Abstract
1. Single barnacle muscle fibres from Megabalanus psittacus (Darwin) were internally perfused with a number of K salt solutions (200 mM) which were made isotonic to the barnacle saline with sucrose.
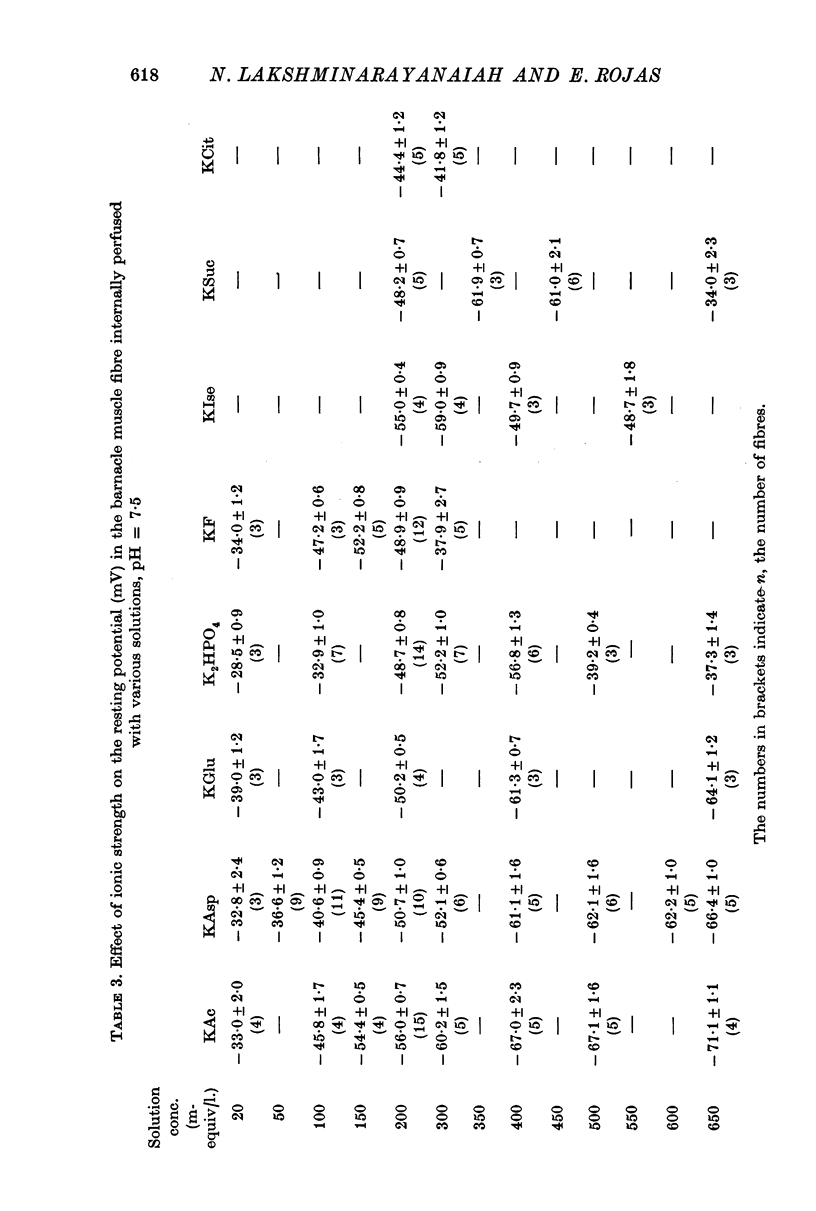
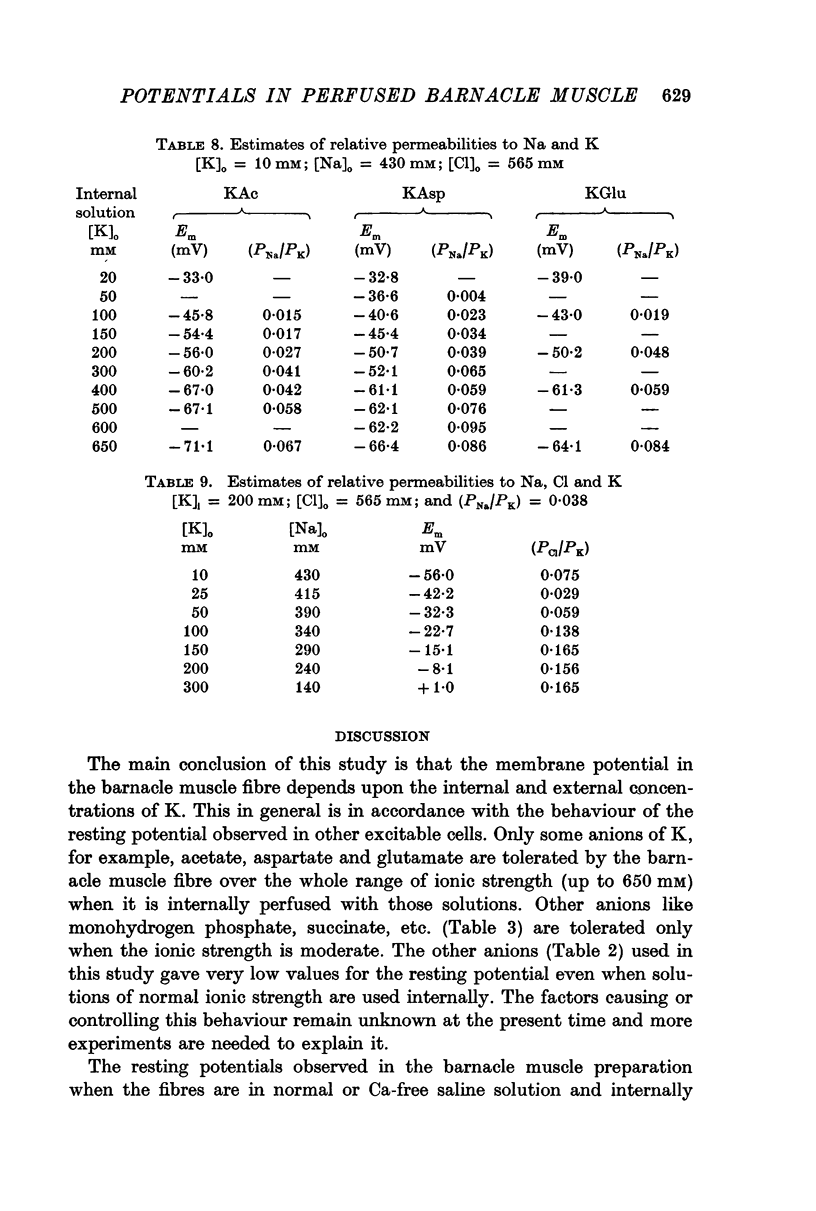
2. 200 mM-K acetate solution, in general, was found to be more effective than other solutions of K salts in generating and maintaining stable resting membrane potential of -56·0 ± 0·7 mV (all potentials are referred to the external solutions as ground). The various K salts, on the basis of the magnitude of the resting potential they generated in the muscle fibres, followed the sequence, acetate > isethionate > aspartate > glutamate > fluoride > monohydrogen phosphate > succinate > citrate > sulphate > oxalate > iodobenzoate > ferrocyanide > chlorate > nitrate > chloride > thiocyanate > iodide > bromide > cyanide.
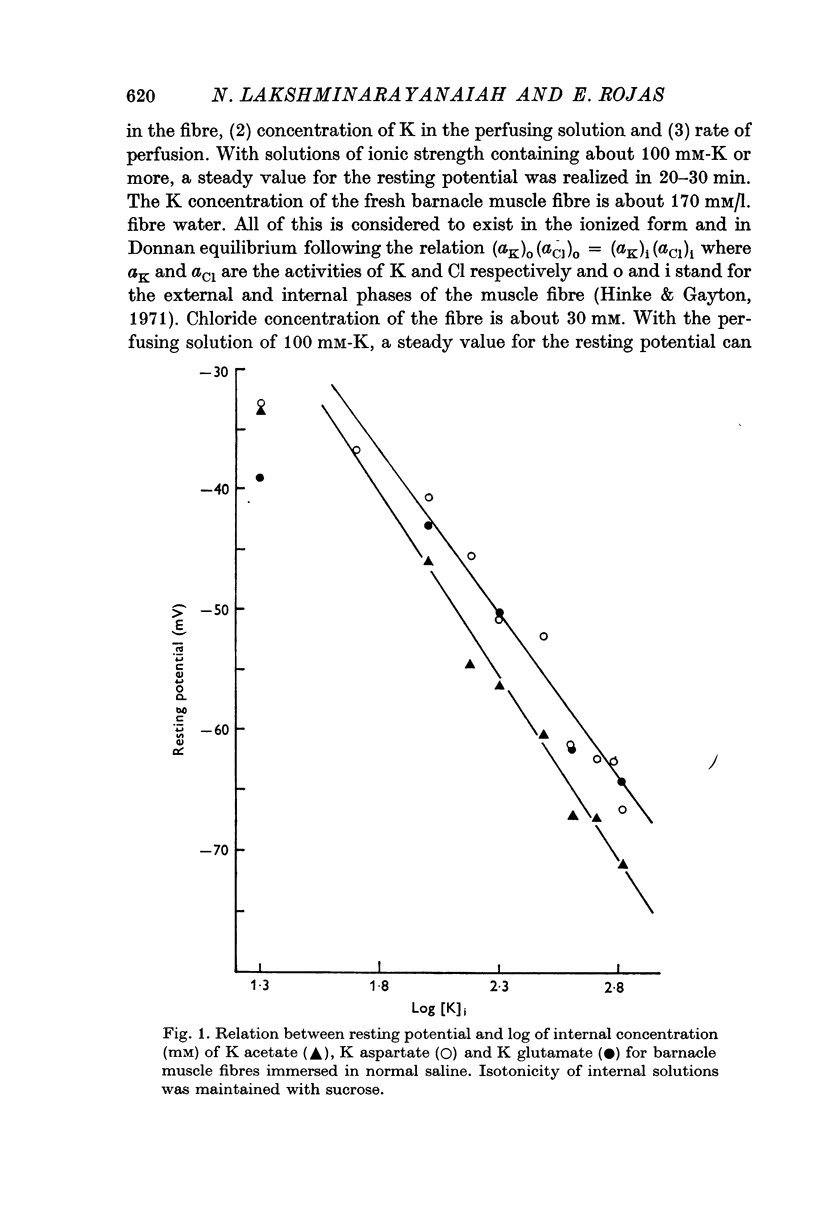
3. The resting potential in muscle fibres perfused with solutions of acetate, aspartate and glutamate increased linearly with the logarithm of the K concentration (slope = 30·4 mV for K acetate and 27·4 for K aspartate and glutamate) when the ionic strength of the solutions was progressively increased from 50 to 650 mM. On the other hand, similar increase of ionic strength beyond 200 mM of solutions of K isethionate, fluoride, monohydrogen phosphate, succinate and citrate depolarized the muscle fibres.
4. Perfusion of acetate solutions of other alkali metal ions gave low values for the resting potential and followed the sequence K > Na > Rb > Li > Cs. Also NH4 and Tris ions gave low values for the resting potential which underwent oscillations associated with the twitching of the fibre and occasionally became positive in value (action potential).
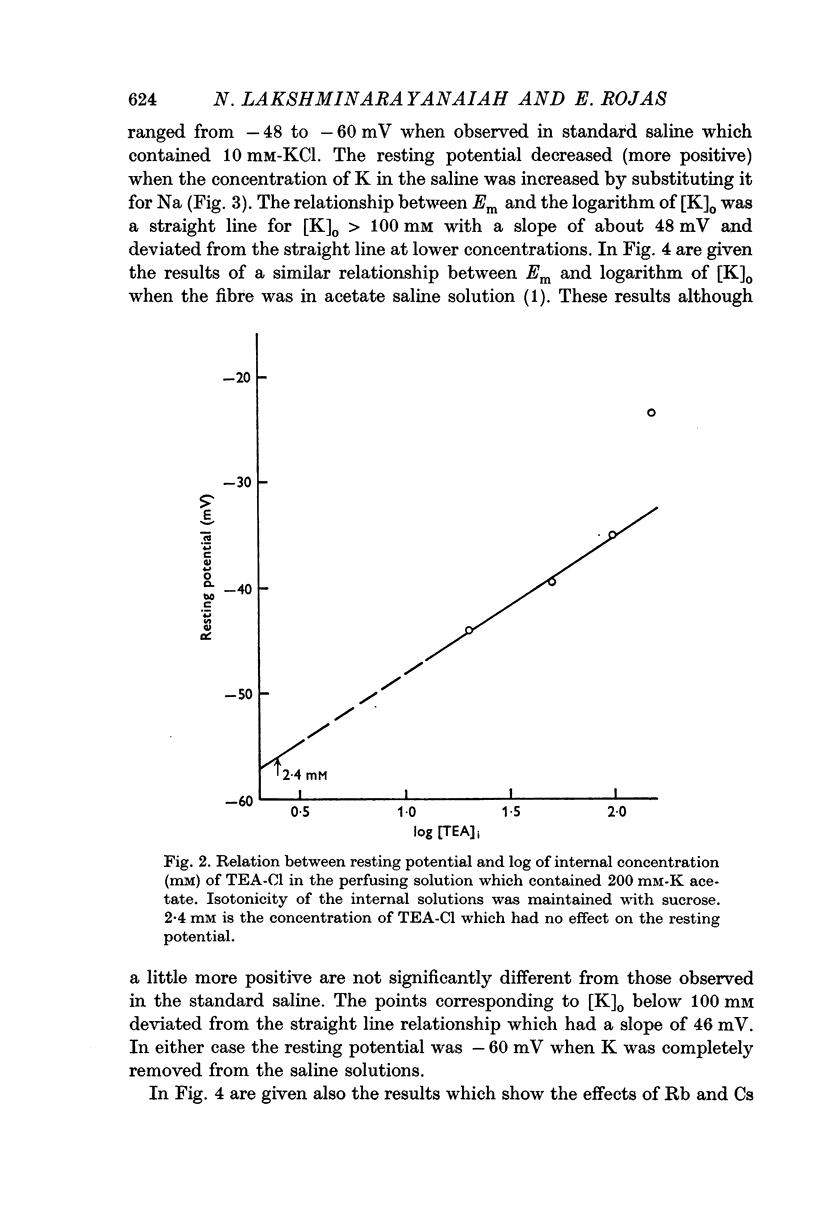
5. Addition of tetraethyl ammonium chloride (TEA-Cl), 20-100 mM, to K acetate solutions (200 mM) depolarized the fibre membrane and the consequent reduction of resting potential varied linearly with the logarithm of TEA concentration.
6. Replacement of chloride ion by acetate or isethionate in the external solution did not change significantly the resting potential although the values were consistently lower by about 2 mV.
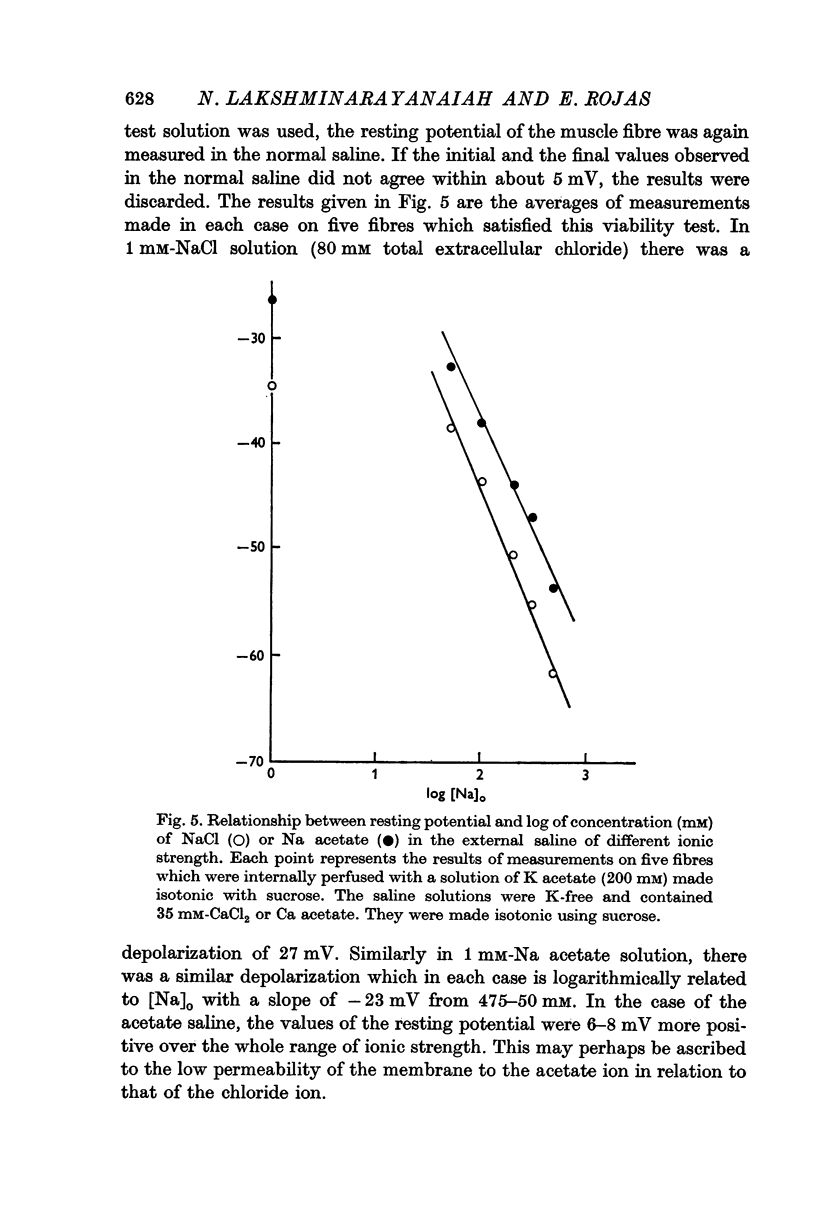
7. Complete elimination of K in the external solution and reduction of its ionic strength using sucrose depolarized the muscle fibres by about 27 mV when Na was changed from 475 to 1 mM. Under these conditions, external solutions completely in acetate form gave resting potentials which were more positive than those observed in completely chloride solutions by 6-8 mV.
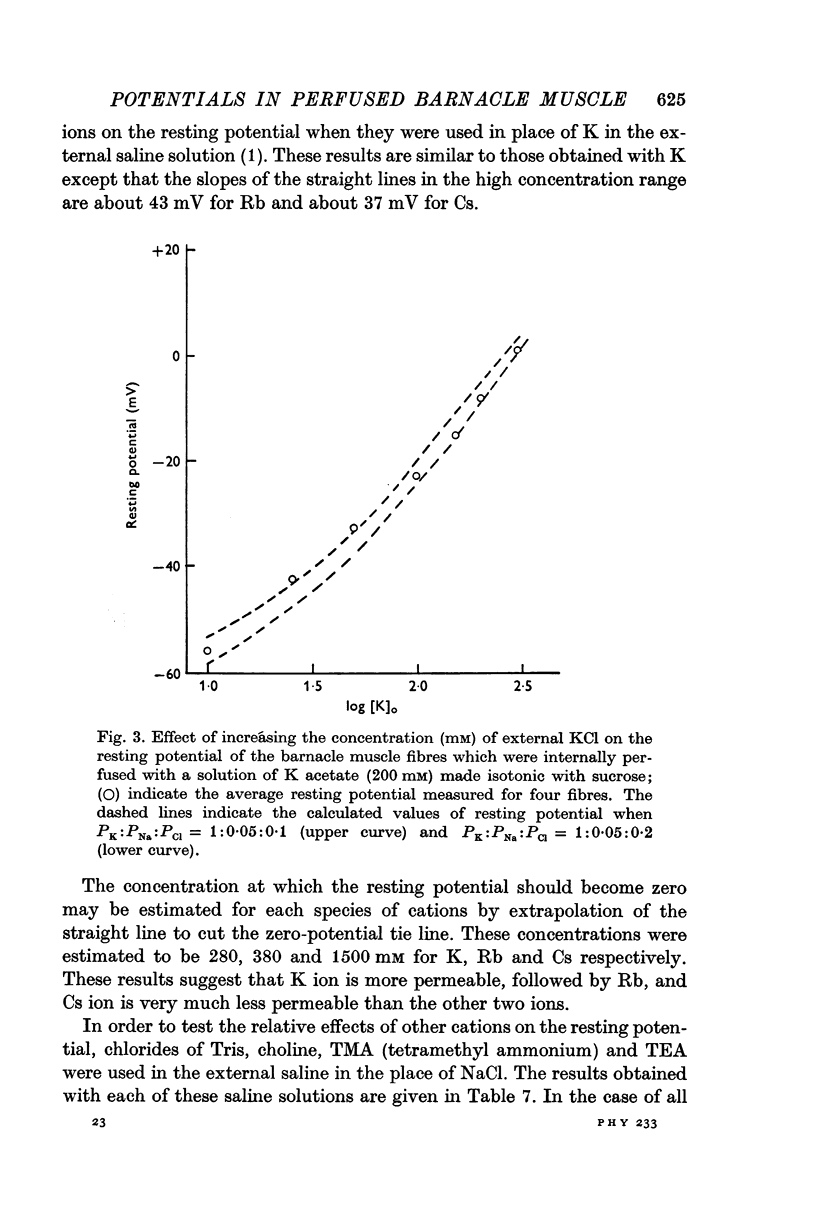
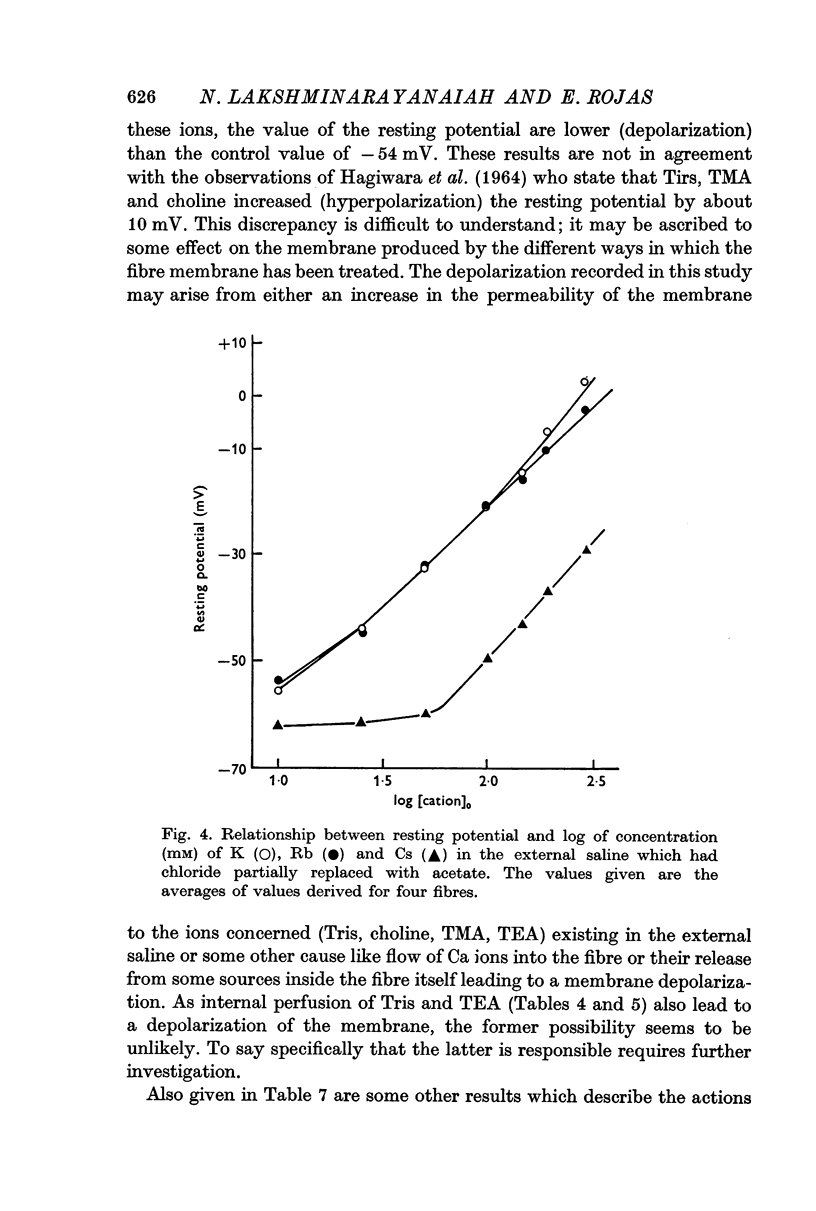
8. Replacement of Na by Li, Tris, choline, tetramethyl or tetraethyl ammonium ion in the external solution made the values of the resting potential more positive (depolarization). Similarly increasing the concentration of K (or Cs or Rb in place of K) by correspondingly decreasing the concentration of Na in the outside solution depolarized the fibres and the resting potential became zero at a concentration of 280 mM (or 308 or 1500 mM for Rb or Cs, respectively) on extrapolation.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. Potassium chloride movement and the membrane potential of frog muscle. J Physiol. 1960 Apr;151:154–185. [PMC free article] [PubMed] [Google Scholar]
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMSTRONG C. M., BINSTOCK L. ANOMALOUS RECTIFICATION IN THE SQUID GIANT AXON INJECTED WITH TETRAETHYLAMMONIUM CHLORIDE. J Gen Physiol. 1965 May;48:859–872. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman W. J., Senft J. P. Dynamic asymmetries in the squid axon membrane. J Gen Physiol. 1968 May 1;51(5):102–114. [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., MEVES H. THE EFFECT OF DILUTING THE INTERNAL SOLUTION ON THE ELECTRICAL PROPERTIES OF A PERFUSED GIANT AXON. J Physiol. 1964 Apr;170:541–560. doi: 10.1113/jphysiol.1964.sp007348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the protoplasm of a giant nerve fibre with artificial solutions. Nature. 1961 Jun 3;190:885–887. doi: 10.1038/190885a0. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2303–2331. doi: 10.1085/jgp.50.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr Sodium and potassium fluxes in isolated barnacle muscle fibers. J Gen Physiol. 1968 Apr;51(4):445–477. doi: 10.1085/jgp.51.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R. Chloride fluxes in isolated dialyzed barnacle muscle fibers. J Gen Physiol. 1972 Oct;60(4):471–497. doi: 10.1085/jgp.60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R., Latorre R. Effect of temperature on membrane potential and ionic fluxes in intact and dialysed barnacle muscle fibres. J Physiol. 1972 Sep;225(2):255–273. doi: 10.1113/jphysiol.1972.sp009939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., GINSBORG B. L. The ionic requirements for the production of action potentials in crustacean muscle fibres. J Physiol. 1958 Aug 6;142(3):516–543. doi: 10.1113/jphysiol.1958.sp006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. The electrical properties of crustacean muscle fibres. J Physiol. 1953 Apr 28;120(1-2):171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D. E. POTENTIAL, IMPEDANCE, AND RECTIFICATION IN MEMBRANES. J Gen Physiol. 1943 Sep 20;27(1):37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., CHICHIBU S., NAKA K. I. THE EFFECTS OF VARIOUS IONS ON RESTING AND SPIKE POTENTIALS OF BARNACLE MUSCLE FIBERS. J Gen Physiol. 1964 Sep;48:163–179. doi: 10.1085/jgp.48.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I., CHICHIBU S. MEMBRANE PROPERTIES OF BARNACLE MUSCLE FIBER. Science. 1964 Mar 27;143(3613):1446–1448. doi: 10.1126/science.143.3613.1446. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., NAKA K. I. THE INITIATION OF SPIKE POTENTIAL IN BARNACLE MUSCLE FIBERS UNDER LOW INTRACELLULAR CA++. J Gen Physiol. 1964 Sep;48:141–162. doi: 10.1085/jgp.48.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of sodium ions on the electrical activity of giant axon of the squid. J Physiol. 1949 Mar 1;108(1):37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE G., SMYTH T., Jr NEUROMUSCULAR PHYSIOLOGY OF GIANT MUSCLE FIBERS OF A BARNACLE, BALANUS NUBILUS DARWIN. Comp Biochem Physiol. 1963 Dec;10:291–314. doi: 10.1016/0010-406x(63)90229-9. [DOI] [PubMed] [Google Scholar]
- HUTTER O. F., NOBLE D. The chloride conductance of frog skeletal muscle. J Physiol. 1960 Apr;151:89–102. [PMC free article] [PubMed] [Google Scholar]
- Hille B. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J Gen Physiol. 1967 May;50(5):1287–1302. doi: 10.1085/jgp.50.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinke J. A., Gayton D. C. Transmembrane K + and Cl - activity gradients for the muscle fiber of the giant barnacle. Can J Physiol Pharmacol. 1971 Apr;49(4):312–322. doi: 10.1139/y71-034. [DOI] [PubMed] [Google Scholar]
- Holtzman D. The effect of low ionic strength extracellular solutions on the resting potential in skeletal muscle fibers. J Gen Physiol. 1967 Jul;50(6):1485–1497. doi: 10.1085/jgp.50.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle G., Smyth T., Jr Giant Muscle Fibers in a Barnacle, Balanus nubilus Darwin. Science. 1963 Jan 4;139(3549):49–50. doi: 10.1126/science.139.3549.49. [DOI] [PubMed] [Google Scholar]
- Kao C. Y., Stanfield P. R. Actions of some cations on the electrical properties and mechanical threshold of frog sartorius muscle fibers. J Gen Physiol. 1970 May;55(5):620–639. doi: 10.1085/jgp.55.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Rojas E., Taylor R. E., Vergara J. Calcium and potassium systems of a giant barnacle muscle fibre under membrane potential control. J Physiol. 1973 Mar;229(2):409–455. doi: 10.1113/jphysiol.1973.sp010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Some factors influencing sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2333–2355. doi: 10.1085/jgp.50.10.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIKAWA T., SPYROPOULOS C. S., TASAKI I., TEORELL T. Methods for perfusing the giant axon of Loligo pealii. Acta Physiol Scand. 1961 Jun;52:195–196. doi: 10.1111/j.1748-1716.1961.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R. The differential effects of tetraethylammonium and zinc ions on the resting conductance of frog skeletal muscle. J Physiol. 1970 Jul;209(1):231–256. doi: 10.1113/jphysiol.1970.sp009164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield P. R. The effect of the tetraethylammonium ion on the delayed currents of frog skeletal muscle. J Physiol. 1970 Jul;209(1):209–229. doi: 10.1113/jphysiol.1970.sp009163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERMAN R., GRUNDFEST H. Graded and all-or-none electrogenesis in arthropod muscle. II. The effects of alkali-earth and onium ions on lobster muscle fibers. J Gen Physiol. 1961 May;44:997–1027. doi: 10.1085/jgp.44.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]


