship of interest between valve pressure setting and ventricular volume | Slope ± SD | Intercept ± SD | Correlation coefficient | P value |
|---|---|---|---|---|
| Individual values of P and V across all subjects | 0.456 ± 0.22 | 71.03 ± 23 | 0.342 | P = 7.2E-05 |
| Individual values of ΔP and corresponding values of ΔV across all subjects | 0.262 ± 0.159 | -4.786 ± 5.02 | 0.327 | P = 1.5E-03 |
| Average individual values of P and V within each subject | 0.353 ± 0.534 | 88.71 ± 55.7 | 0.231 | P = 1.9E-01 |
| Average values for ΔP and ΔV within each subject | 0.533 ± 0.226 | 0.358 ± 6.32 | 0.648 | P = 3.4E-05 |
| Average ΔP/ΔT and ΔV/ΔT for each subject | 0.549 ± 0.178 | 0.039 ± 0.61 | 0.744 | P = 4.6E-07 |
All subjects with at least two computed tomographic scans for volumetric analysis excluding the 17 outliers as listed under "Correlation of Ventricular Volume with Adjustable Valve Pressure."
SD, standard deviation.
The first relationship of interest examines the individual values of adjustable valve pressure settings and the corresponding values of ventricular volume across all patients. The slope ± SD for this equation is 0.456 ± 0.22, and the intercept± SD is 71.03 ± 23. The correlation coefficient for this relationship was 0.342 (P < 0.05), indicating that the absolute values of pressure and volume vary so greatly that the correlation between them is only moderate.
To normalize variability across individuals, we examined the changes in valve pressure (ΔP) and the corresponding changes in ventricular volume (ΔV) across all patients and observed a slope ± SD of 0.262 ± 0.159 and an intercept ± SD of –4.786 ± 5.02. The correlation coefficient for this relationship was 0.327 (P < 0.05), not much stronger than above. We then sought to smooth the variability in individual pressure and volume values (that is, not Δ values) for each patient by examining the average valve pressure settings and average ventricular volume measurements for each patient and observed a slope ± SD of 0.353 ± 0.534 and an intercept ± SD of 88.71 ± 55.7, with a correlation coefficient of 0.231 (P = 0.19), the only one of these observations that does not reach statistical significance. We then examined the relationship between average changes in valve pressure settings (ΔP) and the corresponding average ventricular volume changes (ΔV) for each patient and observed a slope ± SD of 0.533 ± 0.226 and an intercept ± SD of 0.358 ± 6.32. The correlation coefficient for this relationship was 0.648 (P < 0.05).
Finally, we included a measure of pressure and volume change over time by examining the relationship between average changes in valve pressure setting over time (ΔP/ΔT) and average changes in ventricular volume over time (ΔV/ΔT) and observed a slope ± SD of 0.549 ± 0.178 and an intercept ± SD of 0.039 ± 0.61. For this relationship, we observed a high correlation coefficient of 0.744 (P < 0.05).
Variability of Ventricular Volume
We next sought to describe the high variability observed in ventricular volumes for given valve pressure settings by analyzing the values of ventricular volume change (ΔV) for the 39 data points across all 51 patients (now including those patients previously excluded, as described above) where the valve pressure setting did not change, that is where ΔP = 0. The mean ± SD for ΔV where ΔP = 0 was –4.5 ± 30.2. The medianΔV was –3.7. The minimum ΔV was –129.4 and the maximum ΔV was 67.8. Thus, when valve pressure does not change, ventricular volume can vary from –129.4 to 67.8 ml. However, as one can see in Figure 4, which illustrates the frequency distribution of these ΔV values, in most instances when ΔP = 0, ventricular volume only fluctuates between 0 and 20 ml.
FIGURE 4.
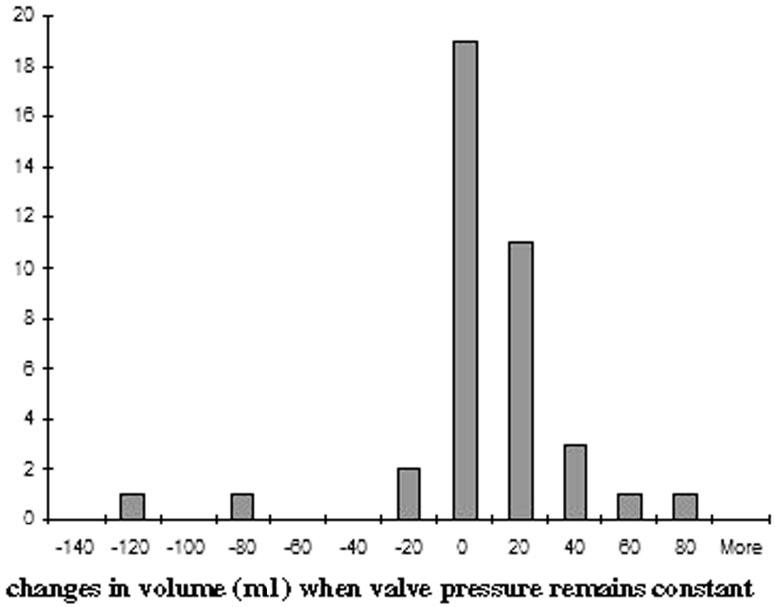
Frequency distribution curve illustrating the number of times across all subjects that ventricular volume was observed to either increase or decrease when the adjustable valve pressure remained constant. This bar graph illustrates the variability in ventricular volume at a given pressure, but it also illustrates that the majority of these variations in volume at a constant pressure are very small, between 0 and 20 ml.
Differences in Pressure—Volume Correspondence for Positive and for Negative Clinical Responders
Finally, we examined the differences in ventricular volume response to valve pressure changes for two different groups: 1) those whose weighted factor score representing clinical improvement in all three symptom categories was 100% (i.e., those who experienced improvement for every symptom they had had before surgery); and 2) those whose weighted factor score was not 100% (i.e., those who either failed to improve at all or who experienced only partial improvement in their symptoms). These results are summarized in Tables 4 and 5. The groups performed similarly for every pressure—volume relationship except for the average ΔP and average ΔV for each patient. For this relationship in the group showing 100% clinical improvement, the slope ± SD was 0.619 ± 0.226 and the intercept ± SD was 3.111 ± 7.08. The correlation coefficient was high at 0.843 (P < 0.05). For the group experiencing no or only partial improvement, the slope ± SD was 0.266 ± 055, the intercept ± SD was –6.452 ± 13.91, and the correlation coefficient was 0.257 (P = 0.32), indicating no correlation between average ΔV and average ΔP for each patient.
TABLE 4.
Ventricular volume response to valve pressure changes for those who experienced 100% (weighted response factor) improvement in symptomsa
| Relationship of interest between valve pressure setting and ventricular volume | Slope ± SD | Intercept ± SD | Correlation coefficient | P value |
|---|---|---|---|---|
| Individual values of P and V across all subjects | -0.487 ± 0.227 | 65.72 ± 23.8 | 0.466 | P = 6.31E-05 |
| Individual values of ΔP and corresponding values of ΔV across all subjects | 0.279 ± 0.247 | -3.582 ± 7.02 | 0.305 | P = 2.77E-02 |
| Average individual values of P and V within each subject | 0.278 ± 0.73 | 32.49 ± 72.5 | 0.212 | P = 4.31E-01 |
| Average values for ΔP and ΔV within each subject | 0.619 ± 0.226 | 3.111 ± 7.08 | 0.843 | P = 4.13E-05 |
SD, standard deviation.
TABLE 5.
Ventricular volume response to valve pressure changes for those who experienced either no or only partial improvement in symptoms as determined by weighted response scorea
| Relationship of interest between valve pressure setting and ventricular volume | Slope ± SD | Intercept ± SD | Correlation coefficient | Statistical significance |
|---|---|---|---|---|
| Individual values of P and V across all subjects | 0.357 ± 0.435 | 90.728 ± 46.7 | 0.231 | P = 1.06E-01 |
| Individual values of ΔP and corresponding values of ΔV across all subjects | 0.367 ± 0.326 | -4.978 ± 10.07 | 0.382 | P = 2.83E-02 |
| Average individual values of P and V within each subject | 0.366 ± 0.865 | 89.860 ± 95 | 0.227 | P = 3.81E-01 |
| Average values for ΔP and ΔV within each subject | 0.266 ± 0.55 | -6.452 ± 13.91 | 0.257 | P = 3.20E-01 |
SD, standard deviation.
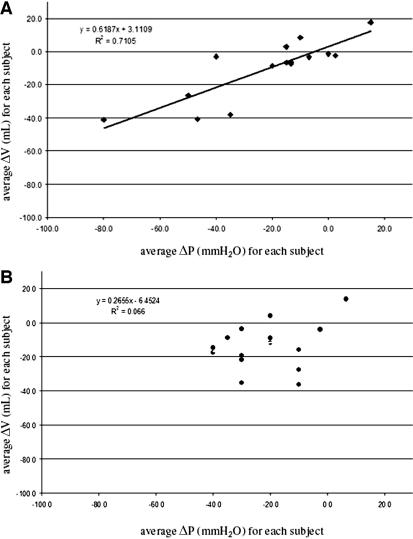
Figure 5, A and B, graphs the lines defined by these two regression analyses. In Figure 5A, the data points adhere closely to the line with slope 0.619, R2 = 0.71. In Figure 5B, the data points are scattered and R 2 = 0.07, indicating that for this group, average ΔP and average ΔV do not correlate.
FIGURE 5.
A , diagram plotting the average changes in ventricular volume (ΔV) for the corresponding changes in valve pressure setting (ΔP ) for those patients who experienced 100% clinical improvement. B , diagram plotting the average changes in ventricular volume (ΔV ) for the corresponding changes in valve pressure setting (ΔP ) for those patients who experienced only partial clinical improvement in presenting symptoms.
Analysis of Clinical Response Indicators
In examining clinical outcomes data, it was desirable to include as many patients as possible; therefore, the previously excluded patients who were considered outliers in terms of their pressure—volume data were reincluded for this analysis so that the total number of patients for this portion of the analysis included the original 51. Table 6 lists all 18 patient variables that were evaluated as potential clinical response indicators.
TABLE 6.
Variables analyzed as clinical response predictors
| Variable no. | Description |
|---|---|
| 0 | Subject identification number (used to rerandomize sets) |
| 1 | Sex |
| 2 | Age at time of surgery |
| 3 | Date of shunt valve surgery |
| 4 | Presence of subdural collection |
| 5 | Average ΔP/ΔT |
| 6 | Average ΔV/ΔT |
| 7 | Initial valve pressure setting (Pi) |
| 8 | Final valve pressure setting (Pf) |
| 9 | Valve pressure difference (Pf – P1) |
| 10 | Initial ventricular volume (Vi) |
| 11 | Final ventricular volume (Vf) |
| 12 | Ventricular volume difference (Vf – Vi) |
| 13 | Maximum valve pressure setting (Pmax) |
| 14 | Minimum valve pressure setting (Pmin) |
| 15 | Difference in maximum and minimum valve pressure settings (Pmax – Pmin) |
| 16 | Maximum ventricular volume (Vmax) |
| 17 | Minimum ventricular volume (Vmin) |
| 18 | Difference in maximum and minimum ventricular volumes (Vmax – Vmin) |
With the exception of average ΔP/ΔT and average ΔV/ΔT per patient, all variables distributed in a random pattern, indicating no apparent segregation of the population into clinical responders and nonresponders by the remaining 18 variables. For average ΔP/ΔT, we found two groups: those whose average ΔP/ΔT was low (–11.01 to –1.06) and those whose ΔP/ΔT was high (–0.997 to +3 [P < 0.05]). The population with higher average ΔP/ΔT (i.e., with gradual decreases or even slight increases in valve pressure) tended to demonstrate significantly improved clinical response in all three symptom categories. For gait, 96.3% of the population with high average ΔP/ΔT values improved, whereas only 75.0% of the group with low average ΔP/ΔT values did (P < 0.05). For incontinence, 92.3% of the population with high average ΔP/ΔT values improved, whereas only 45.8% of the group with low average ΔP/ΔT values did (P < 0.05). For dementia, 88.0% of the population with high average ΔP/ΔT values improved, whereas only 47.8% of the group with low average ΔP/ΔT values did (P < 0.05).
For average ΔV/ΔT, we also found two groups: those whose average ΔV/ΔT was low and slightly positive (–135 to 0.58 ml) and those whose ΔV/ΔT was high (0.677 to +3 [P < 0.05]). A greater percentage of the population with higher average ΔV/ΔT (i.e., ventricular volume increased) improved for all three symptom categories as well as weighted response score than for the population whose average ΔV/ΔT was lower. For gait, 100% of the population with high average ΔP/ΔT values improved, whereas only 83.3.0% of the group with low average ΔP/ΔT values did (P = 0.32). For incontinence, 100% of the population with high average ΔP/ΔT values improved, whereas only 63.4% of the group with low average ΔP/ΔT values did (P < 0.05). For dementia, 100% of the population with high average ΔP/ΔT values improved, whereas only 61.5% of the group with low average ΔP/ΔT values did (P < 0.05).
DISCUSSION
Many studies have demonstrated that ventriculoperitoneal shunting is an effective treatment for INPH, and adjustable valves seem to produce better results than both differential pressure systems and constant flow valves (2, 9, 13, 15, 20, 22, 28–30). Adjustable valves are more effective in the prevention and management of complications after shunting, primarily nontraumatic subdural collections (7, 31). In their 2001 comprehensive literature review of studies of INPH, Hebb and Cusimano (12) found an overall 59% improvement (29% long-term improvement) in symptoms for the 44 articles they reviewed, all of which used either the differential pressure or the constant flow valves. Zemack and Romner (31), in their 2002 retrospective study of 218 patients implanted with adjustable valves, reported that 78.9% of INPH patients experienced a good or excellent response to shunting. These figures are comparable with the weighted factor score of clinical outcomes for the current study, 73.8%. Anderson et al. (1) observed better results at 100% positive response, but their patient population included only 11 patients, which likely undermines the statistical power of their study for the purpose of determining clinical response.
As mentioned above, in dealing with INPH, clinicians have been working under the assumption that INPH compromises brain function in ways that are similar to these processes in secondary hydrocephalus and thus have been approaching these conditions with similar treatment strategies. The advent of adjustable valves has refined the treatment of INPH in that ventricular pressure now can be reduced slowly over time, which seems to work better for those patients who have been hydrocephalic for long periods and whose brain parenchyma has been deformed by chronically increased ventricular pressure and volume. But how the brain parenchyma of INPH patients responds to these changes in valve pressure has not been examined, nor has the relationship between parenchymal response, the observed pressure—volume curves, and clinical outcome of ventriculoperitoneal shunting.
This study advances previous studies of ventricular volume changes in the setting of shunting for INPH in that we have followed up a large patient population (n = 51) over several weeks and, in some cases, several years. We also have examined these changes in ventricular volume as they relate to adjustments in valve pressure settings over time, so that for each patient we have been able to plot the changes in ventricular volumes resulting from valve pressure adjustments and to produce a pressure—volume curve for each patient that describes the response of that patient’s brain parenchyma to shunting over time. Because our study includes both positive and negative clinical responders, we have also been able to observe differences in the pressure volume curves of those patients experiencing positive clinical response from those who did not or who responded to shunting only partially. There similarly are some weaknesses inherent in this retrospective study (to be corrected in a prospective trial), the most important being that we did not have a standardized method for tracking clinical improvement. With such a measure, we would have been able to track degrees of clinical improvement along these pressure—volume curves. In a prospective trial, we also plan to gather information concerning the duration of symptoms before treatment, because such data may very well correlate with the degree of parenchymal damage caused by chronic hydrocephalus and also may predict patient response to shunting. If this theory proves valid, it would support the argument for earlier rather than later surgical intervention for INPH. Also, because many patients were referred to the Brigham and Women’s hydrocephalus clinic by outside physicians, we did not have preoperative CT scans, and thus we were not able to assess ventricular volume before surgery. Such a measure would have supplied an additional variable in our assessment of potential predictors of clinical response to shunting, or even a presurgical predictor of parenchymal response to decreasing ventricular pressure.
Pressure—Volume Curves
For this study, we followed up 51 patients for whom CT scans were available for an average of 51 weeks. Thus, we were able to follow changes in ventricular volume after adjustments in valve pressure for an average of 4 years over the course of each patient’s treatment. We examined several relationships between valve pressure and ventricular volume, both across and within individuals, all of which are listed in Table 3. We found that individual ventricular volumes and pressure vary widely among individuals with INPH and that even when valve pressure does not change, ventricular volume can vary anywhere from –129.4 to +67.8 ml, although most vary only from 0 to 20 ml. Similarly, there was wide variability in patients’ valve adjustments and ventricular volume responses over time. All of these factors contributed to the variability and thus the low correlation coefficients observed in the several pressure—volume curves we examined. We should point out that although our correlation coefficients for these curves were low, the P values accompanying these regression analyses were very strong, indicating that we had adequate numbers of comparisons to define these curves accurately, but that the data were too variable to find a strong correlation. This means that for future studies, collecting data for more than 51 patients is unlikely to improve these pressure—volume correlations, but analyzing the pressure—volume curves of 50 or so patients over a longer time using more than two CT scans and ventricular volume measurements per patient probably will produce stronger correlation coefficients. To overcome this variability, we decided 1) to normalize these data across individuals by examining only the changes in valve pressure settings and ventricular volumes, or ΔV s andΔP s within individuals; and 2) to smooth the variability in each patient’s valve adjustments and volume responses over time by taking the average of these values. Having normalized and smoothed the pressure and volume measurements, we found a moderately strong positive correlation for all patients, both positive and negative clinical responders, of 0.65 for average ΔP and average ΔV. That is, for each patient, the average changes in valve pressure settings over time caused a corresponding average ventricular volume change over time predictable by the pressure—volume curve described in the fourth row of Table 3.
We then sought to compare the pressure—volume curves of patients who had responded positively to shunting with the same curves of patients either who had not responded at all or who had had only a partial response to shunting (Tables 4 and 5). We found that for the group of positive clinical responders, average ΔV correlated strongly with average ΔP (correlation coefficient, 0.843) and that this observation was highly statistically significant (P < 0.05). In contrast, for the group of negative or incomplete clinical responders, average ΔV did not correlate with average ΔP (correlation coefficient, 0.257), nor was this observation consistent enough to reach statistical significance (P = 0.32). These observations suggest that the brain parenchyma of patients who experience a significant improvement in their symptoms after shunt implantation responds differently and more predictably to adjustments in ventricular pressure than does the brain parenchyma of those patients who do not respond well to shunting.
The brain parenchyma has been described biomechanically as an open-cell sponge made of viscoelastoplastic material having two major fluid compartments, the cerebrospinal fluid (CSF) compartment and the intracranial venous compartment, which includes the intraparenchymal veins and capillaries, subarachnoid veins, and venous sinuses (3, 11). Because they are connected to the atmosphere through the extracranial venous system in the neck, the intracranial venous system and the extracellular parenchyma are the only portions of the brain capable of deformation. By collapsing, these compartments are able to dissipate increased ventricular pressure through the extracranial venous system to the atmosphere. Under normal circumstances, the brain parenchyma experiences two equal and opposite pressures: the intraparenchymal venous pressure and the CSF pressure. As long as these pressures are in steady-state, the brain parenchyma is subjected to very little stress and structural distortion. When the CSF pressure increases, the brain parenchyma distorts by collapsing the intracranial venous and intraparenchymal extracellular compartments, thus dissipating increased pressure to the atmosphere through the extracranial venous system. In doing this, the brain parenchyma is distorted by the collapsing of these fluid compartments, so that the ventricular system enlarges. If Force = Area × Pressure, even though this dissipation of pressure effectively lowers the pressure on the brain parenchyma, the distortion of the ventricular system increases the area over which that pressure is applied, thereby maintaining the distorting and damaging force on the brain parenchyma. Until both the size of the ventricular system decreases and the balance of pressures between CSF and parenchyma again reach steady-state, the parenchyma will experience damaging mechanical distortion. If these distorting forces persist, brain parenchyma will be damaged irreversibly both bioelastically and functionally.
In the case of the group of positive clinical responders such as those described above, that their brain parenchyma was able to reexpand and decrease ventricular volume after ventricular pressure decreased suggests that the brain parenchyma of these positive responders had not been permanently damaged by the distorting forces exerted by the chronically increased ventricular volumes characteristic of INPH. The opposite holds true for the group of nonresponders in whom changes in ventricular pressure did not correlate with changes in ventricular volume, indicating that their parenchyma was not able to reexpand in response to shunting. Although it is beyond the scope of this article to explore the pathophysio-logical explanation of our results, the above observations strongly suggest a link between preserved structural and functional integrity.
Clinical Outcomes
In an attempt to find a way to predict clinical response to shunting, we subjected the 18 variables listed in Table 6 to a contingency analysis and found that of those 18, only 2, ΔP/ΔT and ΔV/ΔT, nonrandomly distributed clinical outcome. In terms ofΔV and ΔP, we observed that the most precipitous drops in valve pressure and ventricular volume occurred in those patients who were not responding clinically. Based on standard treatment protocol for INPH, this is exactly what one would expect to find, because neurosurgeons typically continue to decrease valve pressure, thus decreasing ventricular volume for those patients who are not responding clinically to ensure that those patients are not being underdrained.
This is a carefully analyzed modeling study of INPH treatment made possible only by adjustable valve technology. We found that 86.3% of patients experienced improvement in gait symptoms, 50% in incontinence, and 68.8% in dementia. With careful volumetric analysis, we found that changes in ventricular volume correlate with adjustments in valve pressure settings for those patients who improved clinically after shunting. This suggests that positive clinical responders have retained parenchymal elasticity and emphasizes the importance of dynamic changes in this cohort.
Acknowledgments
We thank E.W. McConnell, Jr., for his help in preparing this article.
Contributor Information
Kathleen A. McConnell, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts
Kelly H. Zou, Departments of Radiology and Health Care Policy, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts
Alexandra V. Chabrerie, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts
Nancy Olsen Bailey, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts
Peter McL. Black, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts
REFERENCES
- 1.Anderson RC, Grant JJ, Paz RDL, Frucht S, Goodman RR. Volumetric measurements in the detection of reduced ventricular volume in patients with normal-pressure hydrocephalus whose clinical condition improved after ventriculoperitoneal shunt placement. J Neurosurg. 2002;97:73–79. doi: 10.3171/jns.2002.97.1.0073. [DOI] [PubMed] [Google Scholar]
- 2.Black PMcL. Idiopathic normal-pressure hydrocephalus: Results of shunting in 62 patients. J Neurosurg. 1980;52:371–377. doi: 10.3171/jns.1980.52.3.0371. [DOI] [PubMed] [Google Scholar]
- 3.Black PMcL, Hakim R, Bailey NO. The use of the Codman-Medos Programmable Hakim valve in the management of patients with hydrocephalus:Illustrative cases. Neurosurgery. 1994;34:1110–1113. doi: 10.1227/00006123-199406000-00040. [DOI] [PubMed] [Google Scholar]
- 4.Black PMcL, Ojemann RG, Tzouras A. CSF shunts for dementia, incontinence, and gait disturbance. Clin Neurosurg. 1985;32:632–651. [PubMed] [Google Scholar]
- 5.Borgensen SE. Conductance to outflow of CSF in normal pressure hydro-cephalus. Acta Neurochir (Wien) 1984;71:1–45. doi: 10.1007/BF01401149. [DOI] [PubMed] [Google Scholar]
- 6.Borgesen SE, Gjerris F. Relationships between intracranial pressure, ventricular size, and resistance to CSF outflow. J Neurosurg. 1987;67:535–539. doi: 10.3171/jns.1987.67.4.0535. [DOI] [PubMed] [Google Scholar]
- 7.Carmel PW, Albright AL, Adelson PD.Incidence and management of subdural hematoma/hygroma with variable- and fixed-pressure differential valves: A randomized, controlled study of programmable compared with conventional valves Neurosurg Focus 19997Article 7 [DOI] [PubMed] [Google Scholar]
- 8.Gering DT, Nabavi A, Kikinis R, Hata N, Odonnell LJ, Grimson WE, Jolesz FA, Black PMcL, Wells WM., III An integrated visualization system for surgical planning and guidance using image fusion and an open MR. J Magn Reson Imaging. 2001;13:967–975. doi: 10.1002/jmri.1139. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg JO, Shenkin HA, Adam R. Idiopathic normal pressure hydro-cephalus: A report of 73 patients. J Neurol Neurosurg Psychiatry. 1977;40:336–341. doi: 10.1136/jnnp.40.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakim S.Algunas observaciones sobre la presion del L.C.R. sundrome hidrocefalio en adulto con "presion normal" del L.C.R. Universidad Javerian[Tesis 957] 1964Universidad Javerian; Bogota: (also available in English)(thesis) [Google Scholar]
- 11.Hakim CA, Hakim R, Hakim S. Normal-pressure hydrocephalus. Neurosurg Clin N Am. 2001;36:761–773. [PubMed] [Google Scholar]
- 12.Hebb AO, Cusimano MD. Idiopathic normal pressure hydrocephalus. Neurosurgery. 2001;49:1166–1186. doi: 10.1097/00006123-200111000-00028. [DOI] [PubMed] [Google Scholar]
- 13.Huckman MS. Normal pressure hydrocephalus: Evaluation of diagnostic and prognostic tests. AJNR AM J Neuroradiol. 1981;2:385–395. [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs L, Kinkel WR, Painter F, Muraski J, Heffner RR., Jr . Computerized tomography in dementia with special reference to changes in size of normal ventricles during aging and normal pressure hydrocephalus. In: Katzman R, Terry RD, Blick KL, editors. Alzheimer’s Disease: Senile Dementia and Related Disorders. Raven Press; New York: 1978. pp. 241–259. [Google Scholar]
- 15.Katzman R. Normal pressure hydrocephalus. In: Katzman R, Terry RD, Blick KL, editors. Alzheimer’s Disease: Senile Dementia and Related Disorders. Raven Press; New York: 1978. pp. 115–124. [Google Scholar]
- 16.Krauss JK, Regel JP. The predictive value of ventricular CSF removal in normal pressure hydrocephalus. Neurol Res. 1997;19:357–360. doi: 10.1080/01616412.1997.11740825. [DOI] [PubMed] [Google Scholar]
- 17.Larsson A, Jensen C, Bilting M, Ekholm S, Stephensen H, Wikkelso C. Does the shunt opening pressure influence the effect of shunt surgery in normal pressure hydrocephalus? Acta Neurochir (Wien) 1992;117:15–22. doi: 10.1007/BF01400629. [DOI] [PubMed] [Google Scholar]
- 18.Lorensen W, Cline H. Marching cube: A high resolution 3-D surface construction algorithm. Comput Graph. 1987;21 [Google Scholar]
- 19.Maksymowicz W, Czosnyka M, Koszewski W, Szymanska A, Traczewski W. The role of cerebrospinal compensatory parameters in the estimation of functioning of implanted shunt system in patients with communicating hydrocephalus (preliminary report) Acta Neurochir (Wien) 1989;101:112–116. doi: 10.1007/BF01410524. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC, Mokri B, Laws ER., Jr Surgical treatment of idiopathic hydro-cephalus in elderly patients. Neurology. 1985;35:307–311. doi: 10.1212/wnl.35.3.307. [DOI] [PubMed] [Google Scholar]
- 21.Raftopoulos C, Massager N, Baleriaux D, Deleval J, Clarysse S, Brotchi J. Prospective analysis by computed tomography and long-term outcome of 23 adult patients with chronic idiopathic hydrocephalus. Neurosurgery. 1996;38:51–59. doi: 10.1097/00006123-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Sahuqillo J, Rubio E, Codina A, Molins A, Guitart JM, Poca MA, Chasampi A. Reappraisal of the intracranial pressure and cerebrospinal fluid dynamics in patients with the so-called "normal pressure hydrocephalus" syndrome. Acta Neurochir (Wien) 1991;112:50–61. doi: 10.1007/BF01402454. [DOI] [PubMed] [Google Scholar]
- 23.Schoonderwaldt HC, Cho Chia Yuen G, Colon EJ, Hommes OR, Notermans SL, Walder HA. The preselection value of Doppler LP test for shunt-therapy in patients with normal pressure hydrocephalus. J Neurol. 1981;225:15–24. doi: 10.1007/BF00313457. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder W, Zarge J, Lorensen W. Decimation of triangle meshes. Comput Graph. 1992;26:65–78. [Google Scholar]
- 25.Tans TJ, Poortvliet DC. Reduction of ventricular size after shunting for normal pressure hydrocephalus related to CSF dynamics before shunting. J Neurol Neurosurg Psychiatry. 1988;51:521–525. doi: 10.1136/jnnp.51.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomsen AM, Borgensen SE, Bruhn P, Gjerris F. Prognosis of dementia in normal pressure hydrocephalus after shunt operation. Ann Neurol. 1986;20:304–310. doi: 10.1002/ana.410200306. [DOI] [PubMed] [Google Scholar]
- 27. Deleted in proof [Google Scholar]
- 28.Turner DA, McGeachie RE. Normal pressure hydrocephalus and dementia:Evaluation and treatment. Clin Geriatr Med. 1988;4:815–830. [PubMed] [Google Scholar]
- 29.Wikkelso C, Andersson H, Blomstrand C, Matousek M, Svendsen P. Computed tomography of the brain in the diagnosis of and prognosis in normal pressure hydrocephalus. Neuroradiology. 1989;31:160–165. doi: 10.1007/BF00698846. [DOI] [PubMed] [Google Scholar]
- 30.Wood JH, Bartlet D, James AE, Jr, Udvarhelyi GB. Normal pressure hydro-cephalus: Diagnosis and patient selection for shunt surgery. Neurology. 1974;24:517–526. doi: 10.1212/wnl.24.6.517. [DOI] [PubMed] [Google Scholar]
- 31.Zemack G, Romner B. Seven years of clinical experience with the programmable Codman Hakim valve: A retrospective study of 583 patients. J Neurosurg. 2000;92:941–948. doi: 10.3171/jns.2000.92.6.0941. [DOI] [PubMed] [Google Scholar]