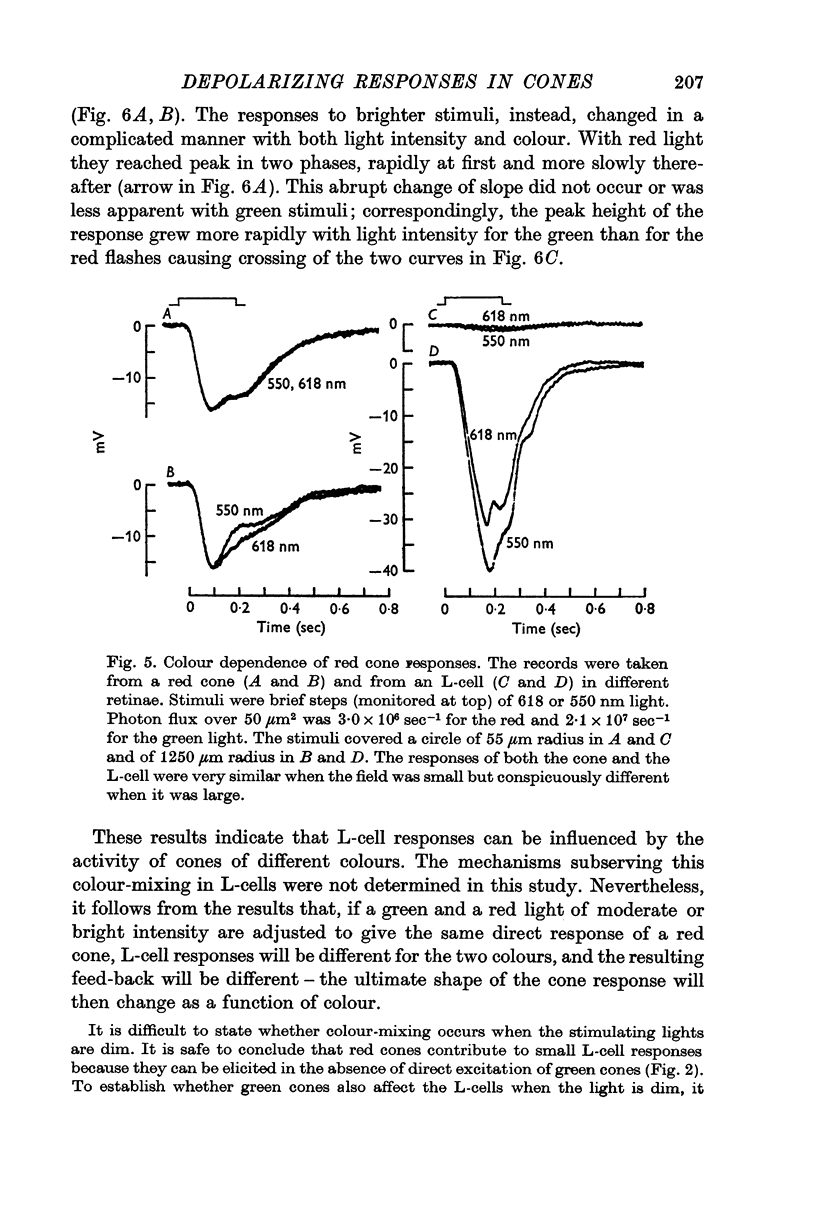
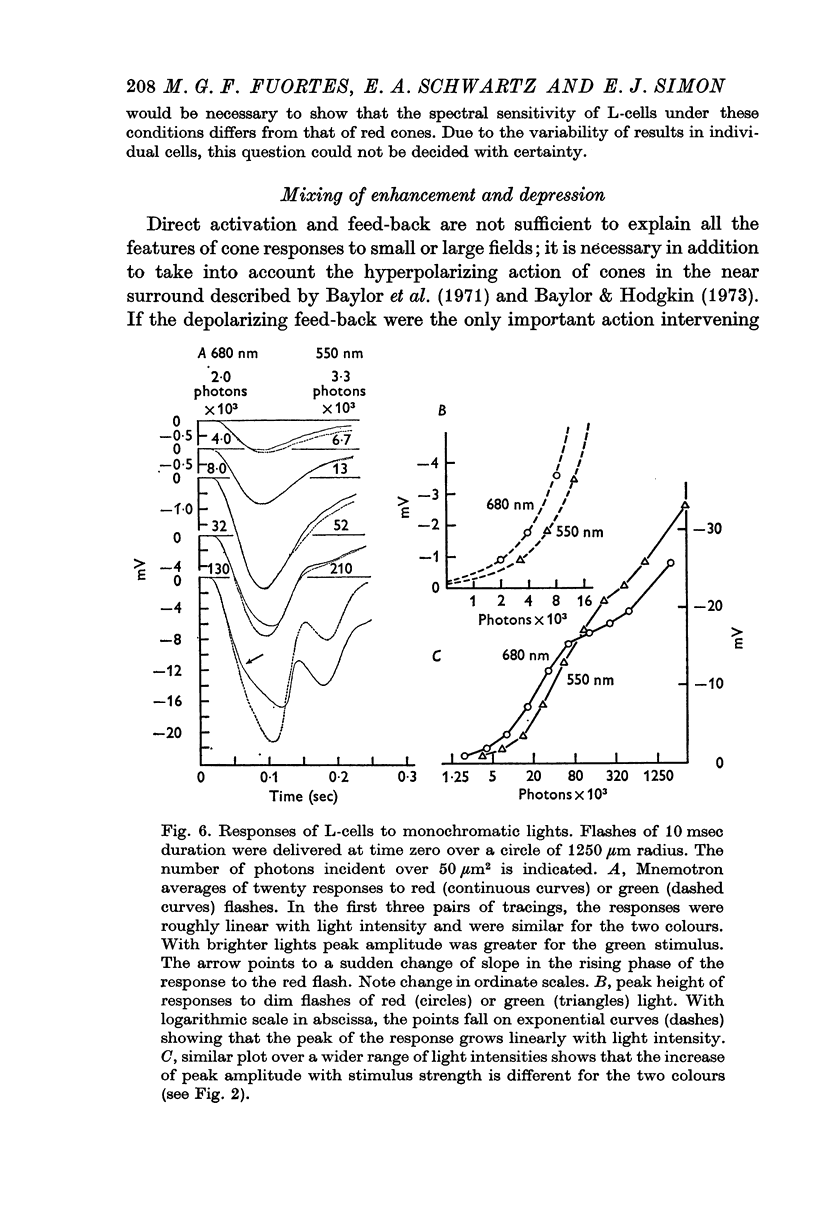
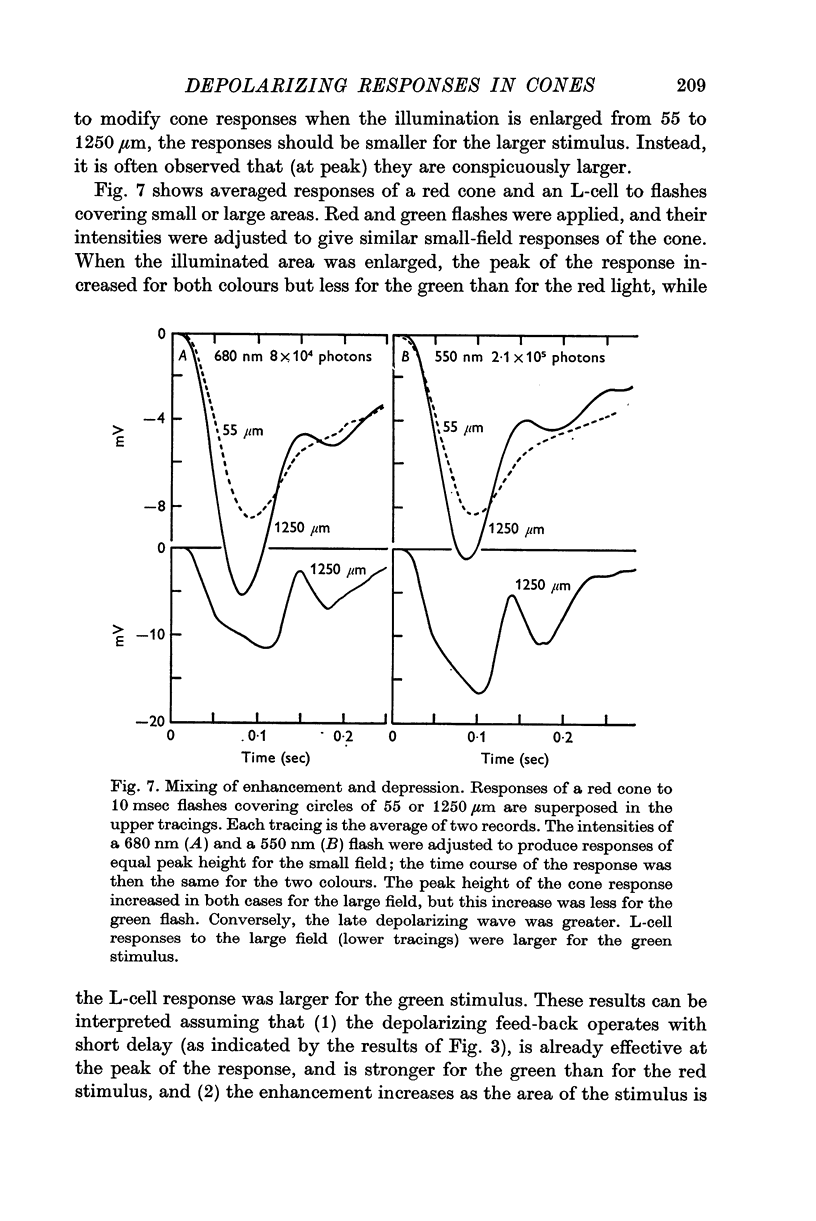
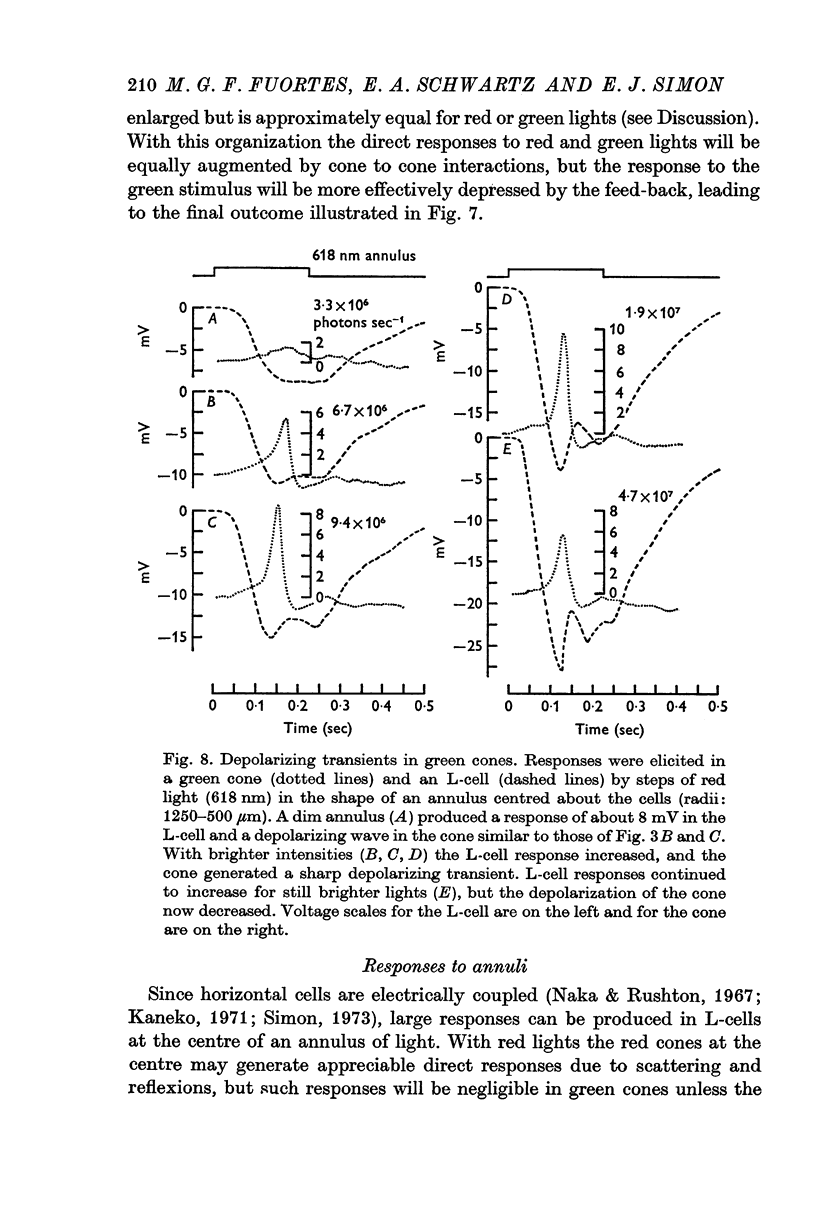
Abstract
1. Responses to monochromatic lights were recorded intracellularly from red cones, green cones, and luminosity horizontal cells (L-cells) in the retinae of turtles.
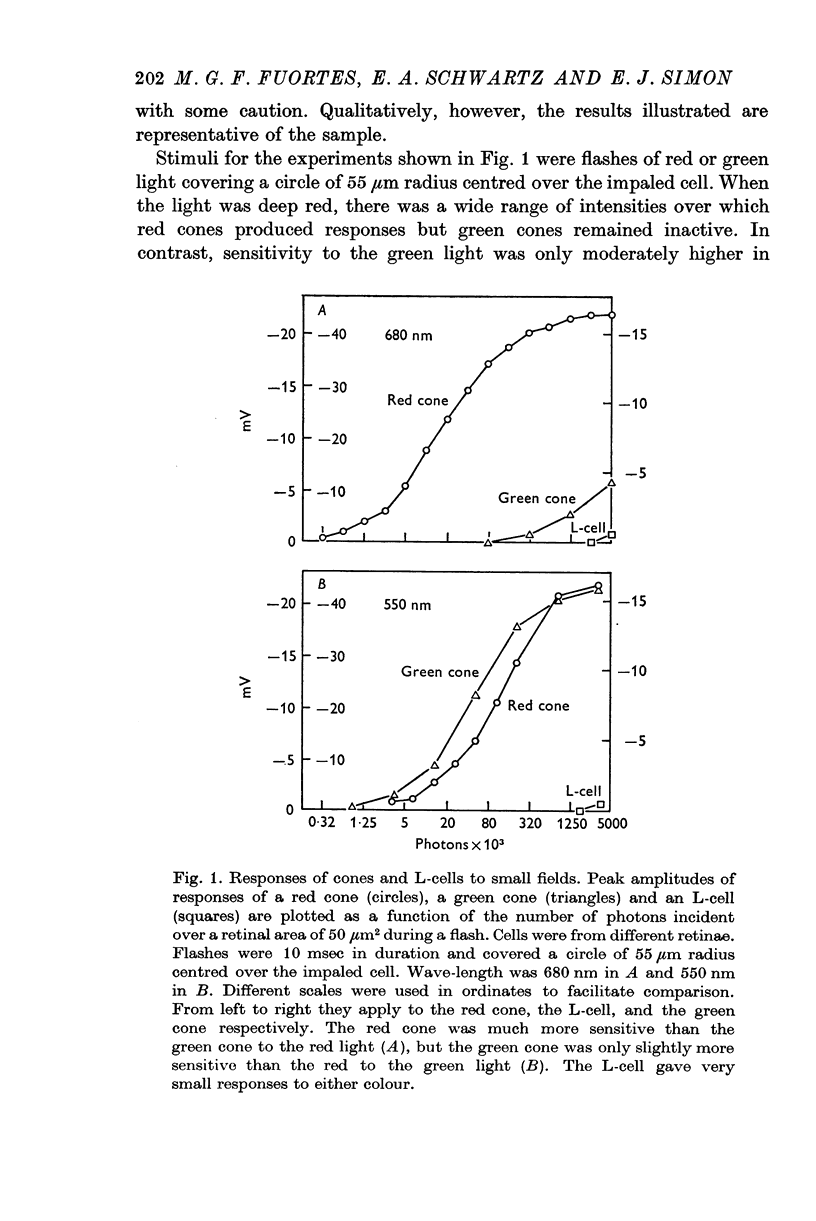
2. Both types of cones responded to small fields of illumination with graded hyperpolarizations. Red cones were only moderately more sensitive to deep red (680 nm) than to green (550 nm) light while green cones were much more sensitive to the green light than to the red. L-cells produced small responses for flashes of either colour covering small fields.
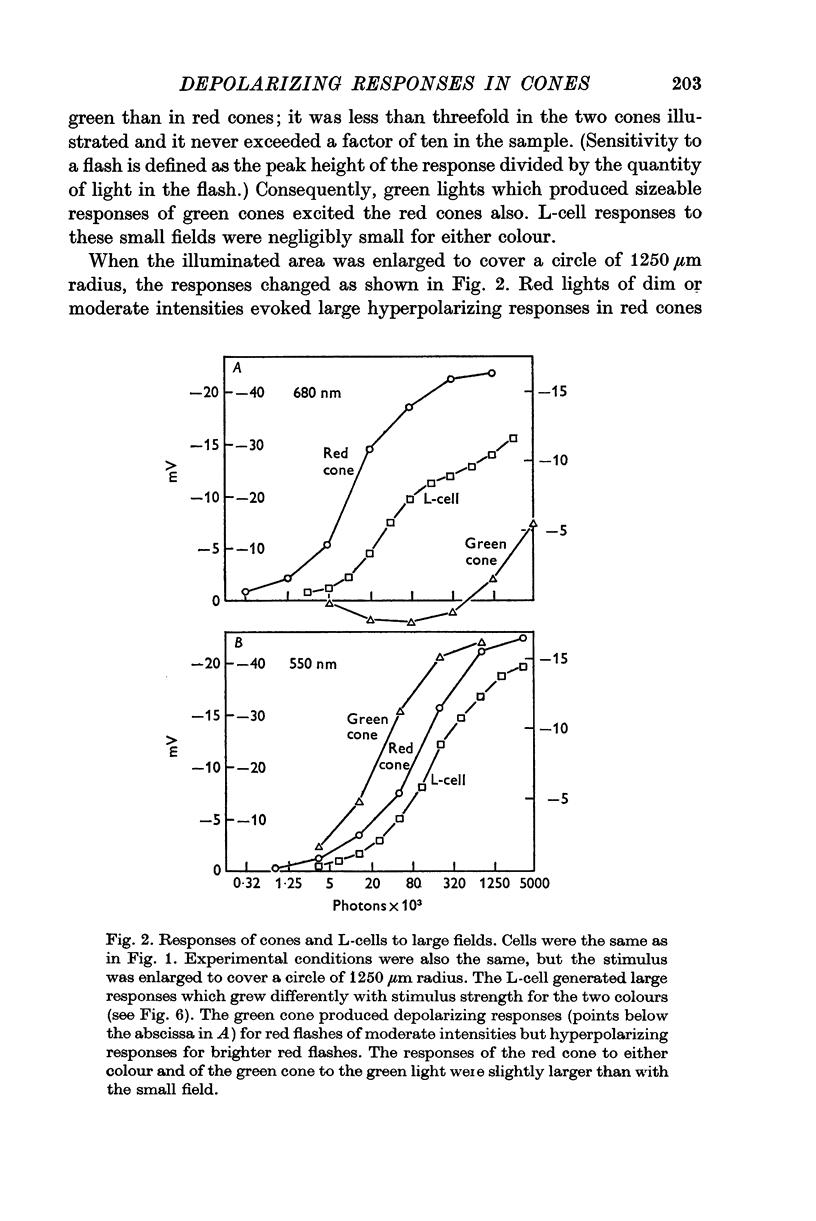
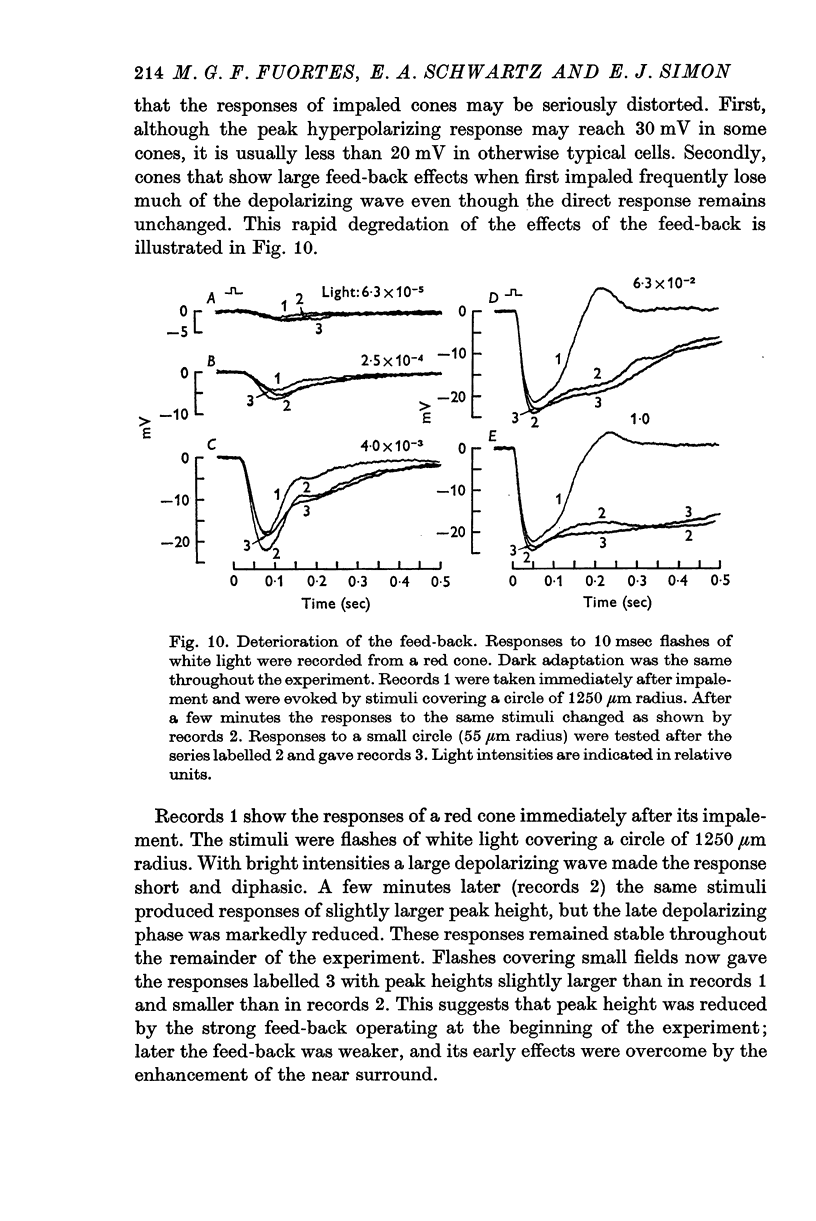
3. Stimulation of large fields with monochromatic lights of moderate or high intensity evoked large L-cell responses and composite responses in cones. These latter include the hyperpolarizing action of the light absorbed by the cone itself (direct response), its enhancement by illumination of the near surround, and the depolarizing effects of L-cell feed-back.
4. L-cells respond primarily to the activity of red cones; with sufficient intensity of the light, however, their responses are influenced also by green cones. As a result, if a red and a green light stimulate red cones equally, the L-cell response is larger for the green stimulus.
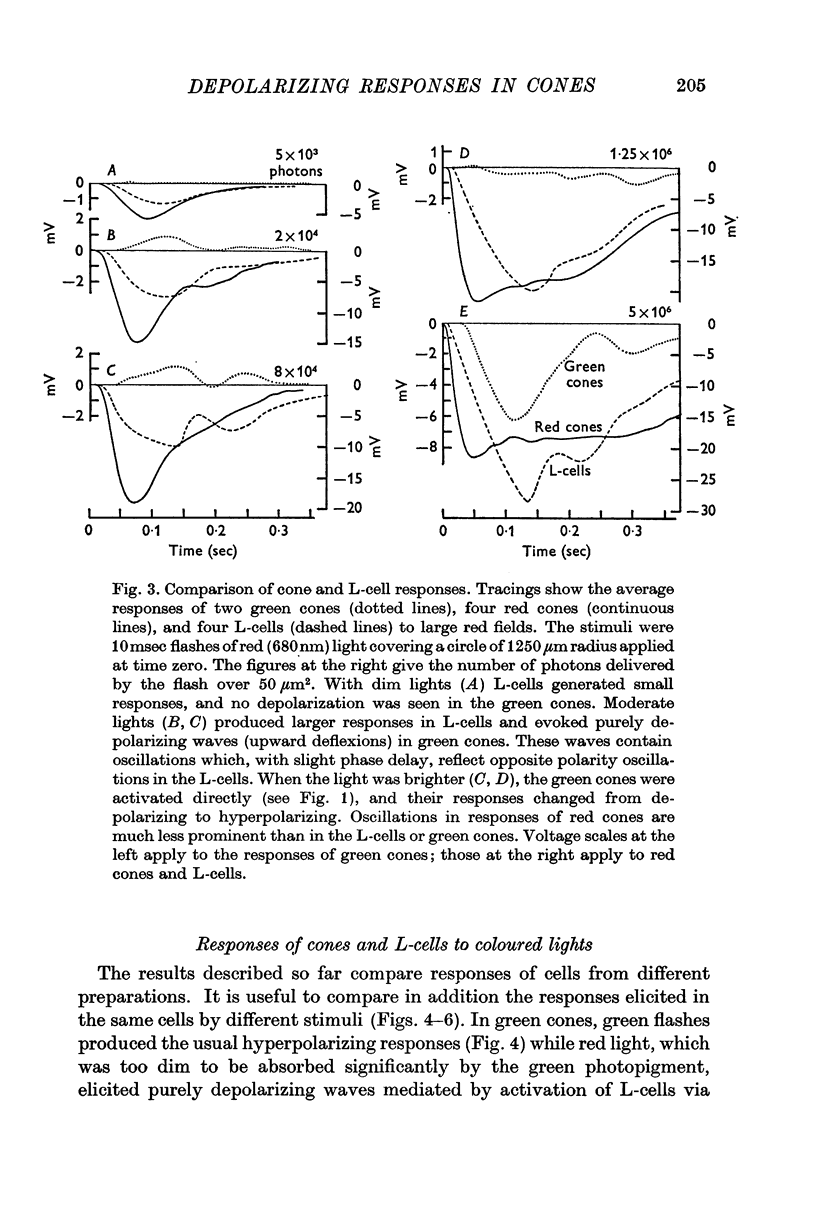
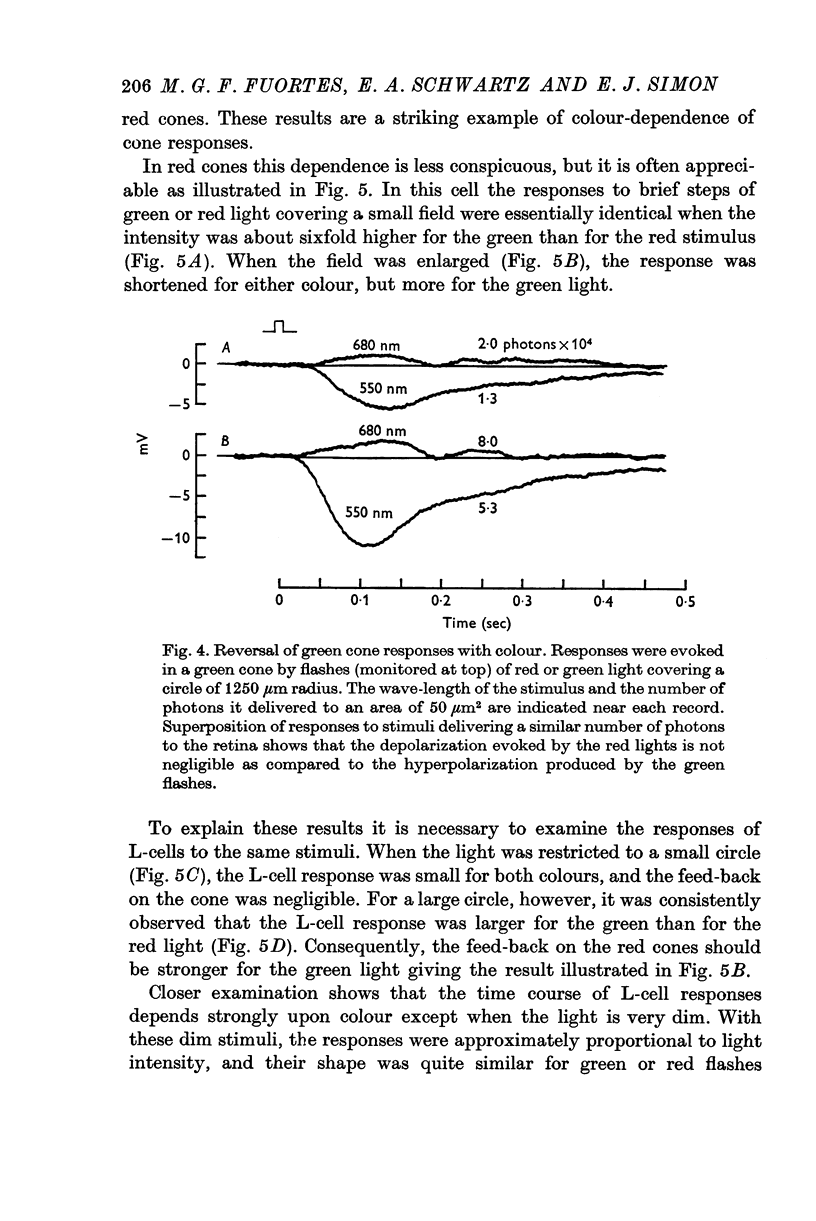
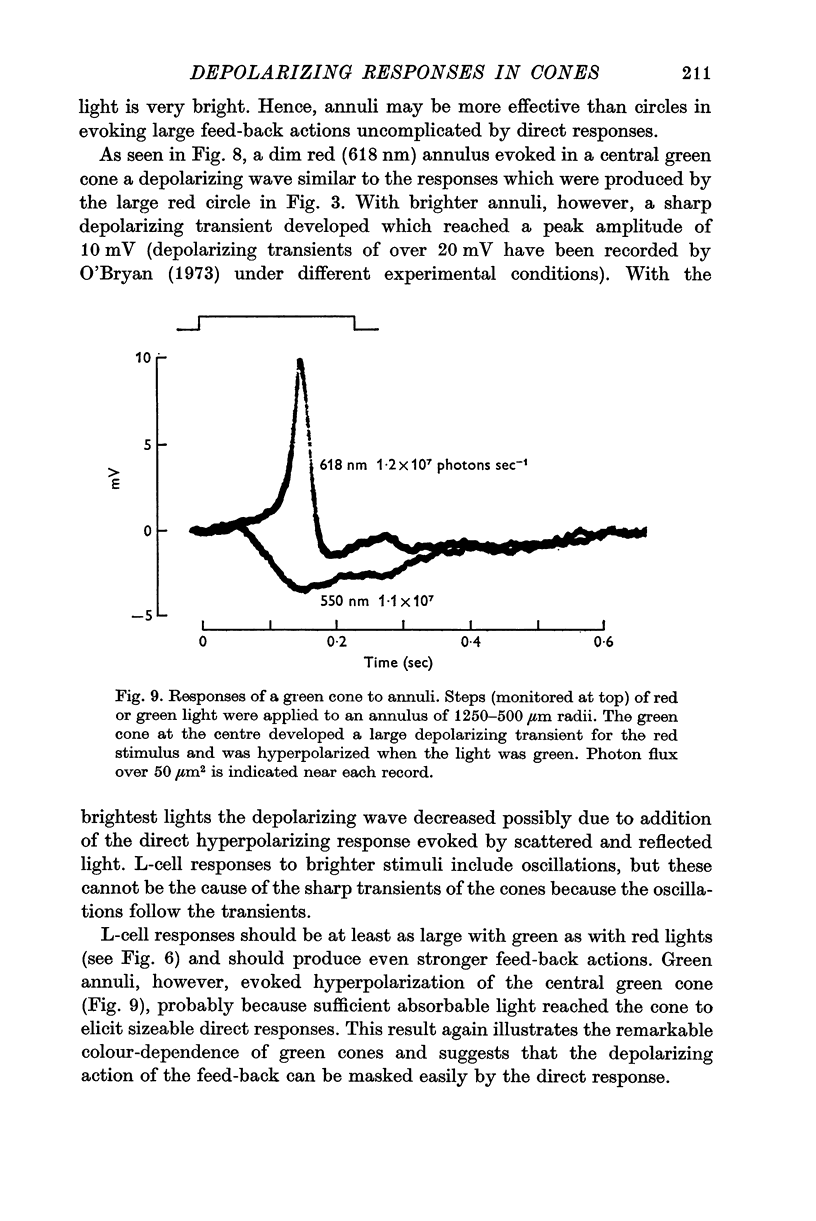
5. Green cones were depolarized by deep red lights of moderate intensity applied over large fields. These depolarizing responses include oscillations which follow closely oscillations in L-cells. Green light applied to the same large fields produced hyperpolarization of green cones.
6. Red cones were hyperpolarized by red or green light covering large fields, but the time course of their responses differed for the two colours, reflecting a corresponding difference in L-cell activity.
7. Red light in the form of an annulus produced large responses in central L-cells without eliciting direct responses in central green cones. In these conditions green cones developed depolarizing waves which included a large, sharp transient.
8. It is concluded from these and other results that the direct response of each cone is modified by two interactions: enhancement only from nearby cones of the same colour and depression controlled (through L-cell feed-back) by cones of all colours. In this way the response of any cone will change as the proportion of responses in cones of different colours changes, this proportion being a function of the wave-length of the light.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoff A., Norton A. L. An electrical model of the vertebrate photoreceptor cell. Vision Res. 1967 Mar;7(3):253–263. doi: 10.1016/0042-6989(67)90089-2. [DOI] [PubMed] [Google Scholar]
- HARTLINE H. K., WAGNER H. G., RATLIFF F. Inhibition in the eye of Limulus. J Gen Physiol. 1956 May 20;39(5):651–673. doi: 10.1085/jgp.39.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol. 1971 Feb;213(1):95–105. doi: 10.1113/jphysiol.1971.sp009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Granda A. M. Microspectrophotometric measurements of visual pigments in two species of turtle, Pseudemys scripta and Chelonia mydas. Vision Res. 1971 Feb;11(2):105–114. doi: 10.1016/0042-6989(71)90227-6. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVAETICHIN G., MACNICHOL E. F., Jr Retinal mechanisms for chromatic and achromatic vision. Ann N Y Acad Sci. 1959 Nov 12;74(2):385–404. doi: 10.1111/j.1749-6632.1958.tb39560.x. [DOI] [PubMed] [Google Scholar]
- Simon E. J. Two types of luminosity horizontal cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):199–211. doi: 10.1113/jphysiol.1973.sp010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Electrophysiological study of the mechanisms subserving color coding in the fish retina. Cold Spring Harb Symp Quant Biol. 1965;30:559–566. doi: 10.1101/sqb.1965.030.01.054. [DOI] [PubMed] [Google Scholar]


