Abstract
1. The adenine nucleotide content of rat jejunal mucosa has been measured. For fresh tissue the concentrations of the three nucleotides in the total water are approximately: ATP, 1·4 mM; ADP, 1·0 mM; and AMP, 0·5 mM.
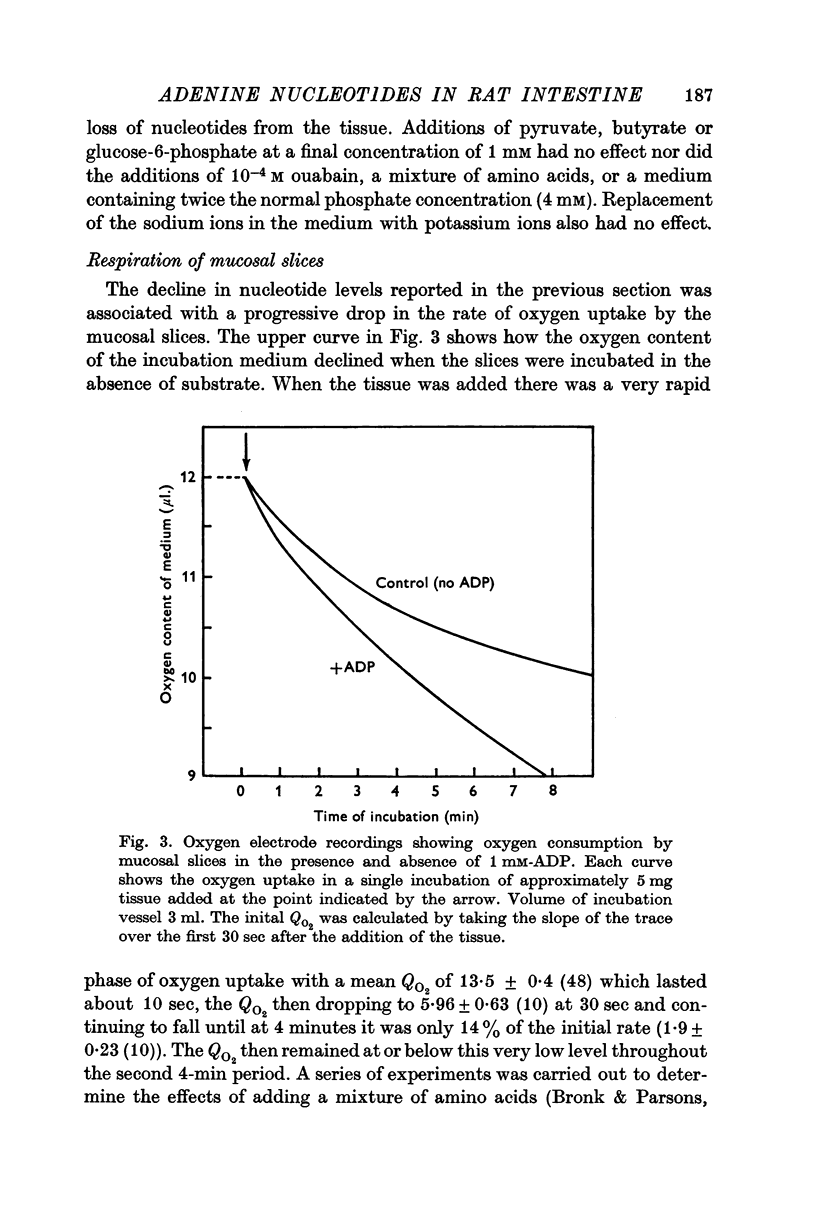
2. The adenine nucleotide content of mucosal slices prepared from rat jejunum was about 80% of that of fresh tissue, but the slices rapidly lost nucleotides when they were incubated in vitro. After a 4 min incubation the mucosal tissue contained about 1 μmole/g dry wt. of each of the three nucleotides. This represented only 14% of the ATP, 20% of the ADP and 36% of the AMP originally present. Further incubation had little effect on the ATP and ADP content, but some additional AMP was lost.
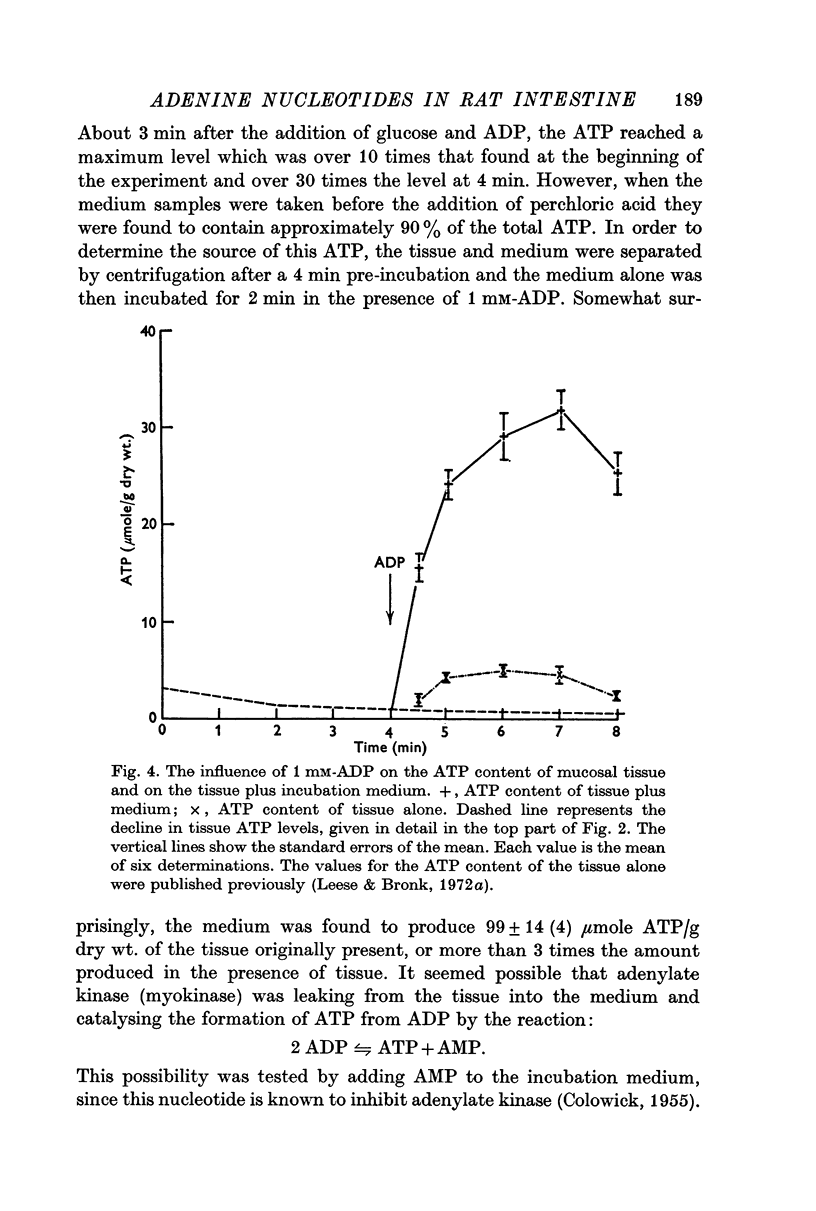
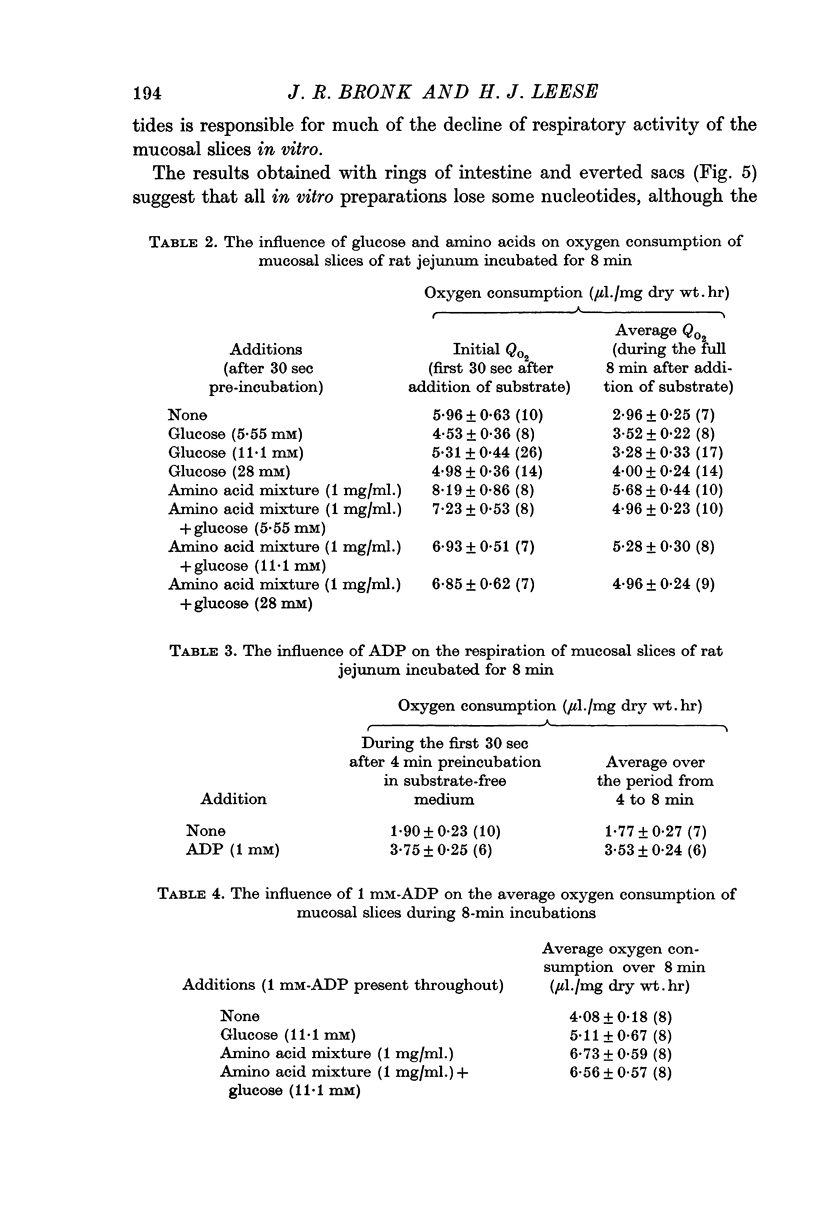
3. Additions of glucose, amino acids, phosphate, pyruvate, butyrate or glucose-6-phosphate did not prevent the loss of nucleotides from the mucosal slices. The addition of ADP (1 mM) did restore the ATP content of slices to a value close to that of fresh tissue within 2 min, but the nucleotide content declined again on further incubation.
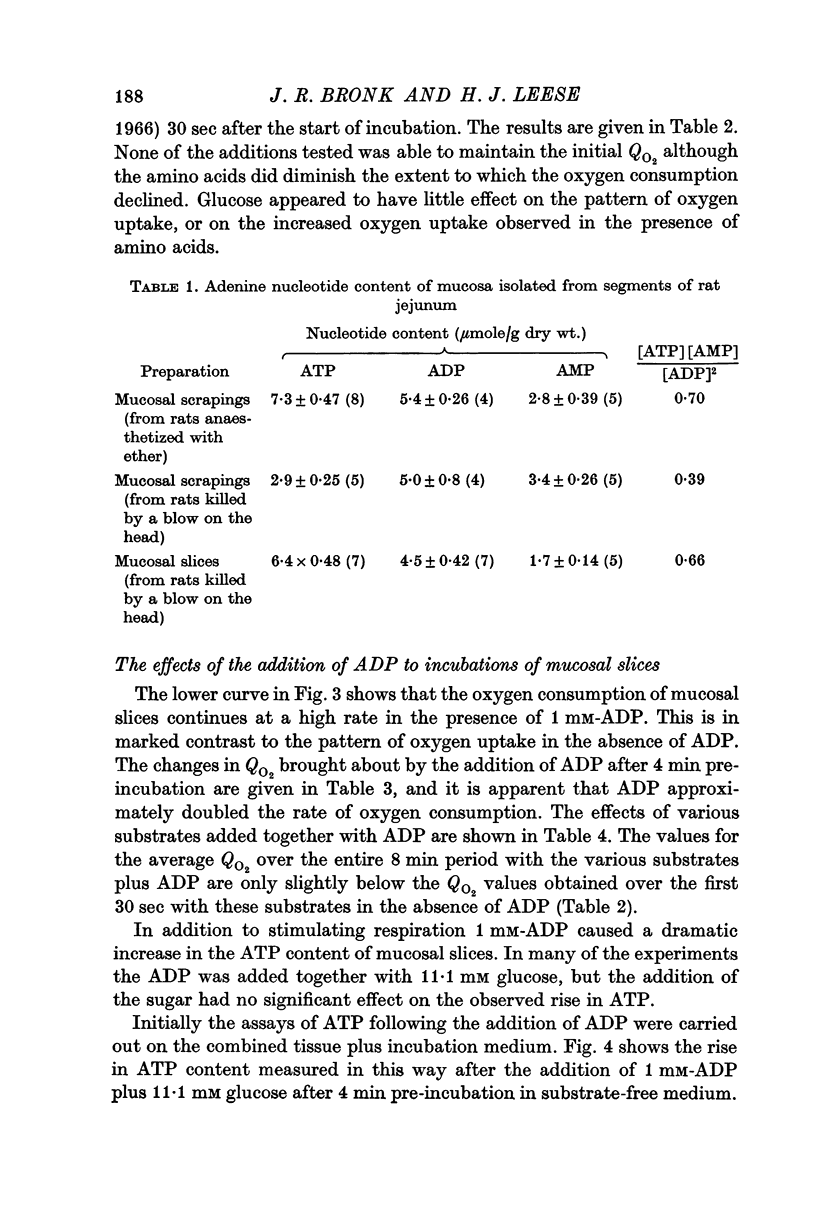
4. The respiration rate of mucosal slices falls progressively during incubation in vitro. This decline was largely prevented by the addition of ADP.
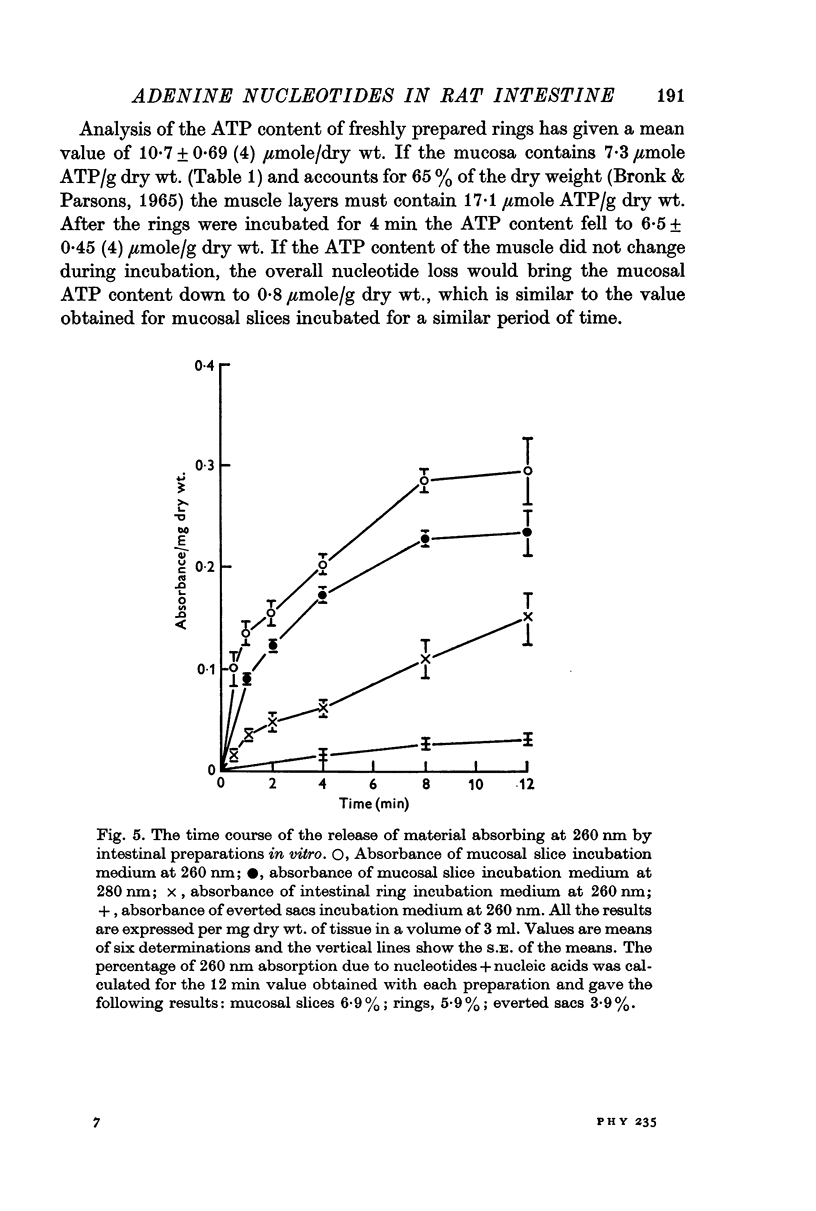
5. Although nucleotide loss occurred most rapidly from mucosal slices, it was also observed with rings of whole intestinal wall and, less rapidly, with everted sacs.
6. ATP added to incubations of mucosal slices disappeared at a rate of about 30 μmole/min. g dry wt.
7. These results suggest that the adenine nucleotide content of preparations of rat small intestine studied in vitro is likely to be severely reduced. The addition of nucleotides to the incubation medium produces only a temporary increase in the nucleotide content of the tissue.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bronk J. R., Parsons D. S. The influence of thyroid gland on amino acid accumulation and protein synthesis by rat small intestine in vitro. J Physiol. 1966 Jun;184(4):942–949. doi: 10.1113/jphysiol.1966.sp007958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Parsons D. S. The polarographic determination of the respiration of the small intestine of the rat. Biochim Biophys Acta. 1965 Oct 18;107(3):397–404. doi: 10.1016/0304-4165(65)90183-2. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerencser G. A., Armstrong W. M. Sodium transfer in bullfrog small intestine. Stimulation by exogenous ATP. Biochim Biophys Acta. 1972 Feb 11;255(2):663–674. doi: 10.1016/0005-2736(72)90169-1. [DOI] [PubMed] [Google Scholar]
- Iemhoff W. G., van den Berg J. W., de Pijper A. M., Hülsmann W. C. Metabolic aspects of isolated cells from rat small intestinal epithelium. Biochim Biophys Acta. 1970 Aug 14;215(2):229–241. doi: 10.1016/0304-4165(70)90020-6. [DOI] [PubMed] [Google Scholar]
- Jasper D. K., Bronk J. R. Studies on the physiological and structural characteristics of rat intestinal mucosa. Mitochondrial structural changes during amino acid absorption. J Cell Biol. 1968 Aug;38(2):277–291. doi: 10.1083/jcb.38.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn P. G., Newey H., Smyth D. H. Electrical potential across the rat small intestine stimulated by adenosine triphosphate. Nature. 1967 Sep 23;215(5108):1395–1395. doi: 10.1038/2151395a0. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Rate control of the tricarboxylic acid cycle. Adv Enzyme Regul. 1970;8:335–353. doi: 10.1016/0065-2571(70)90028-2. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Larson R. E., Davies R. E. The chemical energetics of muscle contraction. I. Activation heat, heat of shortening and ATP utilization for activation-relaxation processes. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):293–313. doi: 10.1098/rspb.1969.0095. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leese H. J., Bronk J. R. Automated fluorometric analysis of micromolar quantities of ATP, glucose, and lactic acid. Anal Biochem. 1972 Jan;45(1):211–221. doi: 10.1016/0003-2697(72)90021-8. [DOI] [PubMed] [Google Scholar]
- Leese H. J., Bronk J. R. Glucose accumulation by rat small-intestinal mucosa after depletion of intracellular adenosine triphosphate. Biochem J. 1972 Jun;128(2):455–457. doi: 10.1042/bj1280455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser S., Christiansen P. A. Inhibition of amino acid uptake by ATP in isolated intestinal epithelial cells. Biochim Biophys Acta. 1971 Apr 13;233(2):480–484. doi: 10.1016/0005-2736(71)90348-8. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 1954 Jan;123(1):116–125. doi: 10.1113/jphysiol.1954.sp005036. [DOI] [PMC free article] [PubMed] [Google Scholar]


