Abstract
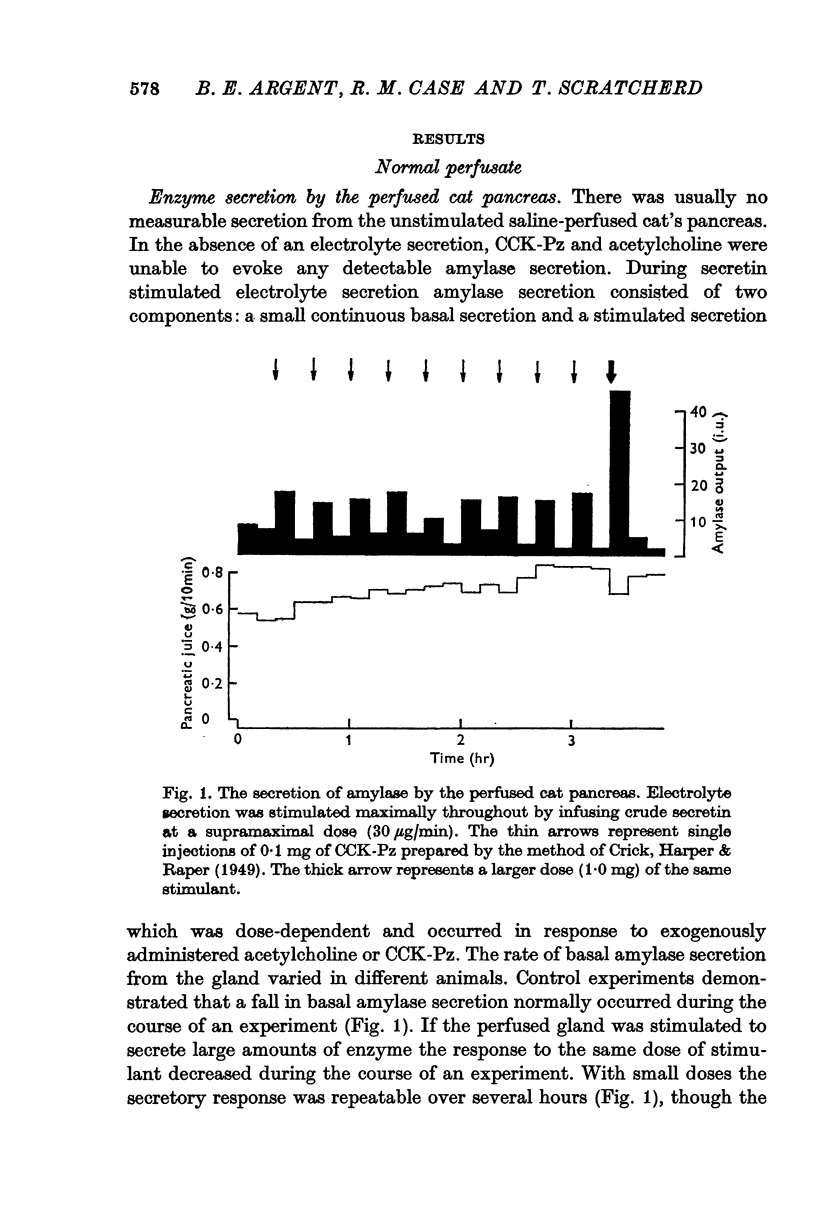
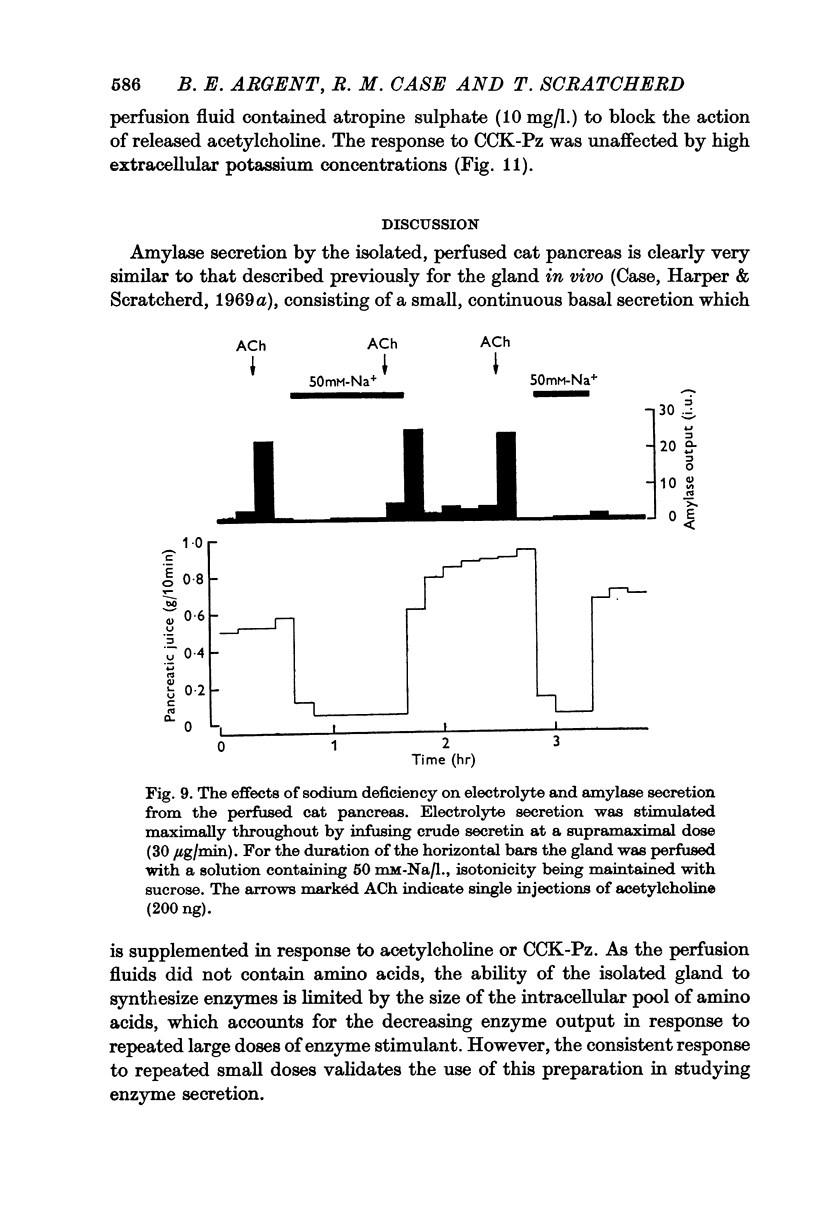
1. Amylase secretion from the perfused pancreas consists of two components: a small continuous basal secretion and a stimulated secretion in response to acetylcholine or cholecystokinin-pancreozymin. The response to small doses of either stimulant was repeatable over several hours.
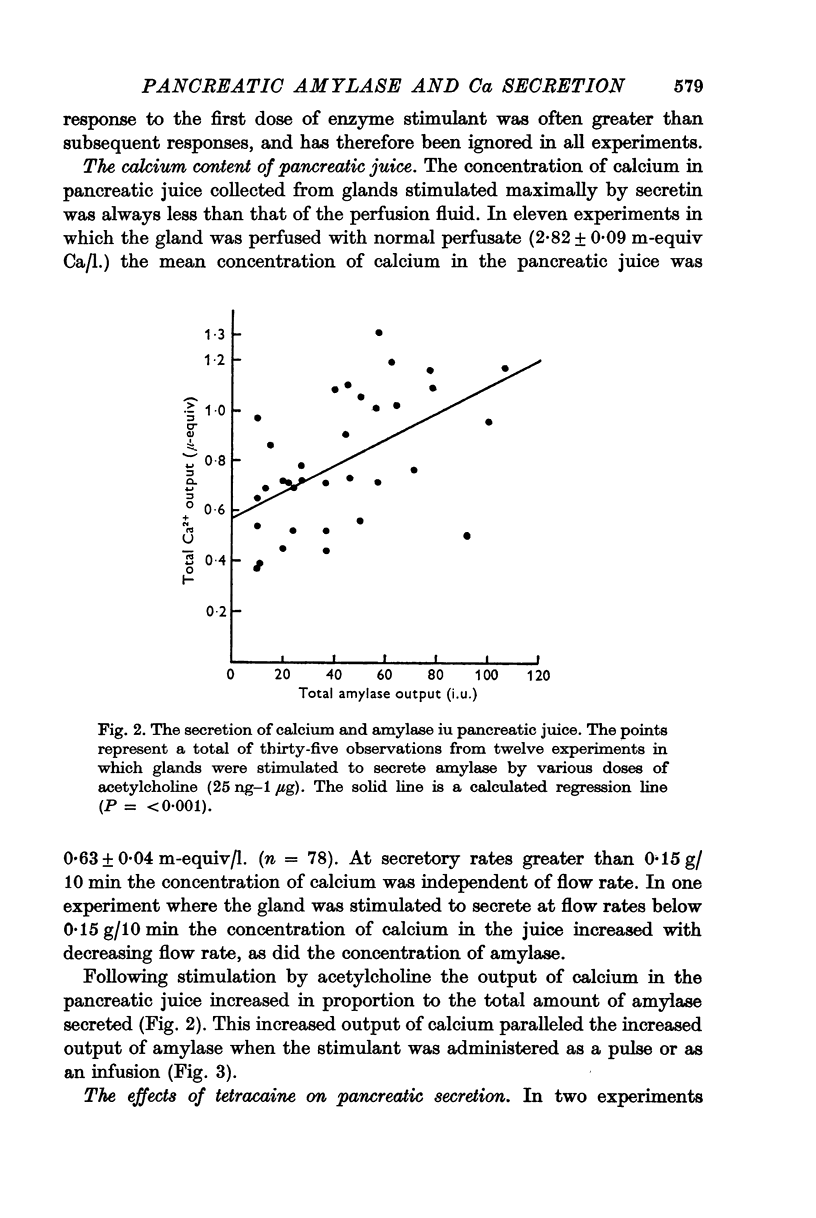
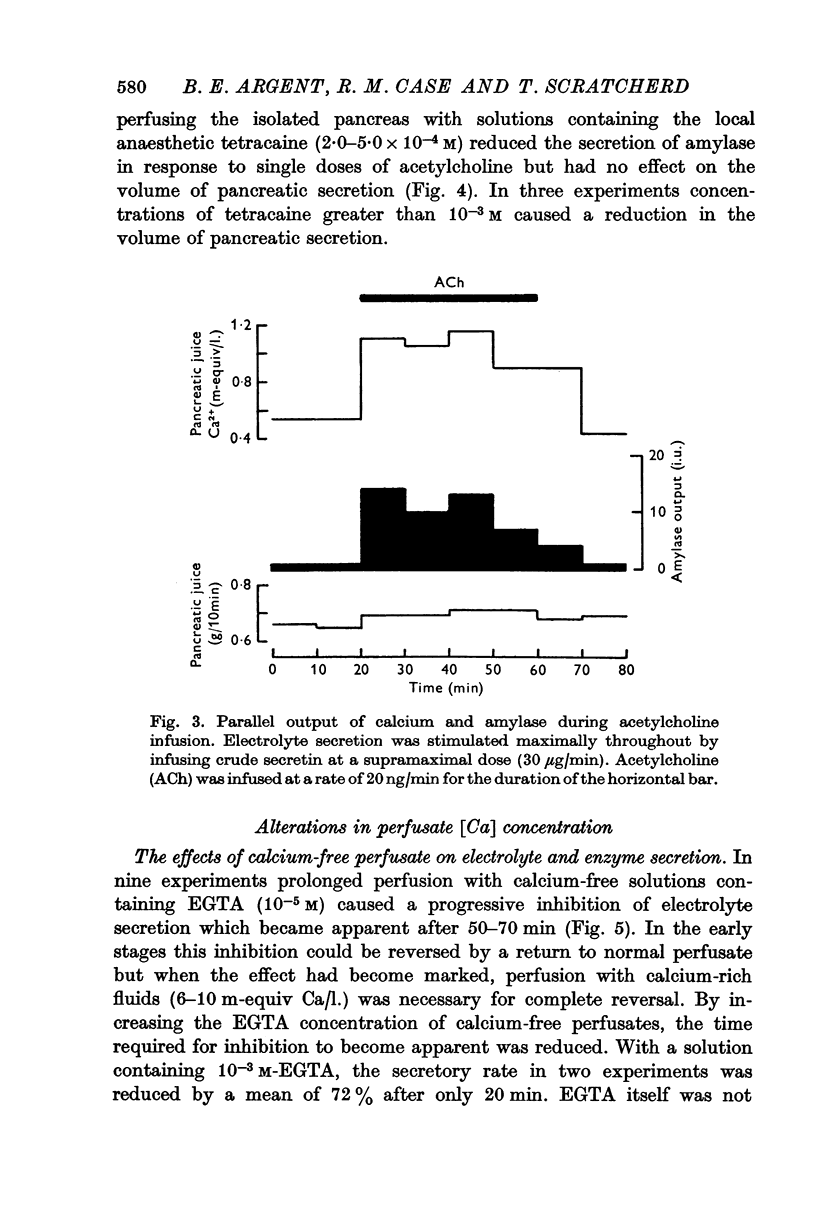
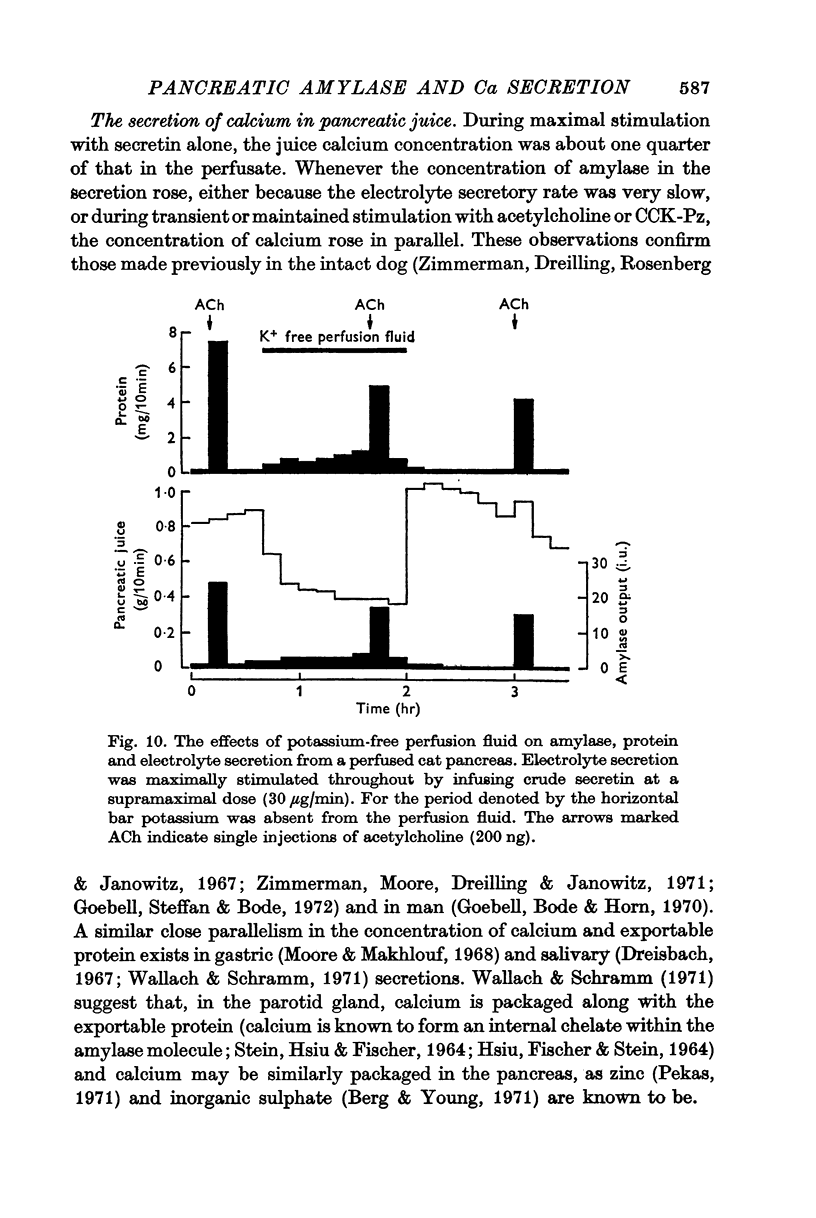
2. The calcium concentration of pancreatic juice, always less than that of the perfusate, was normally constant above secretory rates of 0·15 g/10 min. However, when the concentration of enzymes in the juice rose, either after stimulation or at very low secretory rates, the calcium concentration rose in parallel, suggesting that this calcium is bound to, or is a component of, pancreatic enzymes.
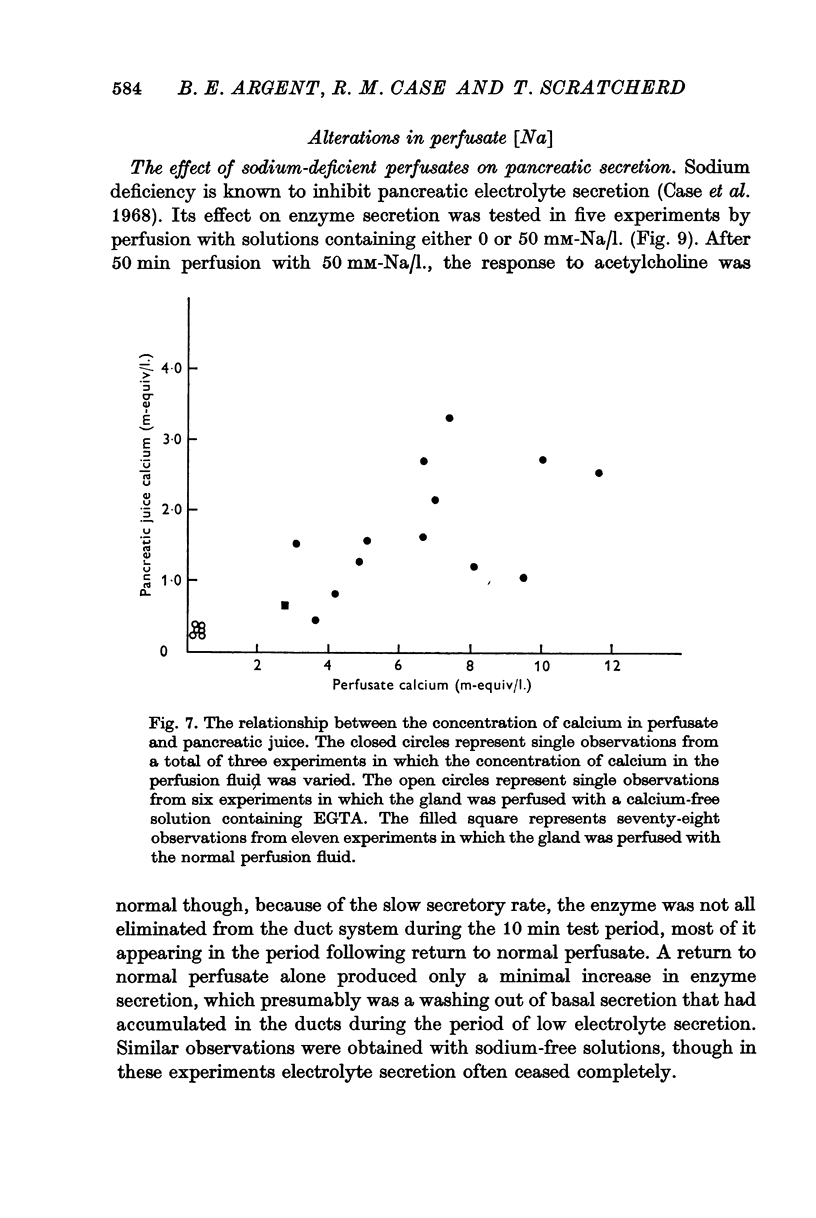
3. Elevation of the perfusate calcium concentration resulted in a parallel increase in the calcium concentration of the pancreatic juice.
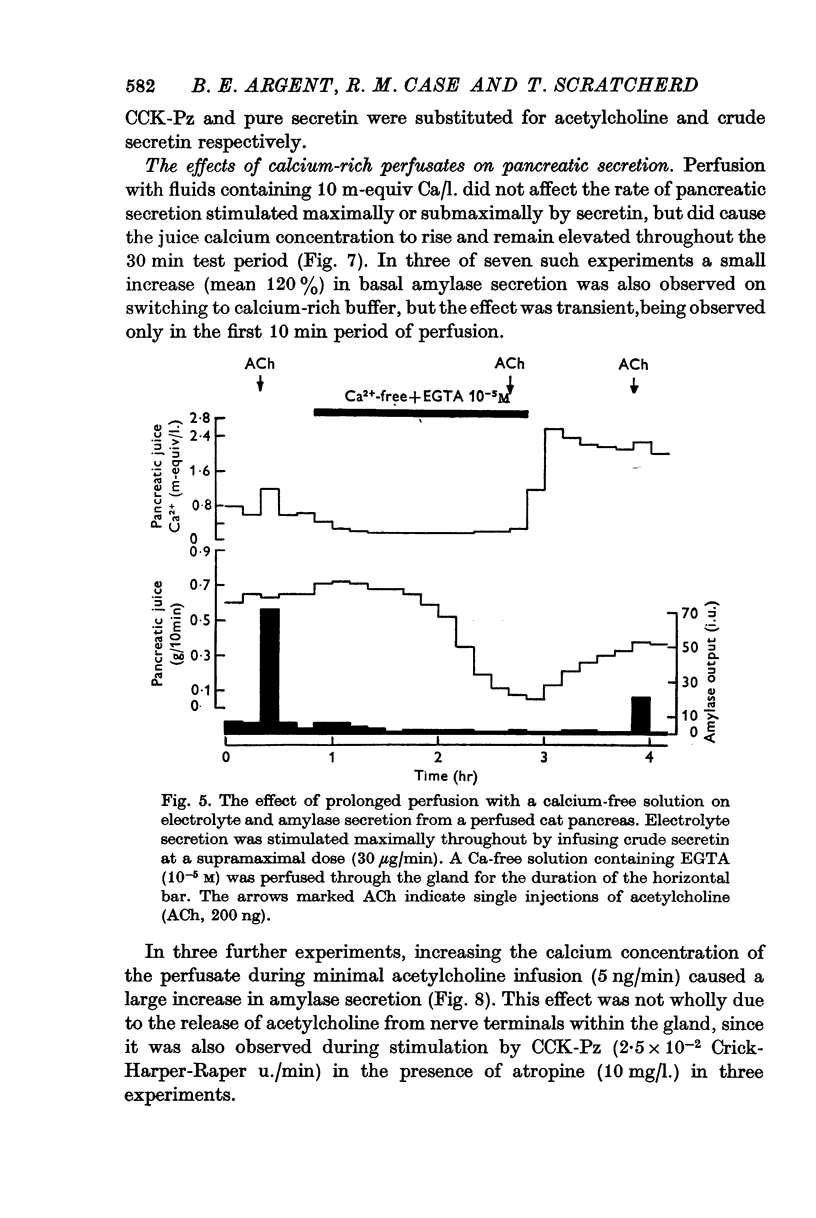
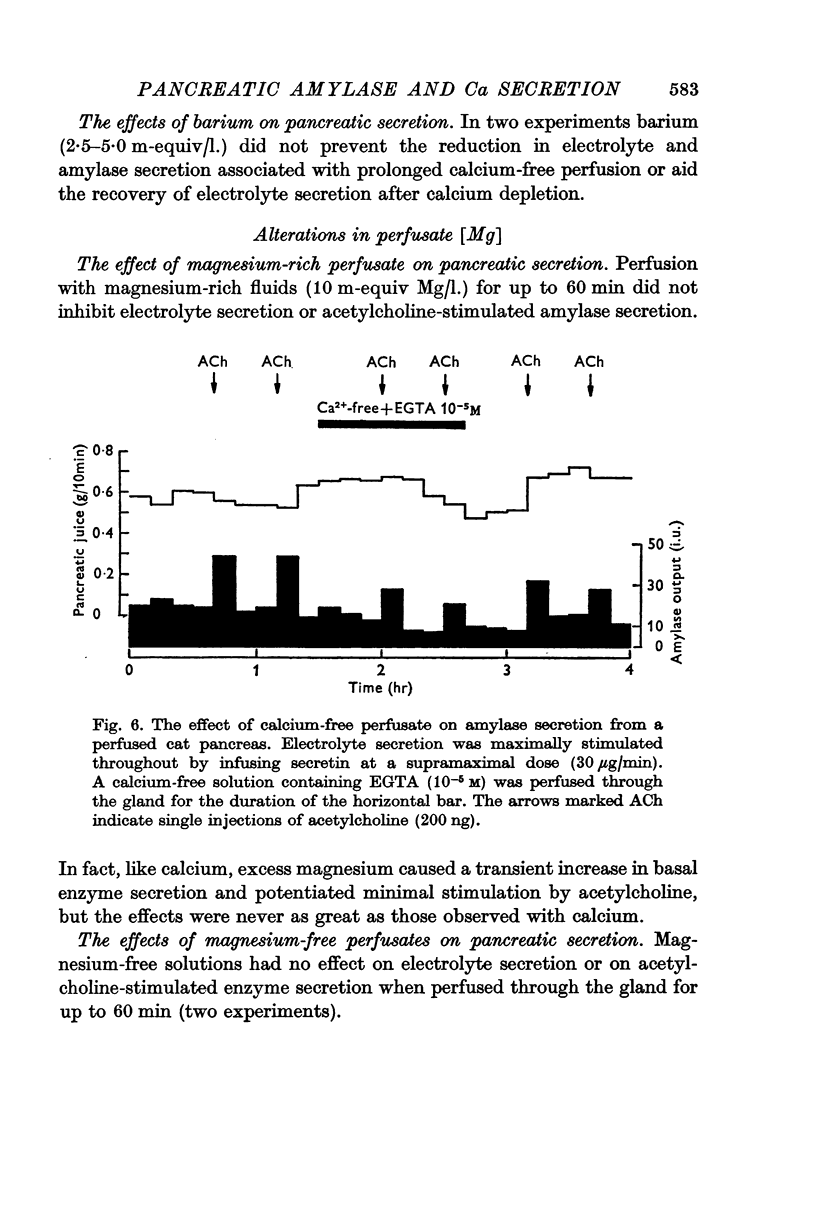
4. Calcium-free solutions initially caused a small reduction in basal and stimulated amylase secretion and, after prolonged periods of perfusion, abolished stimulated secretion and caused a reduction in electrolyte secretion. The latter was completely reversed by calcium-rich perfusates but the effects on enzyme secretion were only partially reversible.
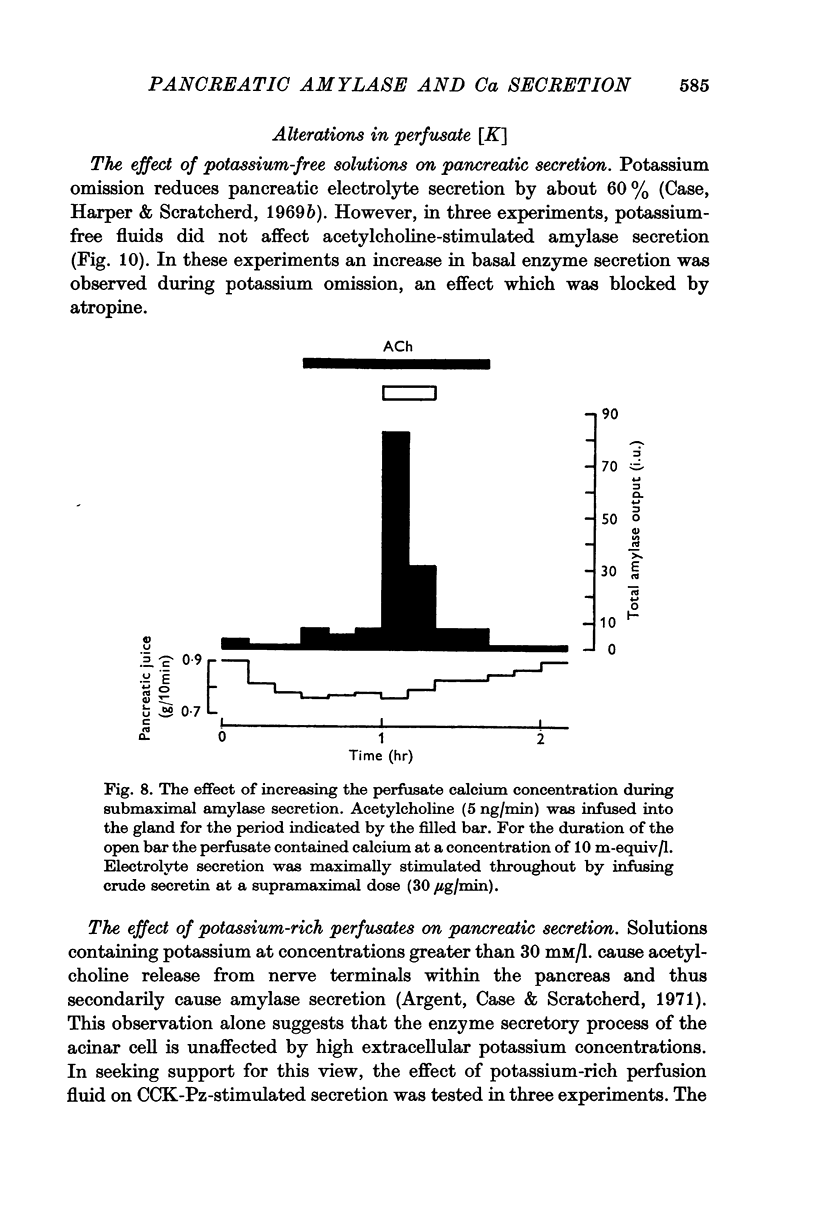
5. Calcium-rich perfusates had no effect on the rate of electrolyte secretion but potentiated submaximally stimulated amylase secretion.
6. Barium did not substitute for calcium in supporting pancreatic secretion.
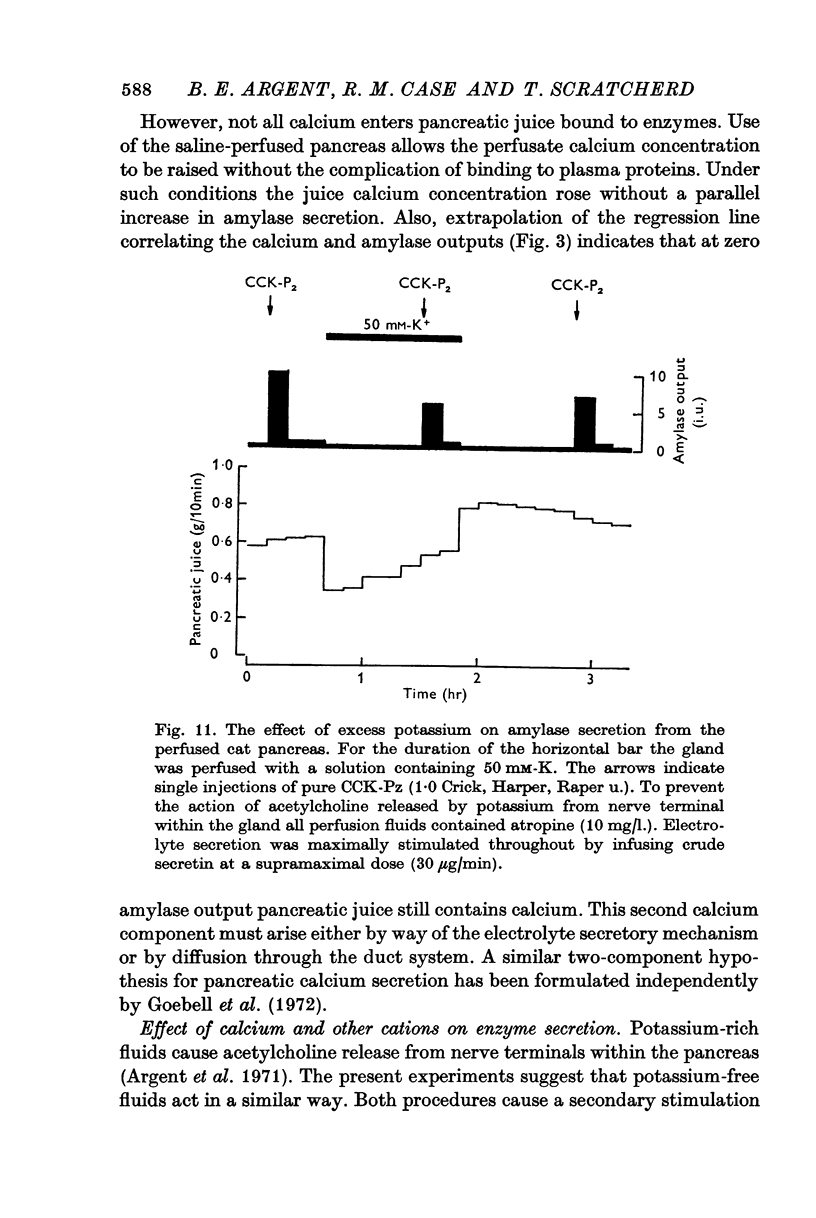
7. Alterations in the extracellular concentrations of sodium, potassium and magnesium had no direct effect on amylase secretion.
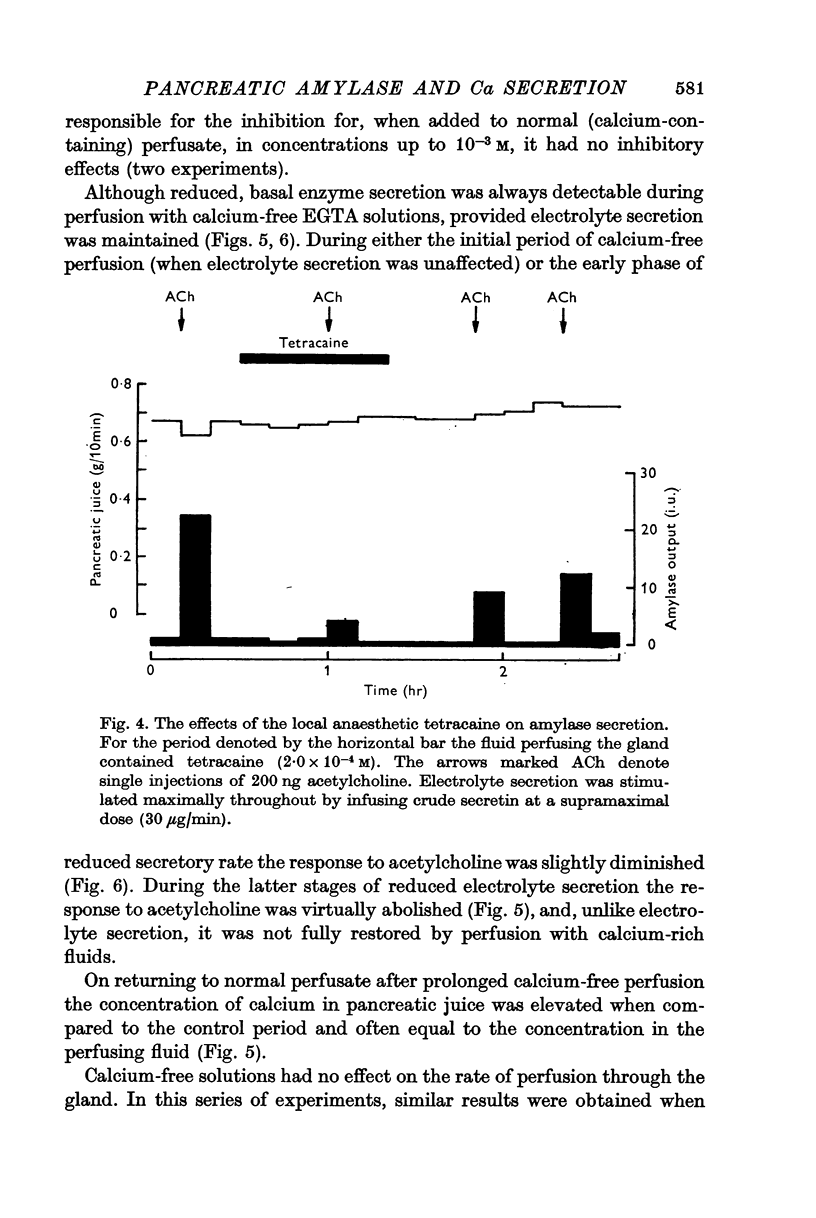
8. The local anaesthetic tetracaine inhibited amylase secretion at a lower concentration than that required to inhibit electrolyte secretion.
9. It is concluded (a) that calcium is secreted into the pancreatic juice in two fractions, one associated with enzymes and the other with the electrolyte component of the juice; and (b) that calcium ions play an important role in the stimulus-secretion coupling of pancreatic acinar cells, but that the effects of calcium depletion on electrolyte secretion may principally be due to alterations in the permeability of the duct system.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argent B. E., Case R. M., Fraser M. P., Scratcherd T. The effects of calcium on volume and amylase secretion from the perfused cat pancreas. J Physiol. 1972 Jul;224(1):29P–30P. [PubMed] [Google Scholar]
- Argent B. E., Case R. M., Scratcherd T. Stimulation of amylase secretion from the perfused cat pancreas by potassium and other alkali metal ions. J Physiol. 1971 Aug;216(3):611–624. doi: 10.1113/jphysiol.1971.sp009543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg N. B., Young R. W. Sulfate metabolism in pancreatic acinar cells. J Cell Biol. 1971 Aug;50(2):469–483. doi: 10.1083/jcb.50.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRICK J., HARPER A. A., RAPER H. S. On the preparation of secretin and pancreozymin. J Physiol. 1949 Dec;110(3-4):367–376. doi: 10.1113/jphysiol.1949.sp004445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Harper A. A., Scratcherd T. The secretion of electrolytes and enzymes by the pancreas of the anaesthetized cat. J Physiol. 1969 Apr;201(2):335–348. doi: 10.1113/jphysiol.1969.sp008759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Harper A. A., Scratcherd T. Water and electrolyte secretion by the perfused pancreas of the cat. J Physiol. 1968 May;196(1):133–149. doi: 10.1113/jphysiol.1968.sp008499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T., Wynne R. D. The origin and secretion of pancreatic juice bicarbonate. J Physiol. 1970 Sep;210(1):1–15. doi: 10.1113/jphysiol.1970.sp009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Pancreatic acinar cells: measurement of membrane potential and miniature depolarization potentials. J Physiol. 1972 Aug;225(1):1–13. doi: 10.1113/jphysiol.1972.sp009926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray G. J. The action of scretin, cholecystokinin-pancreozymin and caerulein on pancreatic secretion in the rat. J Physiol. 1972 Sep;225(3):679–692. doi: 10.1113/jphysiol.1972.sp009963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T. The effect of amethocaine on acetylcholine-induced depolarization and catecholamine secretion in the adrenal chromaffin cell. Br J Pharmacol Chemother. 1967 Aug;30(3):612–619. doi: 10.1111/j.1476-5381.1967.tb02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. The influence of calcium on the secretory response of the submaxillary gland to acetylcholine or to noradrenaline. J Physiol. 1963 Mar;165(3):528–541. doi: 10.1113/jphysiol.1963.sp007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach R. H. Effect of isoproterenol on calcium, protein, and electrolytes of rat submaxillary gland. Proc Soc Exp Biol Med. 1967 Oct;126(1):279–281. doi: 10.3181/00379727-126-32423. [DOI] [PubMed] [Google Scholar]
- FORTE J. G., NAUSS A. H. EFFECTS OF CALCIUM REMOVAL ON BULLFROG GASTRIC MUCOSA. Am J Physiol. 1963 Oct;205:631–637. doi: 10.1152/ajplegacy.1963.205.4.631. [DOI] [PubMed] [Google Scholar]
- Goebell H., Bode C., Horn H. D. Einfluss von Secretin und Pankreozymin auf die Calciumsekretion im menschlichen Duodensalsaft bei normaler und gestörter Pankreasfunktion. Klin Wochenschr. 1970 Nov 15;48(22):1330–1339. doi: 10.1007/BF01485458. [DOI] [PubMed] [Google Scholar]
- Goebell H., Steffen C., Bode C. Stimulatory effect of pancreozymin-cholecystokinin on calcium secretion in pancreatic juice of dogs. Gut. 1972 Jun;13(6):477–482. doi: 10.1136/gut.13.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIU J., FISCHER E. H., STEIN E. A. ALPHA-AMYLASES AS CALCIUM-METALLOENZYMES. II. CALCIUM AND THE CATALYTIC ACTIVITY. Biochemistry. 1964 Jan;3:61–66. doi: 10.1021/bi00889a011. [DOI] [PubMed] [Google Scholar]
- JACOBSON A., SCHWARTZ M., REHM W. S. EFFECTS OF REMOVAL OF CALCIUM FROM BATHING MEDIA ON FROG STOMACH. Am J Physiol. 1965 Jul;209:134–140. doi: 10.1152/ajplegacy.1965.209.1.134. [DOI] [PubMed] [Google Scholar]
- Jaanus S. D., Rosenstein M. J., Rubin R. P. On the mode of action of ACTH on the isolated perfused adrenal gland. J Physiol. 1970 Aug;209(3):539–556. doi: 10.1113/jphysiol.1970.sp009178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manery J. F. Effects of Ca ions on membranes. Fed Proc. 1966 Nov-Dec;25(6):1804–1810. [PubMed] [Google Scholar]
- Martinez J. R., Petersen O. H. The importance of extracellular calcium for acetylcholine-evoked salivary secretion. Experientia. 1972 Feb 15;28(2):167–168. doi: 10.1007/BF01935735. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Saffran M. Steroid production and membrane potential measurement in cells of the adrenal cortex. J Physiol. 1967 Mar;189(1):149–161. doi: 10.1113/jphysiol.1967.sp008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. W., Makhlouf G. M. Calcium in normal human gastric juice. A four-component model with speculation on the relation of calcium to pepsin secretion. Gastroenterology. 1968 Oct;55(4):465–480. [PubMed] [Google Scholar]
- Nielsen S. P., Petersen O. H. Transport of calcium in the perfused submandibular gland of the cat. J Physiol. 1972 Jun;223(3):685–697. doi: 10.1113/jphysiol.1972.sp009869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekas J. C. Pancreatic incorporation of 65Zn and histidine-14C into secreted proteins of the pig. Am J Physiol. 1971 Mar;220(3):799–803. doi: 10.1152/ajplegacy.1971.220.3.799. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Matthews E. K. The effect of pancreozymin and acetylcholine on the membrane potential of the pancreatic acinar cells. Experientia. 1972 Sep 15;28(9):1037–1038. doi: 10.1007/BF01918657. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Tenenhouse A. Cyclic adenosine monophosphate, CA++, and membranes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1364–1370. doi: 10.1073/pnas.59.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderstap A. S., Bonting S. L. Enzyme secretion by the isolated rabbit pancreas: absence of a relation with the Na-K activated ATPase. Studies on Na-K activated ATPase, XXVII. Pflugers Arch. 1969;313(1):53–61. doi: 10.1007/BF00586328. [DOI] [PubMed] [Google Scholar]
- Robberecht P., Christophe J. Secretion of hydrolases by perfused fragments of rat pancreas: effect of calcium. Am J Physiol. 1971 Apr;220(4):911–917. doi: 10.1152/ajplegacy.1971.220.4.911. [DOI] [PubMed] [Google Scholar]
- Rubin R. P., Feinstein M. B., Jaanus S. D., Paimre M. Inhibition of catecholamine secretion and calcium exchange in perfused cat adrenal glands by tetracaine and magnesium. J Pharmacol Exp Ther. 1967 Mar;155(3):463–471. [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- SEDAR A. W., FORTE J. G. EFFECTS OF CALCIUM DEPLETION ON THE JUNCTIONAL COMPLEX BETWEEN OXYNTIC CELLS OF GASTRIC GLANDS. J Cell Biol. 1964 Jul;22:173–188. doi: 10.1083/jcb.22.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIN E. A., HSIU J., FISCHER E. H. ALPHA-AMYLASES AS CALCIUM-METALLOENZYMES. I. PREPARATION OF CALCIUM-FREE APOAMYLASES BY CHELATION AND ELECTRODIALYSIS. Biochemistry. 1964 Jan;3:56–61. doi: 10.1021/bi00889a010. [DOI] [PubMed] [Google Scholar]
- Selinger Z., Naim E. The effect of calcium on amylase secretion by rat parotid slices. Biochim Biophys Acta. 1970 Apr 21;203(2):335–337. doi: 10.1016/0005-2736(70)90148-3. [DOI] [PubMed] [Google Scholar]
- Wallach D., Schramm M. Calcium and the exportable protein in rat parotid gland. Parallel subcellular distribution and concomitant secretion. Eur J Biochem. 1971 Aug 16;21(3):433–437. doi: 10.1111/j.1432-1033.1971.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. J., Dreiling D. A., Rosenberg I. R., Janowitz H. D. Secretion of calcium by the canine pancreas. Gastroenterology. 1967 May;52(5):865–870. [PubMed] [Google Scholar]


