Abstract
1. The discharge of impulses in afferent fibres dissected from the infraorbital and ulnar nerves of anaesthetized cats was recorded during controlled movements of the maxillary and carpal sinus hairs.
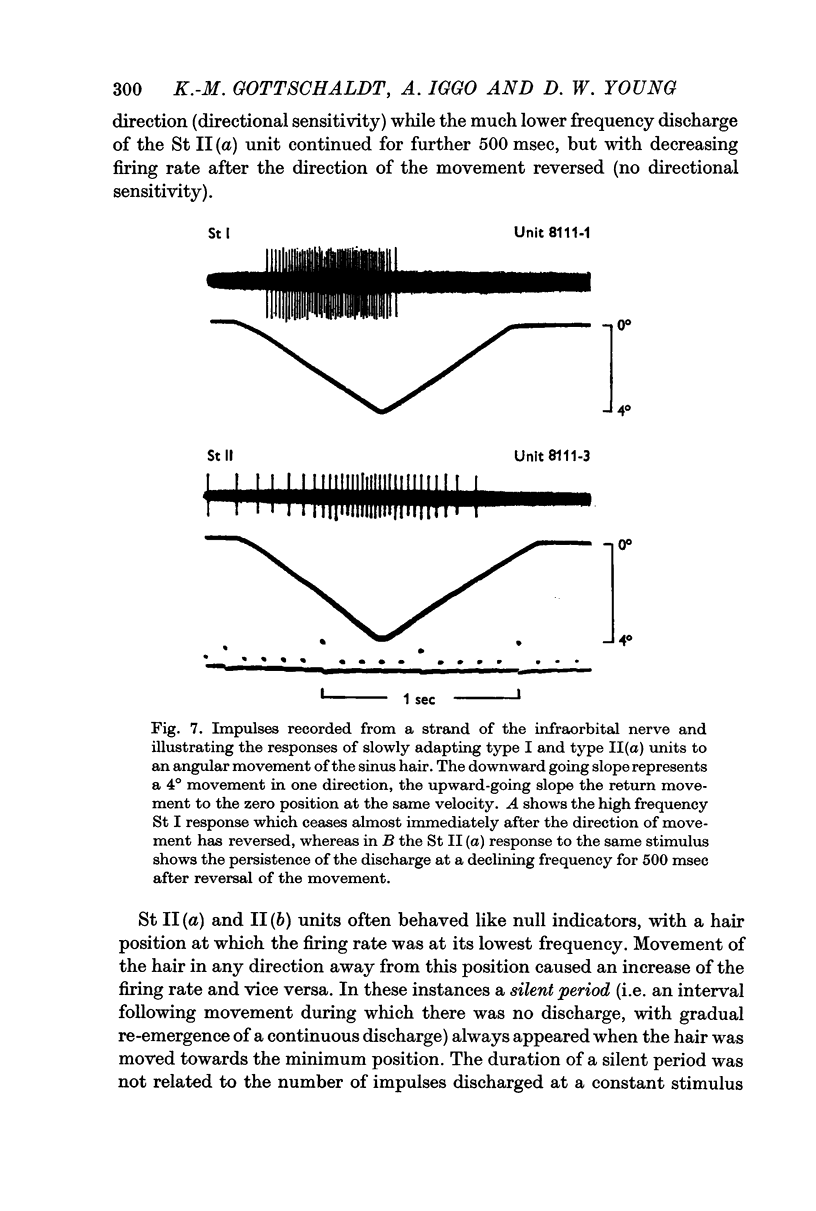
2. Four main types of afferent units were identified. Two had slowly adapting responses characteristic of the epidermal type I, and dermal type II mechanoreceptors of the hairy skin. Two rapidly adapting responses to movement of the sinus hairs were found, one with a high velocity threshold and another with a low velocity threshold.
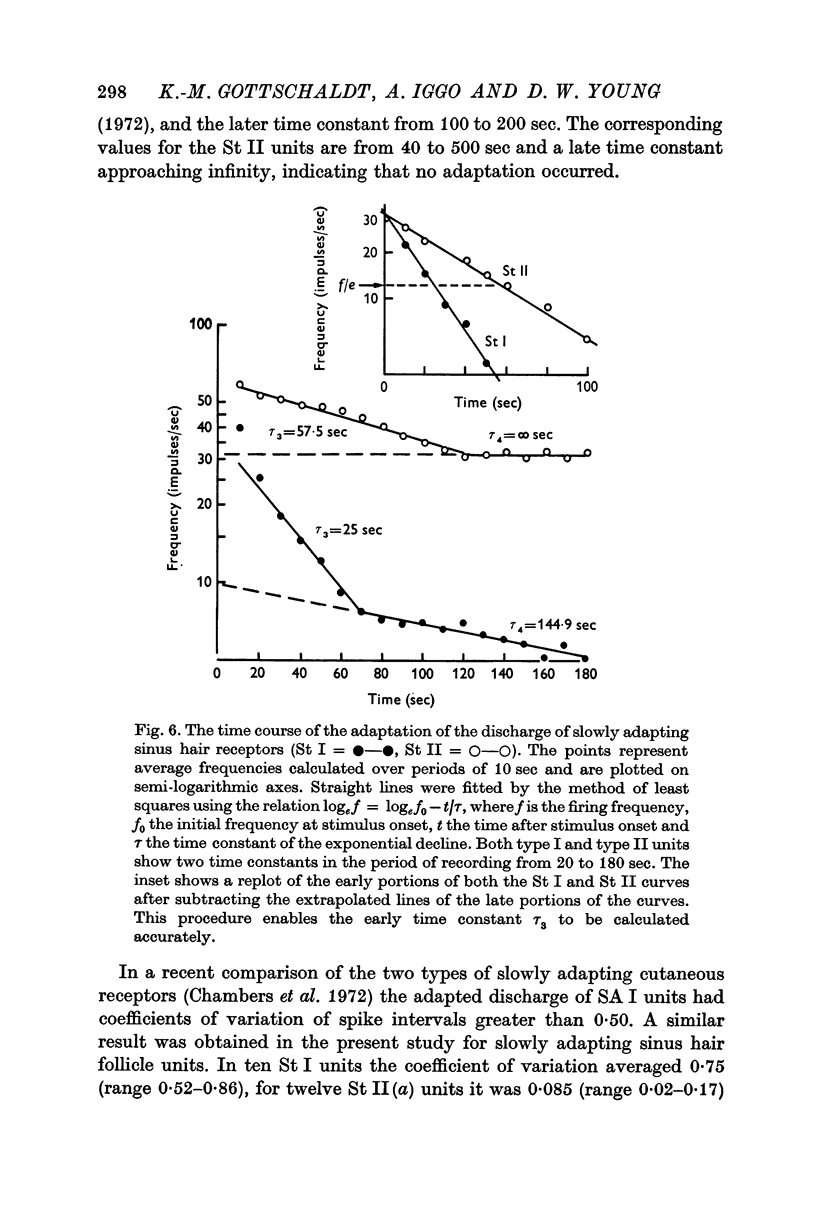
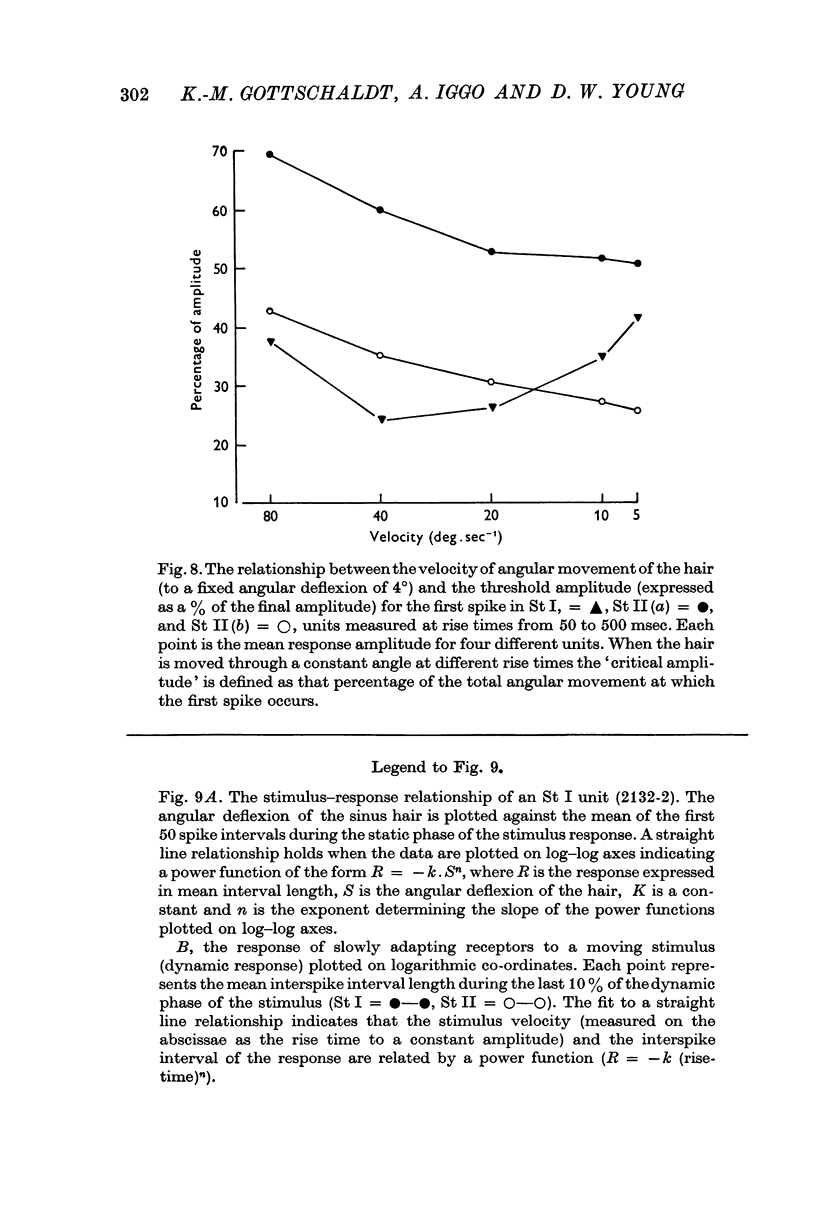
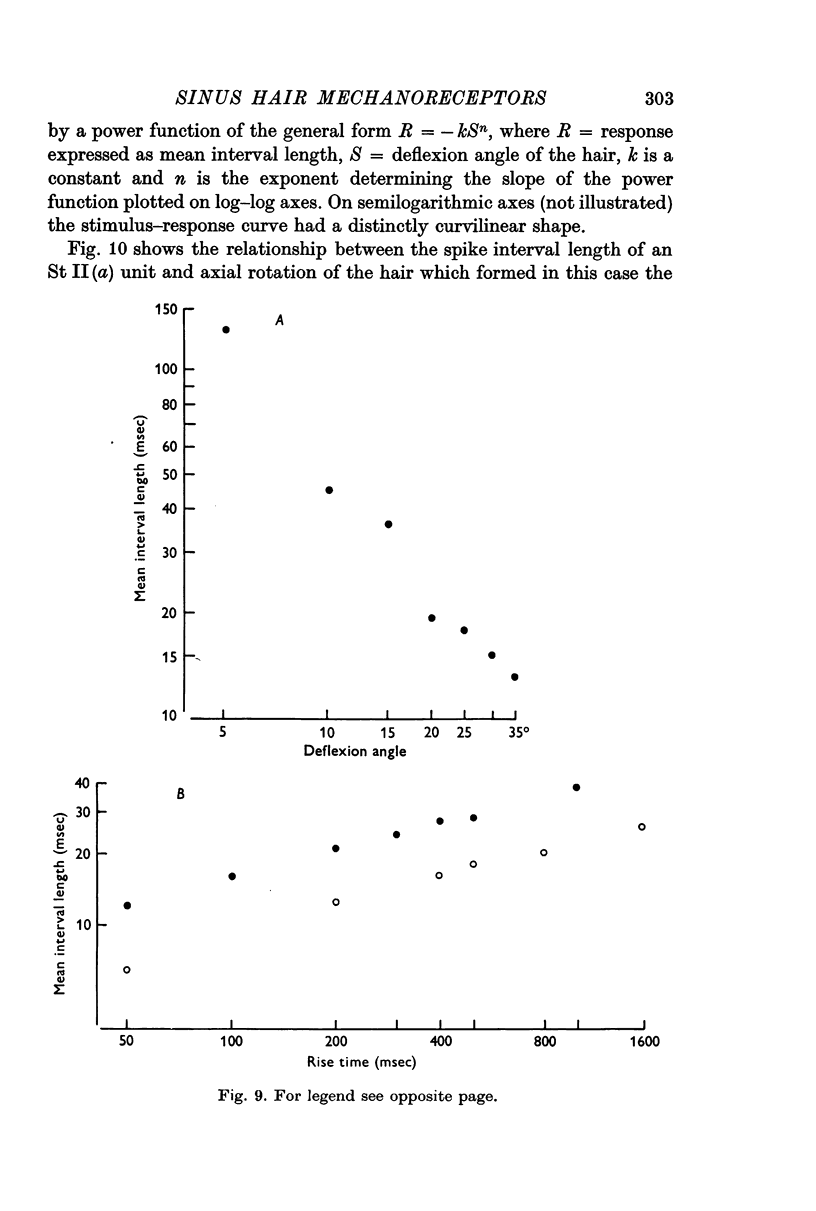
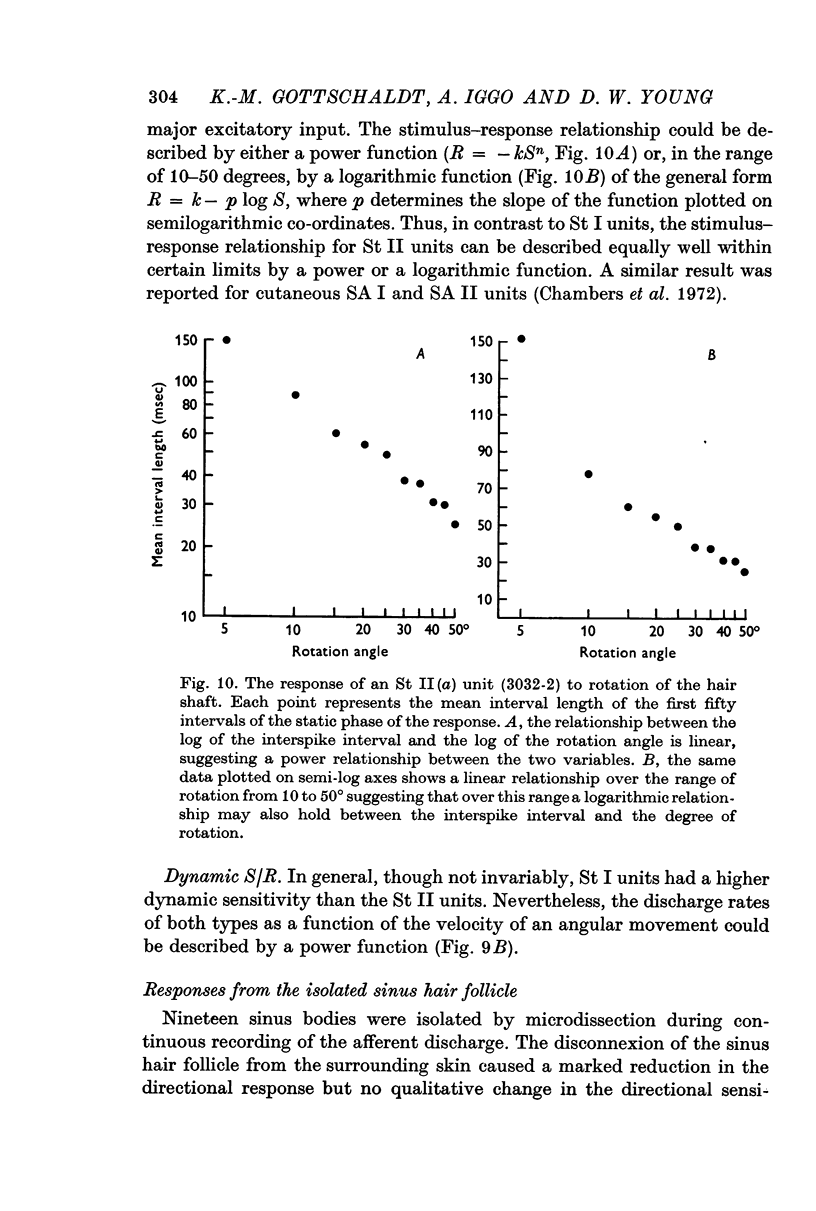
3. The slowly adapting units showed a power relationship between the degree of displacement of the hair and the mean interspike interval of the response. Slowly adapting units also exhibited a power relationship between the velocity of displacement of a hair and the mean interspike interval of the response.
4. The conduction velocities of all types of afferent units were measured and fell in the range of the Aα, fast myelinated fibres.
5. Movements of the carpal sinus hairs yielded both types of slowly adapting response recorded in fibres of the ulnar nerve directly innervating the carpal sinus hair follicles, and rapidly adapting responses from Pacinian corpuscles, found in close association with, but external to, these follicles.
6. On the basis of the findings in this study and the results of anatomical investigations of the receptor structures in the sinus hair follicle a correlation between the distinguishable afferent responses and the morphologically identifiable nerve endings has been proposed.
Full text
PDF


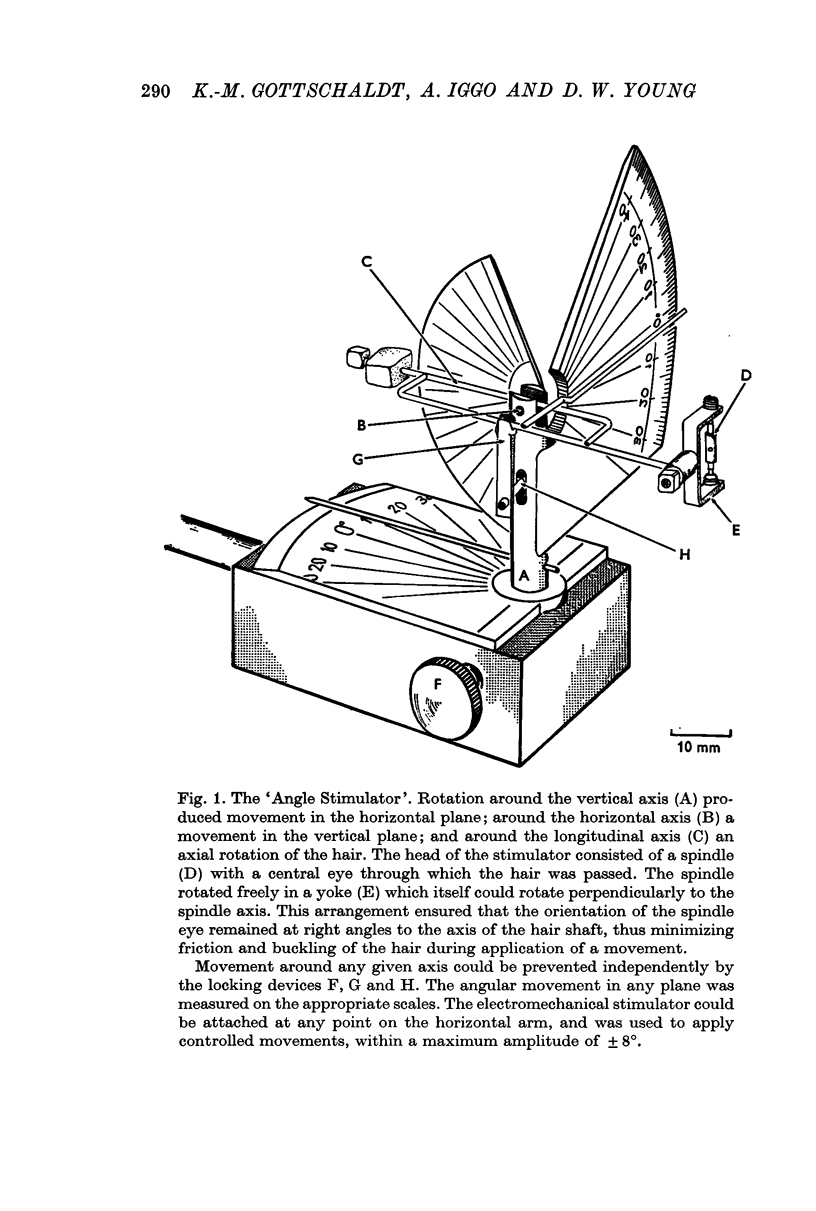



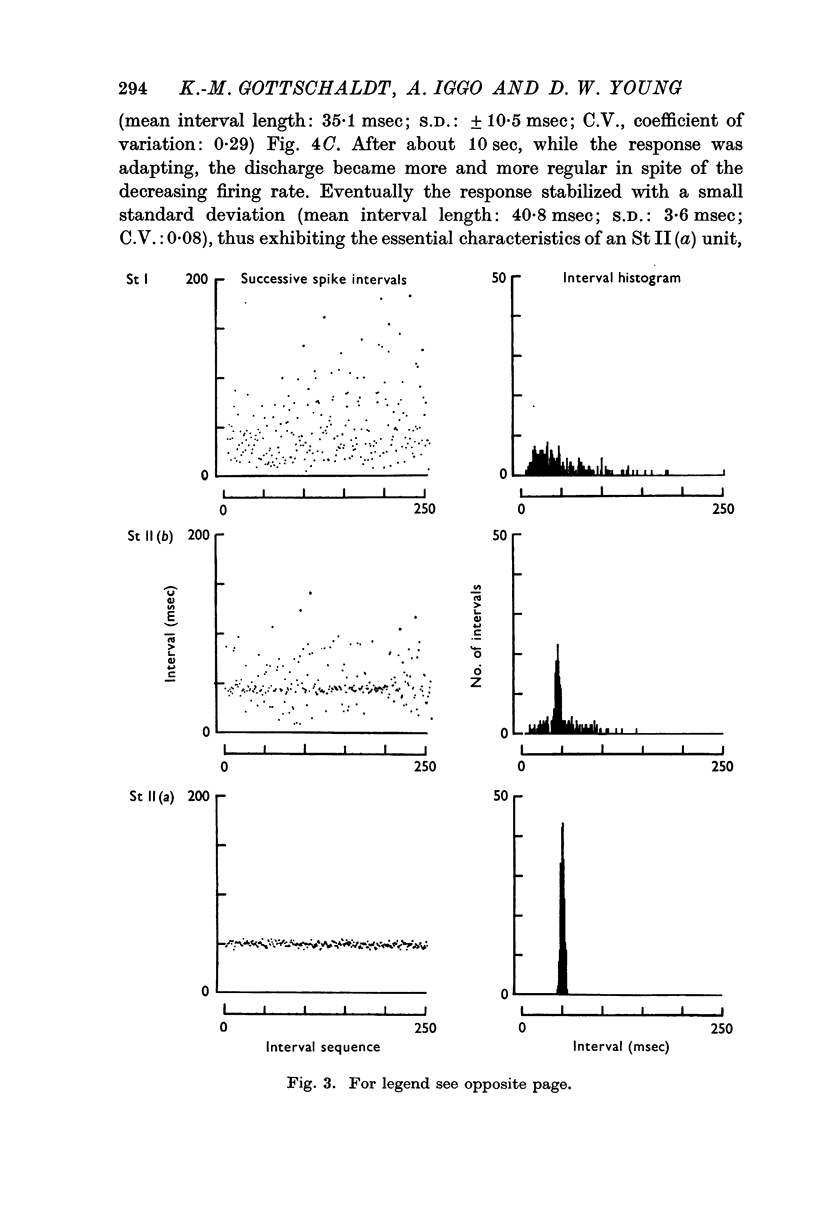

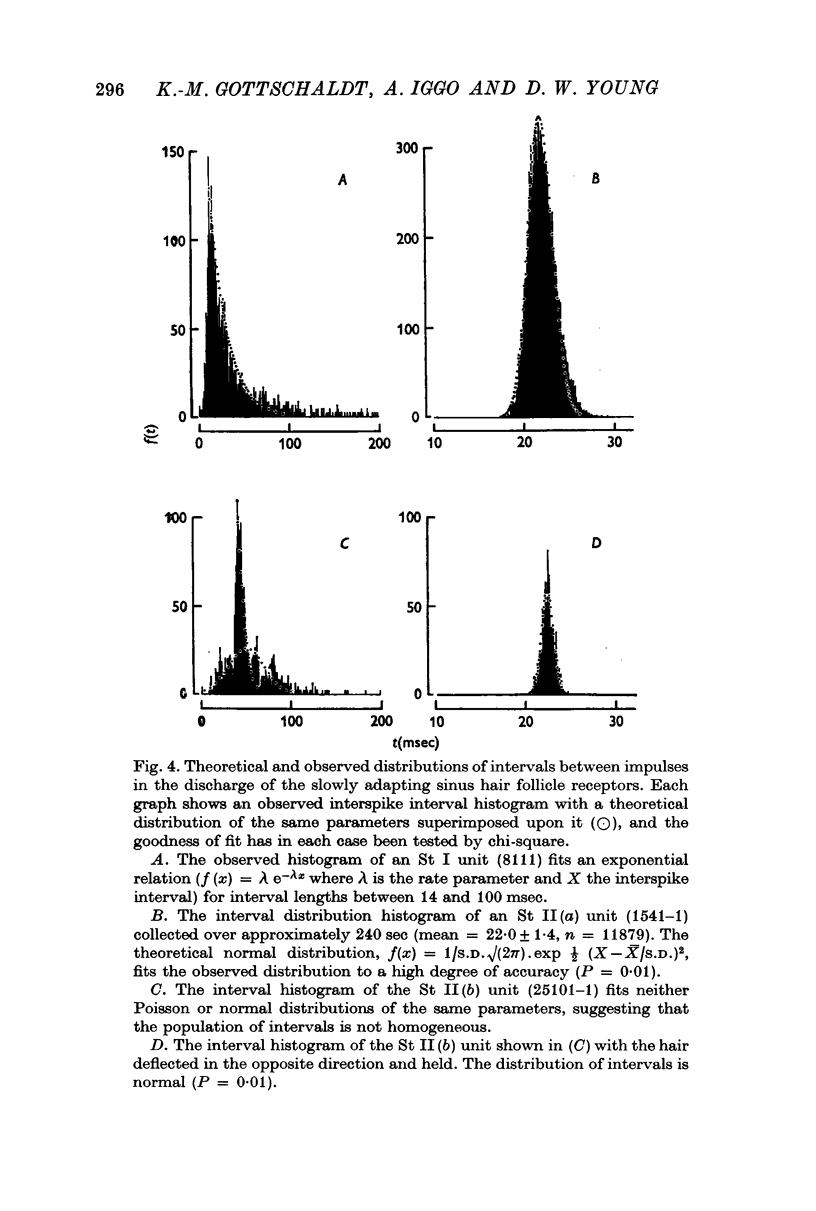
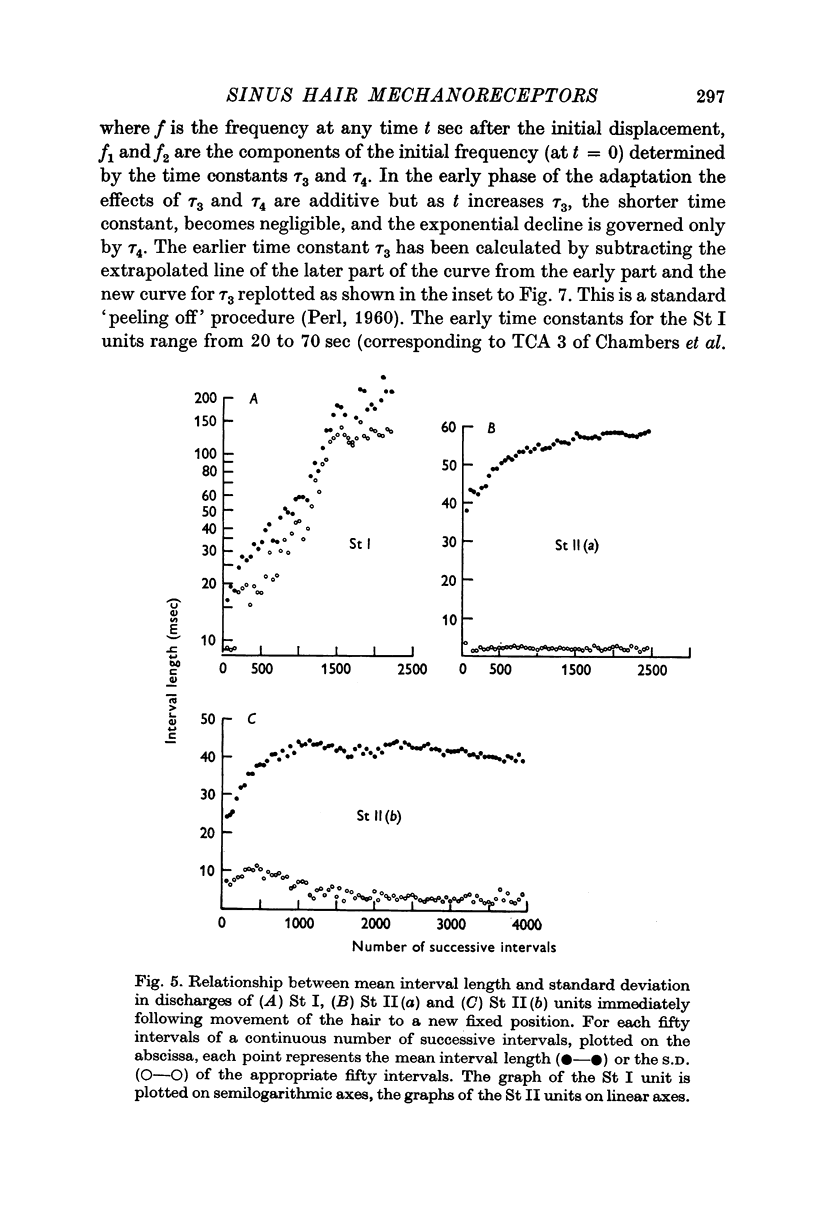












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D. The impulses produced by sensory nerve endings: Part I. J Physiol. 1926 Mar 18;61(1):49–72. doi: 10.1113/jphysiol.1926.sp002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres K. H. Uber die Feinstruktur der Rezeptoren an Sinushaaren. Z Zellforsch Mikrosk Anat. 1966;75(1):339–365. [PubMed] [Google Scholar]
- Brown A. G., Iggo A. A quantitative study of cutaneous receptors and afferent fibres in the cat and rabbit. J Physiol. 1967 Dec;193(3):707–733. doi: 10.1113/jphysiol.1967.sp008390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Petit D., Warren R. M. Receptor types in cat hairy skin supplied by myelinated fibers. J Neurophysiol. 1968 Nov;31(6):833–848. doi: 10.1152/jn.1968.31.6.833. [DOI] [PubMed] [Google Scholar]
- Cauna N. The fine morphology of the sensory receptor organs in the auricle of the rat. J Comp Neurol. 1969 May;136(1):81–98. doi: 10.1002/cne.901360106. [DOI] [PubMed] [Google Scholar]
- Chambers M. R., Andres K. H., von Duering M., Iggo A. The structure and function of the slowly adapting type II mechanoreceptor in hairy skin. Q J Exp Physiol Cogn Med Sci. 1972 Oct;57(4):417–445. doi: 10.1113/expphysiol.1972.sp002177. [DOI] [PubMed] [Google Scholar]
- ERICKSON R. P., KING R. L., PFAFFMANN C. Some characteristics of transmission through spinal trigeminal nucleus of rat. J Neurophysiol. 1961 Nov;24:621–632. doi: 10.1152/jn.1961.24.6.621. [DOI] [PubMed] [Google Scholar]
- Fitzgerald O. Discharges from the sensory organs of the cat's vibrissae and the modification in their activity by ions. J Physiol. 1940 May 14;98(2):163–178. doi: 10.1113/jphysiol.1940.sp003841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON G., LANDGREN S., SEED W. A. The functional characteristics of single cells in the caudal part of the spinal nucleus of the trigeminal nerve of the cat. J Physiol. 1961 Oct;158:544–559. doi: 10.1113/jphysiol.1961.sp006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschaldt K. M., Iggo A., Young D. W. Electrophysiology of the afferent innervation of sinus hairs, including vibrissae, of the cat. J Physiol. 1972 Apr;222(1):60P–61P. [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. An analysis of fibre diameter and receptor characteristics of myelinated cutaneous afferent fibres in cat. J Physiol. 1960 Aug;153:99–112. doi: 10.1113/jphysiol.1960.sp006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. F. Stimulus-response relationships in first-order sensory fibres from cat vibrissae. J Physiol. 1971 Feb;213(1):215–226. doi: 10.1113/jphysiol.1971.sp009377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B. Depolarization of sensory terminals and the initiation of impulses in the muscle spindle. J Physiol. 1950 Oct 16;111(3-4):261–282. doi: 10.1113/jphysiol.1950.sp004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEIDEL W. D., KEIDEL U. O., KIANG N. Y. Peripheral and cortical responses to mechanical stimulation of the cat's vibrissae. Arch Int Physiol Biochim. 1960 Mar;68:241–262. doi: 10.3109/13813456009083545. [DOI] [PubMed] [Google Scholar]
- KERR F. W., LYSAK W. R. SOMATOTOPIC ORGANIZATION OF TRIGEMINAL-GANGLION NEURONES. Arch Neurol. 1964 Dec;11:593–602. doi: 10.1001/archneur.1964.00460240025003. [DOI] [PubMed] [Google Scholar]
- KRUGER L., MICHEL F. A morphological and somatotopic analysis of single unit activity in the trigeminal sensory complex of the cat. Exp Neurol. 1962 Feb;5:139–156. doi: 10.1016/0014-4886(62)90030-4. [DOI] [PubMed] [Google Scholar]
- Kenton B., Kruger L., Woo M. Two classes of slowly adapting mechanoreceptor fibres in reptile cutaneous nerve. J Physiol. 1971 Jan;212(1):21–44. doi: 10.1113/jphysiol.1971.sp009308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANN S. J., STRAILE W. E. TYLOTRICH (HAIR) FOLLICLE: ASSOCIATION WITH A SLOWLY ADAPTING TACTILE RECEPTOR IN THE CAT. Science. 1965 Feb 26;147(3661):1043–1045. doi: 10.1126/science.147.3661.1043. [DOI] [PubMed] [Google Scholar]
- Merzenich M. M., Harrington T. H. The sense of flutter-vibration evoked by stimulation of the hairy skin of primates: comparison of human sensory capacity with the responses of mechanoreceptive afferents innervating the hairy skin of monkeys. Exp Brain Res. 1969;9(3):236–260. doi: 10.1007/BF00234457. [DOI] [PubMed] [Google Scholar]
- Nilsson B. Y. Hair discs and Pacinian corpuscles functionally associated with the carpal tactile hairs in the cat. Acta Physiol Scand. 1969 Dec;77(4):417–428. doi: 10.1111/j.1748-1716.1969.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Nilsson B. Y. Structure and function of the tactile hair receptors on the cat's foreleg. Acta Physiol Scand. 1969 Dec;77(4):396–416. doi: 10.1111/j.1748-1716.1969.tb04584.x. [DOI] [PubMed] [Google Scholar]
- Nord S. G. Somatotopic organization in the spinal trigeminal nucleus, the dorsal column nuclei and related structures in the rat. J Comp Neurol. 1967 Aug;130(4):343–356. doi: 10.1002/cne.901300406. [DOI] [PubMed] [Google Scholar]
- PEASE D. C., QUILLIAM T. A. Electron microscopy of the pacinian corpuscle. J Biophys Biochem Cytol. 1957 May 25;3(3):331–342. doi: 10.1083/jcb.3.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite P. M. The responses of cells in the rat thalamus to mechanical movements of the whiskers. J Physiol. 1973 Jan;228(2):541–561. doi: 10.1113/jphysiol.1973.sp010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker C. Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res. 1971 Mar 5;26(2):259–275. [PubMed] [Google Scholar]
- Wrobel K. H. Bau und Bedeutung der Blutsinus in den Vibrissen von Tupaia glis. Zentralbl Veterinarmed A. 1965 Dec;12(9):888–899. [PubMed] [Google Scholar]
- Yamamoto T. The fine structure of the palisade-type sensory endings in relation to hair follicles. J Electron Microsc (Tokyo) 1966;15(3):158–166. [PubMed] [Google Scholar]
- Zucker E., Welker W. I. Coding of somatic sensory input by vibrissae neurons in the rat's trigeminal ganglion. Brain Res. 1969 Jan;12(1):138–156. doi: 10.1016/0006-8993(69)90061-4. [DOI] [PubMed] [Google Scholar]


