Abstract
Lateralisation is an attractive and intriguing feature of the vertebrate CNS studied for decades in the different disciplines of the neurosciences. Due to the complexity of the phenomena and intrinsic limitations of the approaches used to date, it has been difficult to establish useful links between the different, and usually distant, levels of lateralisation e.g. between genetics, morphology, physiology and behaviour. Recently, the dorsal diencephalon of the teleost zebrafish has emerged as a valuable model to begin addressing this issue and as a result unravel the role of vertebrate CNS lateralisation. Zebrafish is a well-established genetic system that allows a ‘bottom up’ (‘gene to behaviour’) approach to the study of lateralisation. In fact, it is the single vertebrate system to date in which asymmetric gene expression in the brain has been directly linked to asymmetric morphology. Zebrafish offers several experimental advantages that allow the study of brain lateralisation using a wide range of experimental tools, from study of gene function through in vivo analysis of morphology and physiology to behavioural assessments. Altogether, these features will allow the establishment of operational links between lower (genetics and morphology) and upper (physiology and behaviour) levels of brain lateralisation.
Keywords: Asymmetry, CNS, Epithalamus, Genetics, Laterality, Lateralisation, Limbic system, Zebrafish
INTRODUCTION
The terms asymmetry, lateralisation and laterality of the central nervous system refer to the fact that left and right halves of the brain are not identical, a feature that is thought to confer advantages for information processing and ultimately for species survival [1]. Asymmetries in the brain, for example, correlate with enhanced feeding performance [2], predator detection [3] and long term memory [4], and are proposed as the basis of speech and other behavioural traits [5–8]. Although asymmetry and lateralisation are in principle interchangeable concepts, they usually imply different levels of the same basic attribute of the brain. Asymmetry is primarily used to convey structural (e.g. morphological and connectional) differences between left and right sides of the brain whereas lateralisation depicts localisation of neural functions (e.g. speech) in one half of the brain. The term laterality, in contrast, denotes a different aspect of the phenomenon as it specifies the side of the brain (left or right) in which structural or functional asymmetries localise. The conceptual distinction between asymmetry/lateralisation and laterality is vindicated by the fact that these two features of the brain are under the control of separate genetic mechanisms (see below).
Since the nineteenth century, research in asymmetry/lateralisation/laterality has explored different aspects of this phenomenon including genetics, morphology, physiology and behaviour (for historical overviews see [5–10]). In a simplistic model, these aspects may be arranged hierarchically with genetics being the keystone that controls where and how morphological asymmetries are established in the developing brain. Asymmetric morphology is the substrate on which asymmetric functions arise and lateralised behaviour is the outcome of activation of lateralised circuits within the brain (Fig. 1). Closing this path of relationships we find evolution through which asymmetry/lateralisation/laterality is maintained in phylogeny should it offer a competitive advantage for species survival [3,10]. In addition, each of these levels is modulated by epigenetic factors (e.g. hormones [11]) that operate throughout development and adulthood. Taking this model of brain asymmetry/lateralisation/laterality as a framework helps us realize how scattered our knowledge is of each level for a given brain region or behaviour and, more importantly, it reveals how little we know of the relationships between the different levels of this fundamental feature of the brain. For example, we have known for decades that aspects of speech are localised to the left hemisphere in the human brain, however, a clear understanding of the genetic and structural basis of this important function has proven difficult to achieve [6]. Lack of deep insights into this problem may in part be explained by the complexity of the phenomena (e.g. speech involves the complex interplay of several brain functions) and by practical limitations of studying human CNS structures (e.g. difficulties in mapping traits and tracing neuronal connections). It is perhaps more productive to use animal models on which to build our understanding of the genetic and developmental basis of this phenomenon, and then begin making the necessary links to morphology through physiology up to behaviour. Indeed, this bottom up (gene to behaviour) approach has proven useful for the study of brain asymmetry in invertebrate models such as worms [12]. We therefore ought to find and employ vertebrate genetic models that facilitate the analysis of operational links between the distinct – and usually distant – levels of asymmetry/lateralisation/laterality.
Fig. 1.
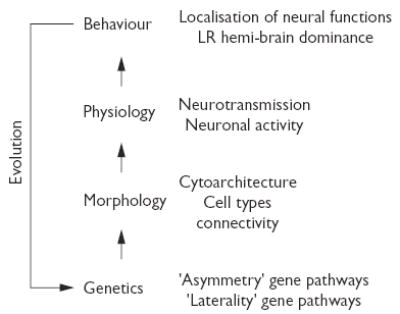
Levels and hierarchies in brain asymmetry/lateralisation/laterality. (Left) Basic model of the relationships between the different levels of brain asymmetry/lateralisation/laterality. Epigenetic factors (not included) modulate asymmetric morphology, function and behaviour. (Right) Key features to be considered for the analysis of each level of brain asymmetry/lateralisation/laterality. For more details see text.
ZEBRAFISH AS A MODEL OF BRAIN ASYMMETRY
The dorsal diencephalon of the teleost zebrafish (Danio rerio) has recently emerged as a valuable model that offers fundamental advantages for the study of vertebrate brain asymmetry/lateralisation/laterality. First, as a well-established genetic system it facilitates the search for genes that control the establishment of several aspects of brain asymmetry. Indeed, zebrafish is the single vertebrate model to date in which asymmetric gene expression in the brain has been directly linked to the development of asymmetric morphology [13–16]. Second, zebrafish is suitable for embryonic experimentation and in vivo visualization of neuronal and axonal development due to the small size and transparency of the embryos. These features have allowed the direct visualisation of structural asymmetries in transgenic animals expressing green fluorescent protein (GFP) in restricted regions of the brain [13,15]. Finally, zebrafish has proven to be suitable for functional approaches such as electrophysiology [17–20] and behavioural analysis [21,22], both of which are fundamental tools to start linking physiology and behaviour with more basal levels of asymmetry. Indeed, preliminary analysis of lateralised behaviours such as eye use in zebrafish fry with situs inversus supports a genetic basis for this lateralised behaviour (Richard Andrew, Ádám Miklósi, Anukampa Barth and Stephen Wilson, unpublished data).
THE DORSAL DIENCEPHALIC CONDUCTION SYSTEM OF ZEBRAFISH
The limbic system of vertebrates is thought to be an integrative centre involved in emotional and sexual behaviours, motivation, memory formation and in modulating appropriate responses to sensory and emotional stimuli [23,24]. Much of the circuitry of the limbic system is conserved among vertebrates. For instance, certain telencephalic projections originate in the anterior portion of the medial forebrain bundle and form the stria medullaris which connects with the epithalamic habenular complex. From the habenulae, axons extend to the ventral midbrain through the fasciculi retroflexus [23,24]. Interestingly, the habenular complex of a wide range of vertebrate species display striking asymmetries in morphology, neurochemistry and connectivity suggesting a fundamental role of habenular asymmetry/lateralisation/laterality in limbic system-related behaviours (reviewed in [25]). In some vertebrate groups such as lampreys, teleosts and lizards the left side of the habenular complex receives afferent connectivity from the photoreceptive parapineal organ (also called the parietal eye) [13,14,26–28]. This observation opens the intriguing possibility that limbic system-related circuits are asymmetrically modulated by light [25]. Although the study of the development of limbic system circuitry is still in its infancy, zebrafish has the potential to elucidate the mechanisms underlying establishment of these neural structures.
In zebrafish, the habenular complex and its associated fibre tracts are already differentiated in the early larvae (Fig. 2). The habenulae are enlarged, contain more neuropil and exhibits more intense expression of a number of genes in the left than in the right nucleus, a feature that is maintained until adulthood [13–15,29]. Habenular neurons project predominantly to the interpeduncular nuclei in the ventral midbrain and current studies are aimed at assessing whether left and right habenular projections are segregated in their target nuclei (Hidenori Aizawa, Isaac Bianco, Stephen Wilson and Hitoshi Okamoto, unpublished observations). The habenulae also receive afferent connectivity from basal telencephalic nuclei [30] and the parapineal organ [13,14]. The parapineal organ develops in close proximity to the left habenula and establishes profuse connectivity with the region of the left habenula that simultaneously develops enlarged neuropil and asymmetric gene expression [13]. Such tight temporal and spatial coordination of parapineal and habenular asymmetries is seen not only in wild type but also in mutants with randomised laterality of CNS asymmetries. Although the molecules involved are still unknown, such coordination depends on mutual interactions between the parapineal organ and habenulae and/or their precursors during embryogenesis [13,15].
Fig. 2.
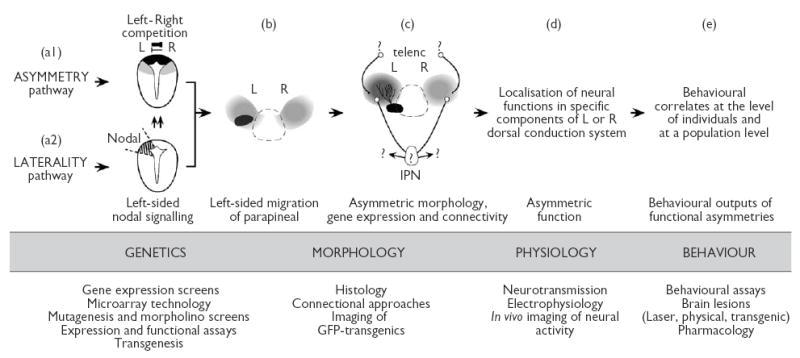
A bottom up approach for the study of asymmetry/lateralisation/laterality in the dorsal diencephalic conduction system of zebrafish. The four basic levels of brain asymmetry/lateralisation/laterality are indicated in capitals. (Top) Summary of the current understanding of each level of asymmetry/lateralisation/laterality in zebrafish. (a1) Genetic control of asymmetry. Transversal view of the epithalamic region of the neural tube at day one of development showing the location of parapineal (black) and habenular (grey) precursors. Competitive interactions between left and right sides of the brain contribute to the initial establishment of CNS asymmetry [13]. (a2) Genetic control of laterality. Transverse view of the epithalamic region of the neural tube at day one of development showing left sided expression of Nodal genes. The role of Nodal signalling is to bias the outcome of left/right competition towards the left side of the brain [13,14]. (b,c) Dorsal views of the epithalamus at 2 (b) and 4 (c) days of development. (b) Morphological asymmetry of the parapineal organ is characterised by left-sided migration and by the development of asymmetric connectivity directed to the left habenula [13–15]. (c) Structural asymmetries of the habenular complex involve differential growth, development of neuropil, strength of gene expression and patterns of connectivity between the left and right sides [13–15]. Details of the subnuclear organisation and connectivity of various components of the dorsal diencephalic conduction system are still unknown (question marks). IPN (interpeduncular nuclei), telenc (telencephalon). Asymmetric morphology may determine the localisation of neural functions in specific components of the system and on a specific side of the brain (d). Lateralisation of neural functions may in turn have behavioural correlates at both individual and population levels (e). (Bottom) Experimental approaches currently available for the study of CNS asymmetry in zebrafish.
The initial establishment of asymmetry and laterality in the zebrafish brain is controlled by independent mechanisms that operate in tandem. Although conclusive data is still lacking, autonomous control of these two aspects may be a widespread feature in vertebrates as it has also been suggested to drive the genetic control of handedness in humans [31]. A primary, genetically based mechanism, ensures that structural asymmetries are established in the brain and this involves competitive interactions by which one side of the brain inhibits the ability of the contralateral side to develop as left [13] (Fig. 2). How this conceptual notion of left-right mutual inhibition translates into molecules and cellular events in the epithalamus is still unknown but likely involves signals that travel across the dorsal midline of the diencephalon during early stages of development.
A second genetic mechanism of CNS development ensures that laterality of asymmetry is consistently biased to the same (left) side of the brain and involves a genetic pathway with a conserved role in the control of asymmetry of the heart and viscera in vertebrates [32]. In zebrafish, asymmetrical expression of several components of the Nodal signalling pathway is initially detected in the left lateral plate mesoderm (L-LPM) and a few hours later in the left epithalamus. It has been proposed that laterality signals associated to Nodal signalling are transferred from the L-LPM to the left epithalamus leading to coordinated organ and brain laterality [33]. Once these signals reach the brain, several components of the Nodal signalling pathway are expressed on the left side of the epithalamus prior to the development of neuroanatomical asymmetries and in close association with the precursors of the habenulae and parapineal organ (Fig. 2) [13,14,16,34]. In embryos carrying mutations that either directly or indirectly affect Nodal signalling, the expression of Nodal genes is either absent or bilateral in the brain [14,16,34]. Interestingly, neuroanatomical asymmetries of the parapineal organ and habenulae still develop but the laterality of these asymmetries in the mutant embryos is randomised [13–15]. The consequence of asymmetric activation of Nodal signalling in the brain is therefore to ensure that laterality of the CNS is biased to the same side within the population [14,25]. As consistency of CNS laterality appears to be more common in social species like zebrafish than in those with solitary life styles [10] it becomes essential to determine the behavioural correlates of epithalamic asymmetries at a population level in zebrafish.
THE WAY FORWARD
The use of a bottom up approach in the study of CNS asymmetry has so far proven very helpful for the understanding of the genetic control of asymmetric morphology in zebrafish. However, this initial link between the genetic and morphological aspects of asymmetry/lateralisation/laterality needs in the future to be extended to upper levels of the hierarchy (physiology and behaviour) for us to understand the role of lateralisation in the dorsal diencephalic conduction system. A plethora of experimental approaches currently available in zebrafish will facilitate the achievement of this goal (summarised in Fig. 2), and it appears only a matter of time before we begin making the necessary links. One important aspect still to be determined is the subnuclear organisation and connectivity of the various components of the dorsal diencephalic conduction system. The search of genetic markers through microarrays and gene expression screens will help delineate neuronal domains within this system and will be the starting point in the generation of GFP-transgenic zebrafish labelling axonal projections within the circuit. In addition, design of mutant screens using GFP-transgenic zebrafish will allow the isolation of mutations with which to study the role of specific nuclei within the dorsal diencephalic conduction system. Once our understanding of the connectivity of circuits is more complete, we can design physiological and behavioural approaches to test the function of genes and specific structures within the dorsal diencephalic conducting system, and also explore the role of asymmetry/lateralisation/laterality in the generation of individual and social behaviours. The availability of in vivo techniques for the analysis of neuronal activity [17,18,20], and the suitability of zebrafish for electrophysiology [19], pharmacology [35] and behavioural assessments [21,22] will facilitate the achievement of these goals. Finally, it is no less relevant that zebrafish is also a suitable system on which to test the role of hormones and other environmental factors in the epigenesis of CNS asymmetry/lateralisation/laterality [36,37].
Acknowledgments
I am very grateful to StephenWilson, Francisco Aboitiz, and members of my group for their comments, and to Stephen Wilson, Hitoshi Okamoto, Richard Andrew and Ádám Miklósi for discussions of unpublished work. Grants from FONDECYT (1020902-7020902), Fundación Andes (C-13760), Iniciativa Científica Milenio (ICM P01-007-F) and WellcomeTrust support my research in this field. I am an International Research Development Award Fellow from the WellcomeTrust.
References
- 1.Rogers LJ. Advantages and disadvantages of lateralization. In: Rogers LJ and Andrew RJ (eds). Comparative Vertebrate Lateralization Cambridge: Cambridge University Press; 2002, pp. 126–153.
- 2.Gunturkun O, Diekamp B, Manns M, Nottelmann F, Prior H, Schwarz A, Skiba M. Asymmetry pays: visual lateralization improves discrimination success in pigeons. Curr Biol. 2000;10:1079–1081. doi: 10.1016/s0960-9822(00)00671-0. [DOI] [PubMed] [Google Scholar]
- 3.Rogers LJ. Evolution of hemispheric specialization: advantages and disadvantages. Brain Lang. 2000;73:236–253. doi: 10.1006/brln.2000.2305. [DOI] [PubMed] [Google Scholar]
- 4.Pascual A, Huang KL, Neveu J, Preat T. Neuroanatomy: brain asymmetry and long-term memory. Nature. 2004;427:605–606. doi: 10.1038/427605a. [DOI] [PubMed] [Google Scholar]
- 5.Toga AW, Thompson PM. Mapping brain asymmetry. Nature Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 6.Hutsler J, Galuske RA. Hemispheric asymmetries in cerebral cortical networks. Trends Neurosci. 2003;26:429–435. doi: 10.1016/S0166-2236(03)00198-X. [DOI] [PubMed] [Google Scholar]
- 7.Sherman GF, Galaburda AM, Geschwind N. Neuroanatomical asymmetries in non-human species. Trends Neurosci. 1982;5:429–431. [Google Scholar]
- 8.Rogers LJ and Andrew RJ. Comparative Vertebrate Lateralization Cambridge: Cambridge University Press; 2002.
- 9.Harrington A. Unfinished business: models of laterality in the nineteenth century. In: Davidson RJ and Hugdahl K (eds). Brain Asymmetry Cambridge: MIT Press; 1998, pp. 3–26.
- 10.Vallortigara G, Rogers LJ, Bisazza A. Possible evolutionary origins of cognitive brain lateralization. Brain Res Rev. 1999;30:164–175. doi: 10.1016/s0165-0173(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 11.Lewis DW and Diamond MC. The influence of gonadal steroids on the asymmetry of the cerebral cortex. In: Davidson RJ and Hugdahl K (eds). Brain Asymmetry Cambridge: MIT Press; 1998, pp. 3–26.
- 12.Hobert O, Johnston RJ, Jr, Chang S. Left-right asymmetry in the nervous system: the Caenorhabditis elegans model. Nature Rev Neurosci. 2002;3:629–640. doi: 10.1038/nrn897. [DOI] [PubMed] [Google Scholar]
- 13.Concha ML, Russell C, Regan JC, Tawk M, Sidi S, Gilmour DT, et al. Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality. Neuron. 2003;39:423–438. doi: 10.1016/s0896-6273(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 14.Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000;28:399–409. doi: 10.1016/s0896-6273(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 15.Gamse JT, Thisse C, Thisse B, Halpern ME. The parapineal mediates left-right asymmetry in the zebrafish diencephalon. Development. 2003;130:1059–1068. doi: 10.1242/dev.00270. [DOI] [PubMed] [Google Scholar]
- 16.Liang JO, Etheridge A, Hantsoo L, Rubinstein AL, Nowak SJ, Izpisua Belmonte JC, Halpern ME. Asymmetric nodal signaling in the zebrafish diencephalon positions the pineal organ. Development. 2000;127:5101–5112. doi: 10.1242/dev.127.23.5101. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi M, Narushima M, Oda Y. In vivo imaging of functional inhibitory networks on the mauthner cell of larval zebrafish. J Neurosci. 2002;22:3929–3938. doi: 10.1523/JNEUROSCI.22-10-03929.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higashijima S, Masino MA, Mandel G, Fetcho JR. Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator. J Neurophysiol. 2003;90:3986–3997. doi: 10.1152/jn.00576.2003. [DOI] [PubMed] [Google Scholar]
- 19.Friedrich RW, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291:889–894. doi: 10.1126/science.291.5505.889. [DOI] [PubMed] [Google Scholar]
- 20.Gleason MR, Higashijima S, Dallman J, Liu K, Mandel G, Fetcho JR. Translocation of CaM kinase II to synaptic sites in vivo. Nature Neurosci. 2003;6:217–218. doi: 10.1038/nn1011. [DOI] [PubMed] [Google Scholar]
- 21.Roeser T, Baier H. Visuomotor behaviors in larval zebrafish after GFP-guided laser ablation of the optic tectum. J Neurosci. 2003;23:3726–3734. doi: 10.1523/JNEUROSCI.23-09-03726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerlai R. Zebra fish: an uncharted behavior genetic model. Behav Genet. 2003;33:461–468. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- 23.Sandyk R. Relevance of the habenular complex to neuropsychiatry: a review and hypothesis. Int J Neurosci. 1991;61:189–219. doi: 10.3109/00207459108990738. [DOI] [PubMed] [Google Scholar]
- 24.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 25.Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J Anat. 2001;199:63–84. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yañez J, Pombal MA, Anadon R. Afferent and efferent connections of the parapineal organ in lampreys: a tract tracing and immunocytochemical study. J Comp Neurol. 1999;403:171–189. doi: 10.1002/(sici)1096-9861(19990111)403:2<171::aid-cne3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Yañez J, Meissl H, Anadon R. Central projections of the parapineal organ of the adult rainbow trout (Oncorhynchus mykiss) Cell Tiss Res. 1996;285:69–74. doi: 10.1007/s004410050621. [DOI] [PubMed] [Google Scholar]
- 28.Engbretson GA, Reiner A, Brecha N. Habenular asymmetry and the central connections of the parietal eye of the lizard. J Comp Neurol. 1981;198:155–165. doi: 10.1002/cne.901980113. [DOI] [PubMed] [Google Scholar]
- 29.Halpern ME, Liang JO, Gamse JT. Leaning to the left: laterality in the zebrafish forebrain. Trends Neurosci. 2003;26:308–313. doi: 10.1016/S0166-2236(03)00129-2. [DOI] [PubMed] [Google Scholar]
- 30.Yañez J, Anadon R. Afferent and efferent connections of the habenula in the rainbow trout (Oncorhynchus mykiss): an indocarbocyanine dye (DiI) study. J Comp Neurol. 1996;372:529–543. doi: 10.1002/(SICI)1096-9861(19960902)372:4<529::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Klar AJ. Human handedness and scalp hair-whorl direction develop from a common genetic mechanism. Genetics. 2003;165:269–276. doi: 10.1093/genetics/165.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schier AF. Nodal signaling in vertebrate development. Annu Rev Cell Dev Biol. 2003;19:589–621. doi: 10.1146/annurev.cellbio.19.041603.094522. [DOI] [PubMed] [Google Scholar]
- 33.Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development. 2003;130:2303–2316. doi: 10.1242/dev.00436. [DOI] [PubMed] [Google Scholar]
- 34.Bisgrove BW, Essner JJ, Yost HJ. Multiple pathways in the midline regulate concordant brain, heart and gut left-right asymmetry. Development. 2000;127:3567–3579. doi: 10.1242/dev.127.16.3567. [DOI] [PubMed] [Google Scholar]
- 35.Yeh JR, Crews CM. Chemical genetics: adding to the developmental biology toolbox. Dev Cell. 2003;5:11–19. doi: 10.1016/s1534-5807(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 36.Shrader EA, Henry TR, Greeley MS, Jr, Bradley BP. Proteomics in zebrafish exposed to endocrine disrupting chemicals. Ecotoxicology. 2003;12:485–488. doi: 10.1023/b:ectx.0000003034.69538.eb. [DOI] [PubMed] [Google Scholar]
- 37.Kishida M, McLellan M, Miranda JA, Callard GV. Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb markers of early development in zebrafish (Danio rerio) Comp Biochem Physiol B Biochem Mol Biol. 2001;129:261–268. doi: 10.1016/s1096-4959(01)00319-0. [DOI] [PubMed] [Google Scholar]


