Abstract
1. Micro-electrode recordings were made from axons of the spinocervical tract in unanaesthetized decerebrate-spinal cats.
2. The effects of stimulation of (1) descending systems at the level of the upper cervical spinal cord and (2) hind limb cutaneous nerves, on discharges of spinocervical tract neurones were examined.
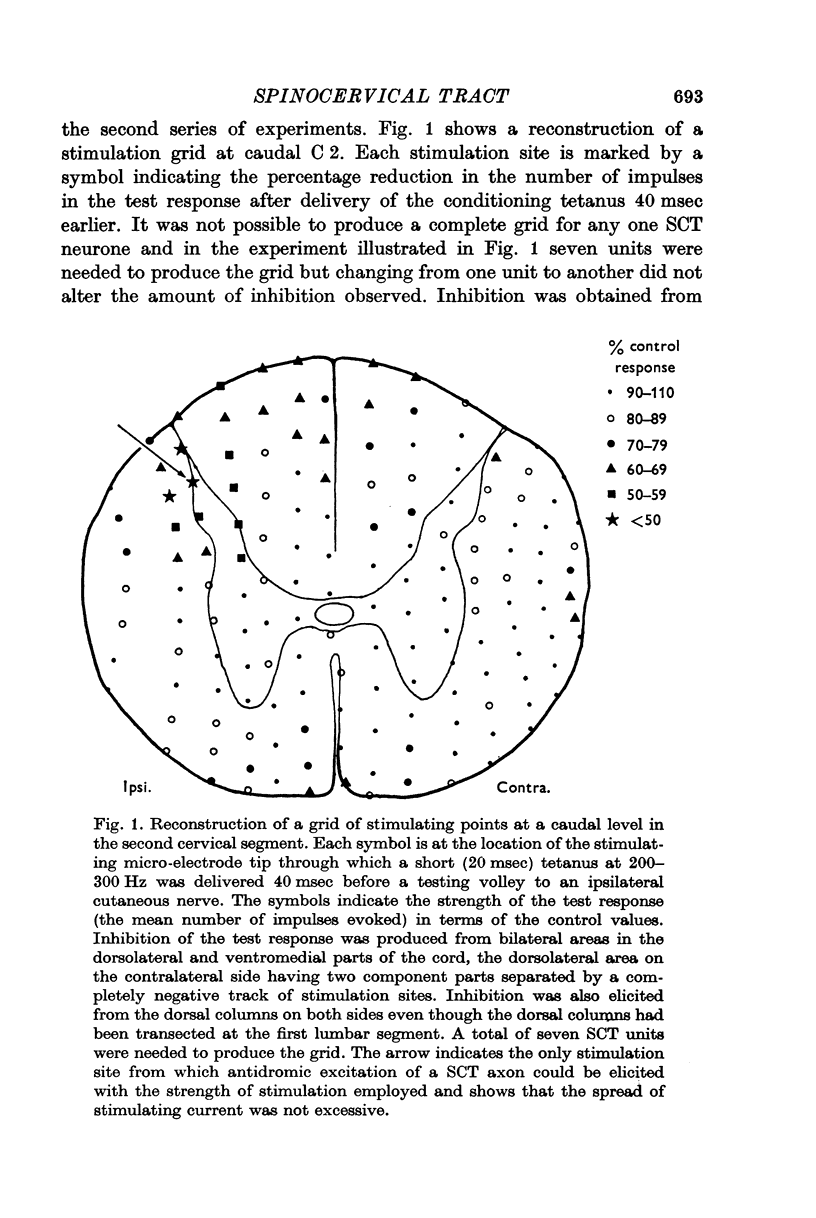
3. Effects were obtained from bilateral spinal cord regions in the dorsolateral funiculi and the most medial and ventral parts of the ventral funiculi and also from the dorsal columns in the upper cervical region even though the columns had been transected at low thoracic and upper lumbar levels.
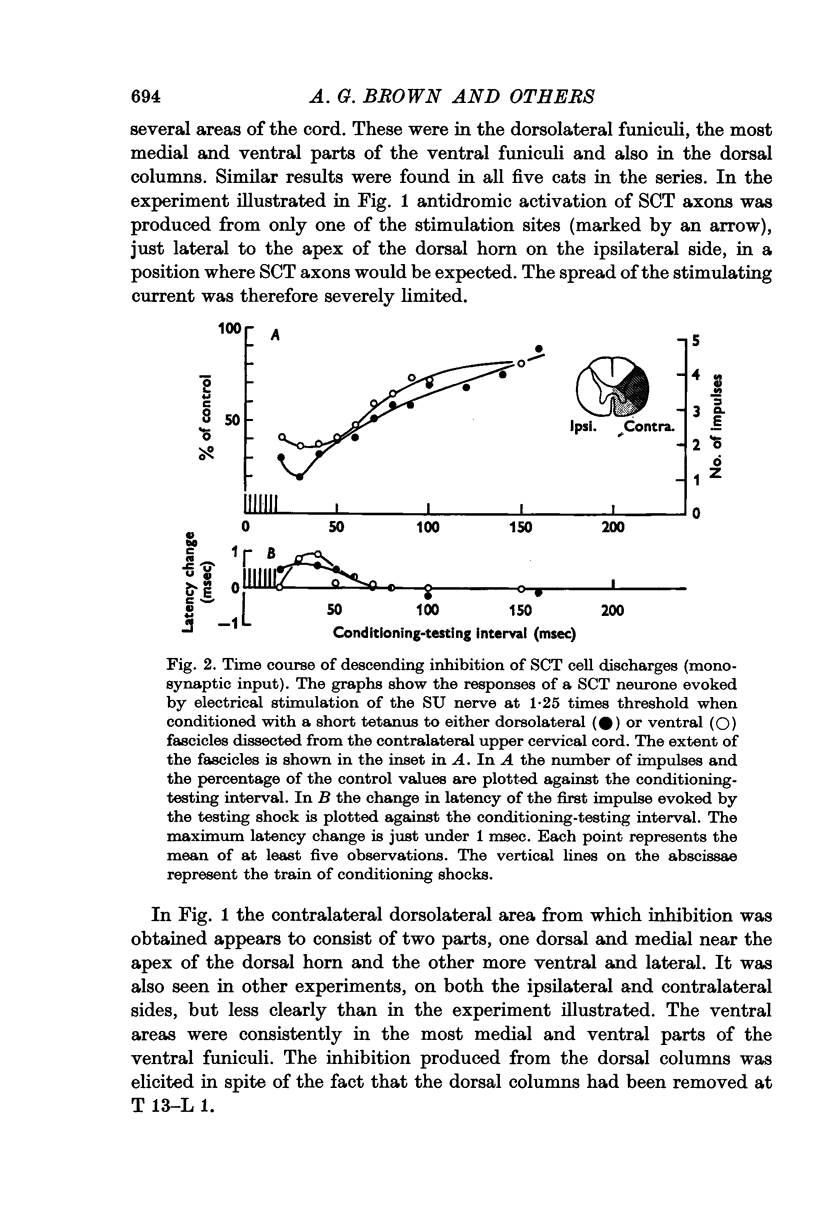
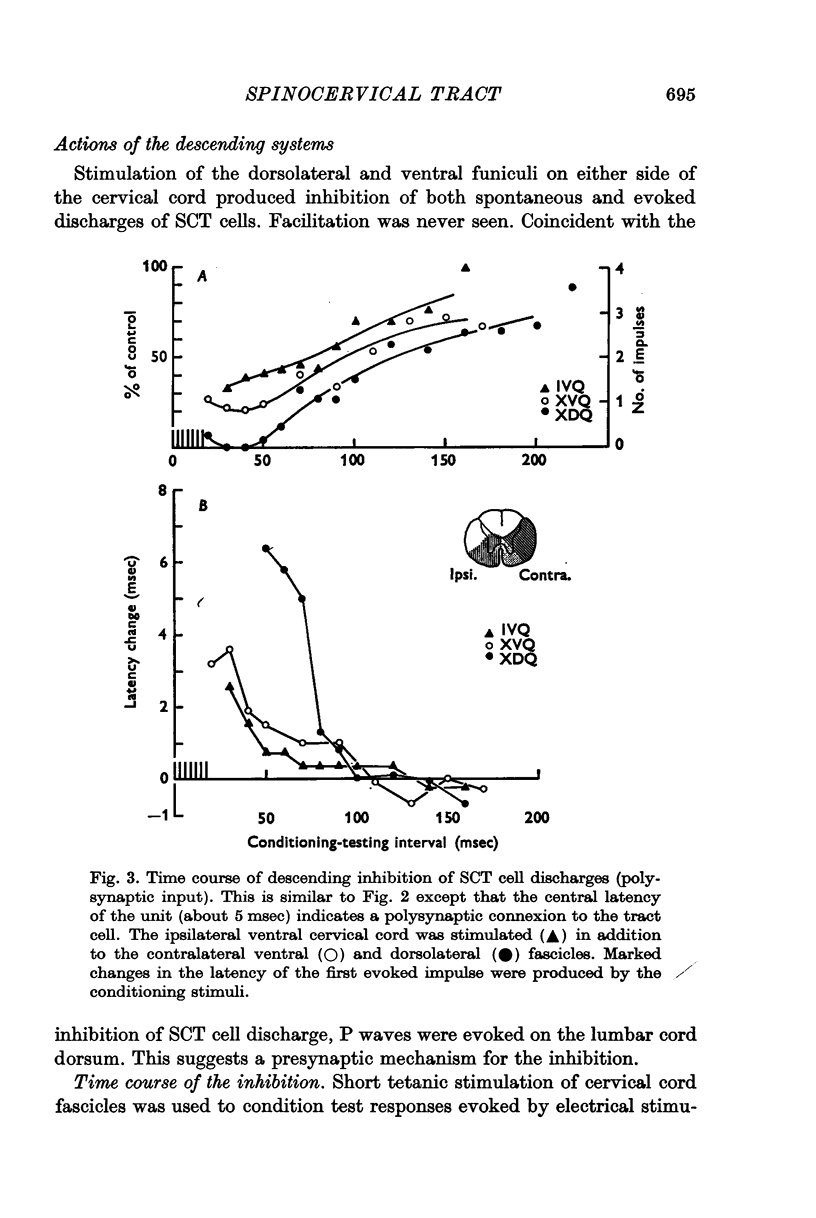
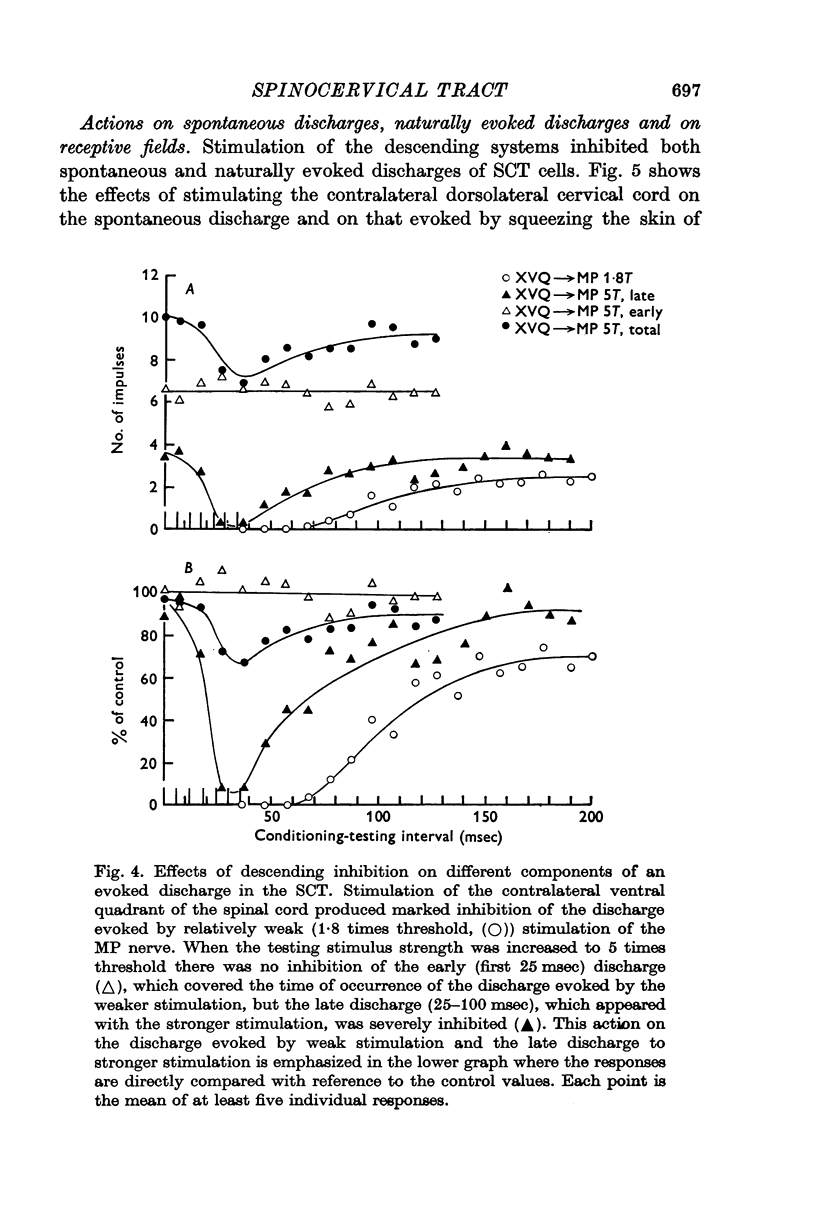
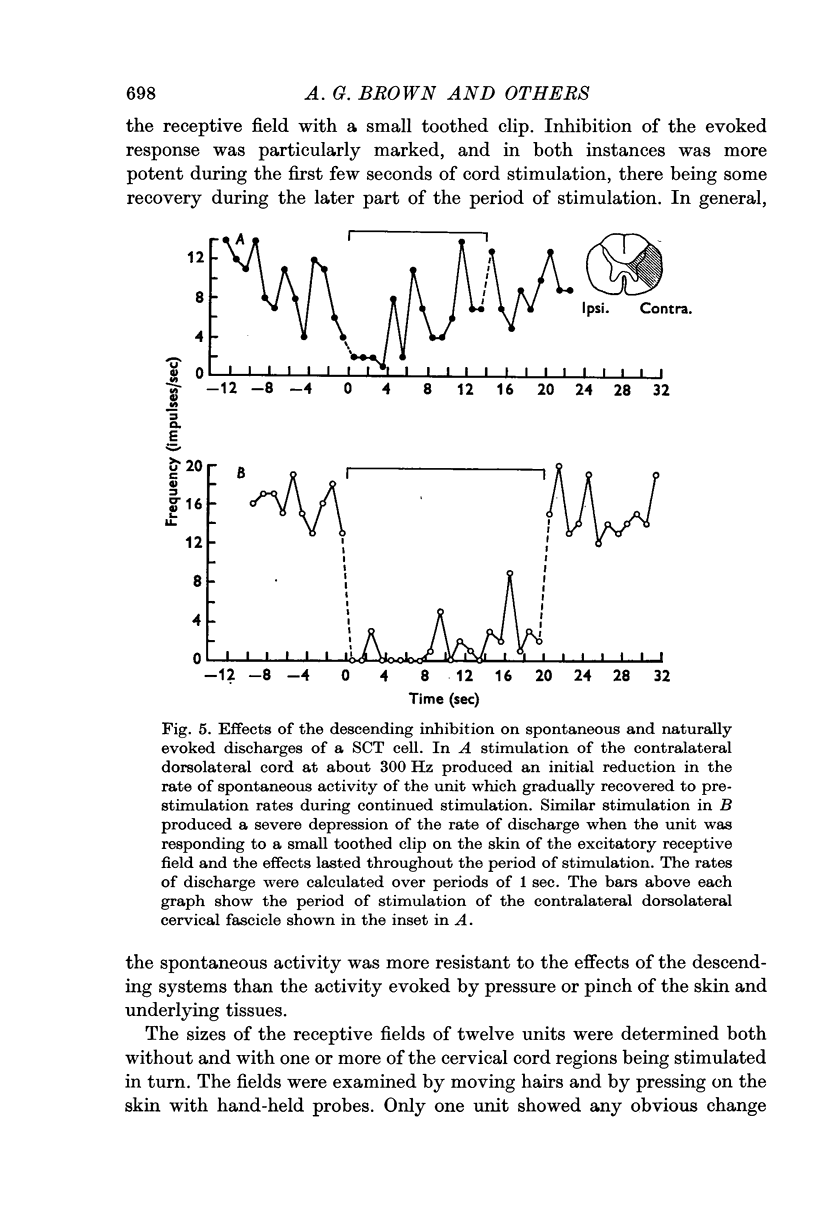
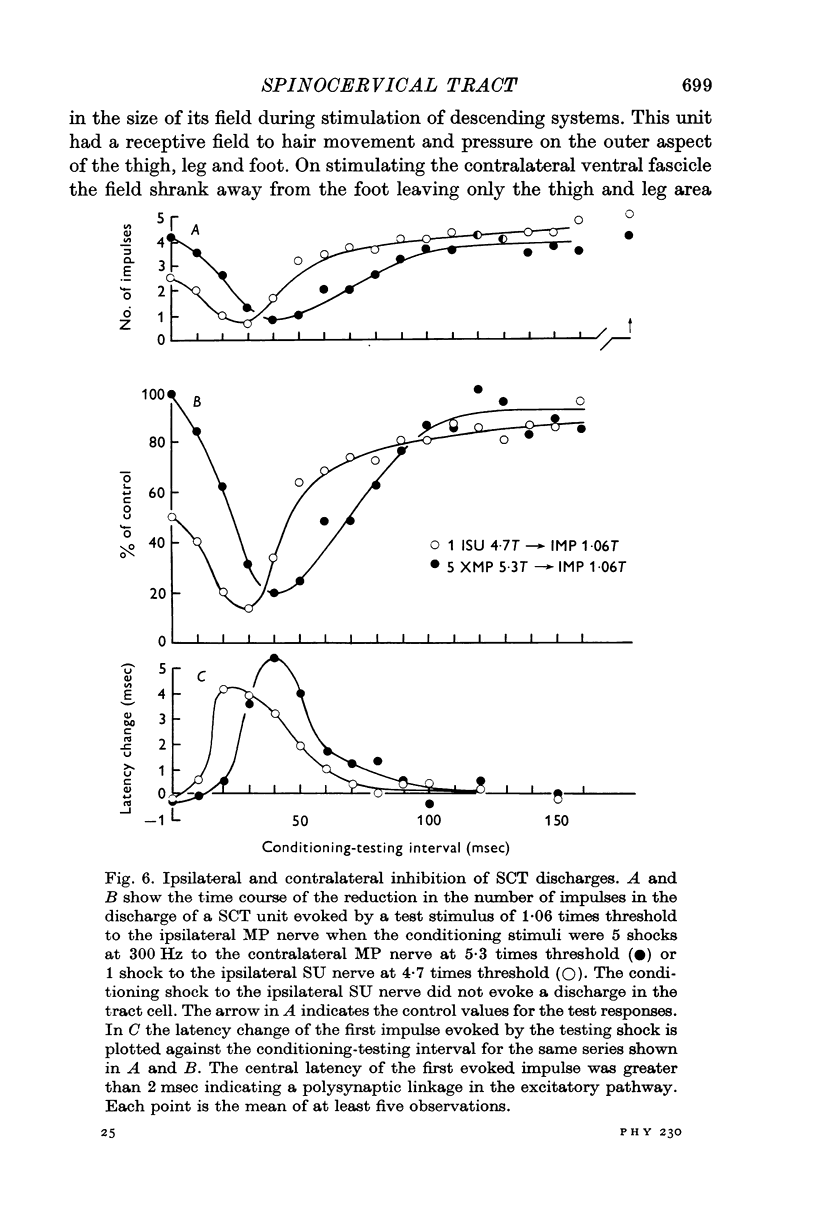
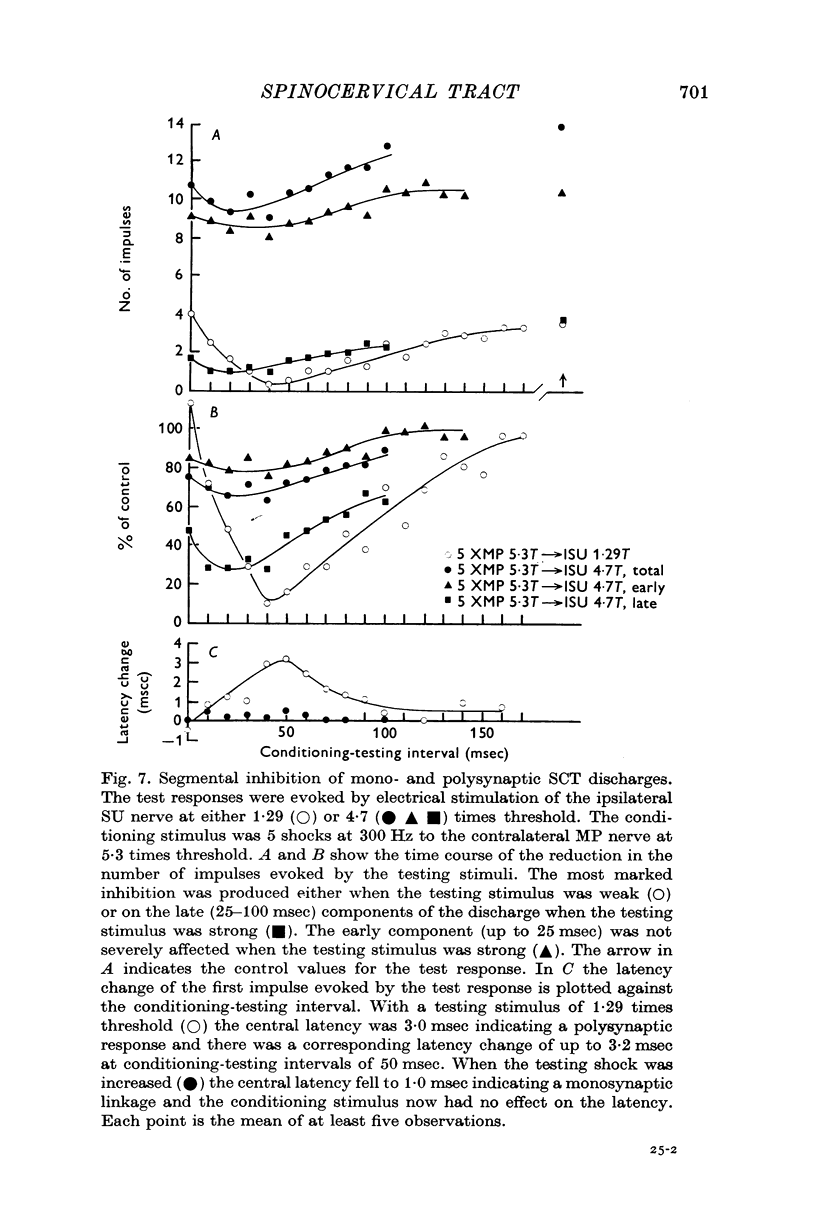
4. Stimulation of either descending or segmental systems inhibited spontaneous and evoked responses. Facilitation was not seen. The inhibition had a time course of up to 250 msec, with maximal action at 20-40 msec and was greatest for polysynaptic responses or those evoked from the smaller myelinated cutaneous axons.
5. It is suggested that the descending and segmental systems converge on to common inhibitory interneurones.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. G. Effects of descending impulses on transmission through the spinocervical tract. J Physiol. 1971 Dec;219(1):103–125. doi: 10.1113/jphysiol.1971.sp009652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., KOSTYUK P. G., SCHMIDT R. F. Presynaptic inhibition of the central actions of flexor reflex afferents. J Physiol. 1962 May;161:258–281. doi: 10.1113/jphysiol.1962.sp006885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Sprague J. M., Whitsel B. L., Dogan S., Jannetta P. J. Organization of the vestibular projection to the spinal cord of the cat. J Neurophysiol. 1966 Jul;29(4):626–664. doi: 10.1152/jn.1966.29.4.626. [DOI] [PubMed] [Google Scholar]
- Fetz E. E. Pyramidal tract effects on interneurons in the cat lumbar dorsal horn. J Neurophysiol. 1968 Jan;31(1):69–80. doi: 10.1152/jn.1968.31.1.69. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Post-synaptic excitation and inhibition from primary afferents in neurones of the spinocervical tract. J Physiol. 1968 Dec;199(3):569–592. doi: 10.1113/jphysiol.1968.sp008669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAPORTE Y., LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. I. Recording of mass discharge in dissected Flechsig's fasciculus. Acta Physiol Scand. 1956 Mar 24;36(1-2):175–187. doi: 10.1111/j.1748-1716.1956.tb01316.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Three ascending spinal pathways in the dorsal part of the lateral funiculus. Acta Physiol Scand. 1961 Jan;51:1–16. doi: 10.1111/j.1748-1716.1961.tb02108.x. [DOI] [PubMed] [Google Scholar]
- RUDIN D. O., EISENMAN G. A method for dissection and electrical study in vitro of mammalian central nervous tissue. Science. 1951 Sep 21;114(2960):300–302. doi: 10.1126/science.114.2960.300-a. [DOI] [PubMed] [Google Scholar]
- Shoumura K. Patterns of fiber degeneration in the lateral wall of the suprasylvian gyrus (Clare-Bishop area) following lesions in the visual cortex in cats. Brain Res. 1972 Aug 11;43(1):264–267. doi: 10.1016/0006-8993(72)90293-4. [DOI] [PubMed] [Google Scholar]
- TAUB A. LOCAL, SEGMENTAL AND SUPRASPINAL INTERACTION WITH A DORSOLATERAL SPINAL CUTANEOUS AFFERENT SYSTEM. Exp Neurol. 1964 Oct;10:357–374. doi: 10.1016/0014-4886(64)90006-8. [DOI] [PubMed] [Google Scholar]
- Wall P. D. The laminar organization of dorsal horn and effects of descending impulses. J Physiol. 1967 Feb;188(3):403–423. doi: 10.1113/jphysiol.1967.sp008146. [DOI] [PMC free article] [PubMed] [Google Scholar]


