Abstract
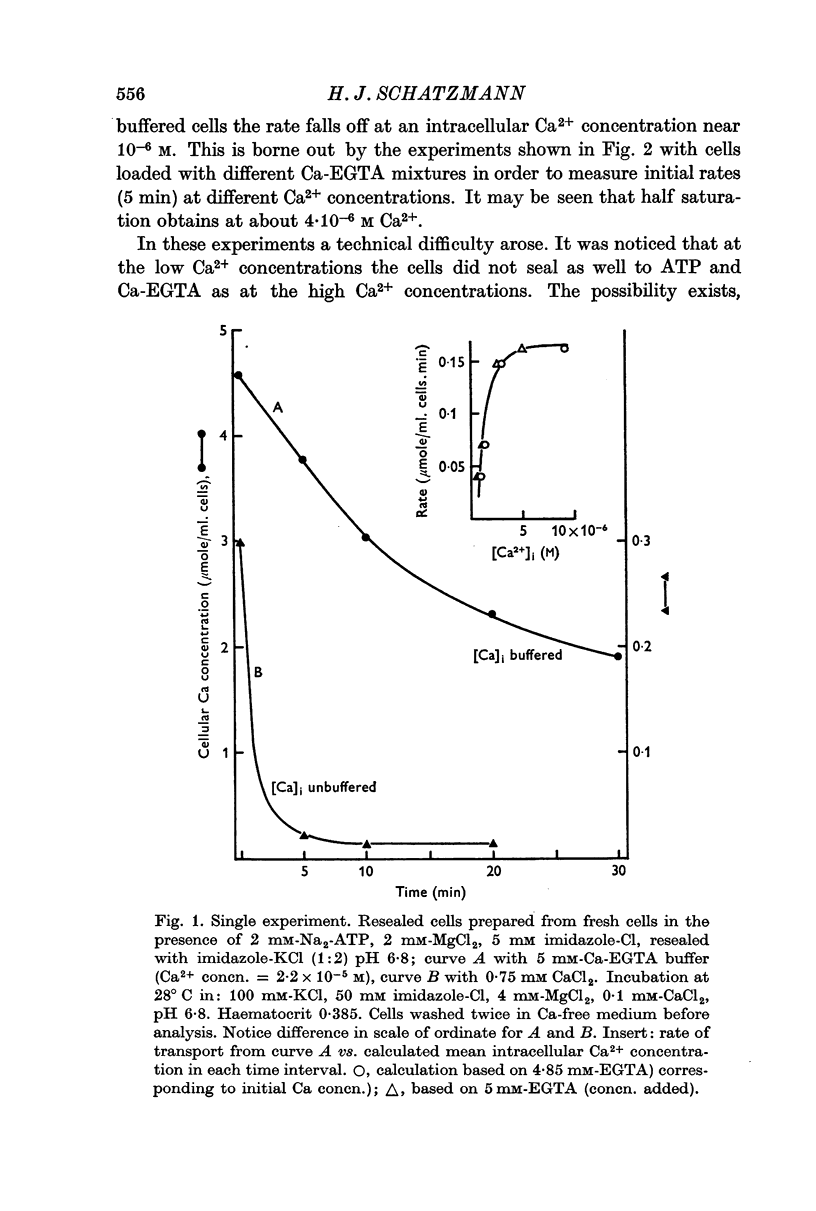
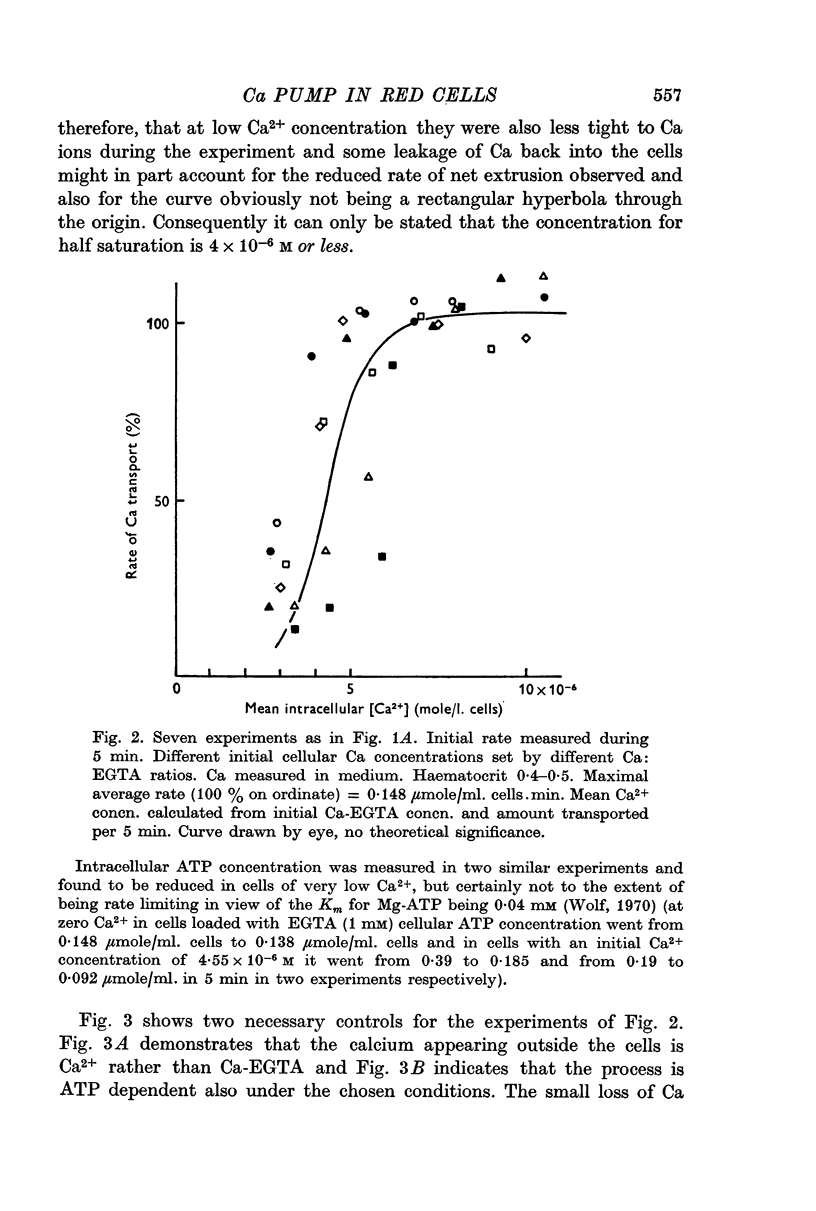
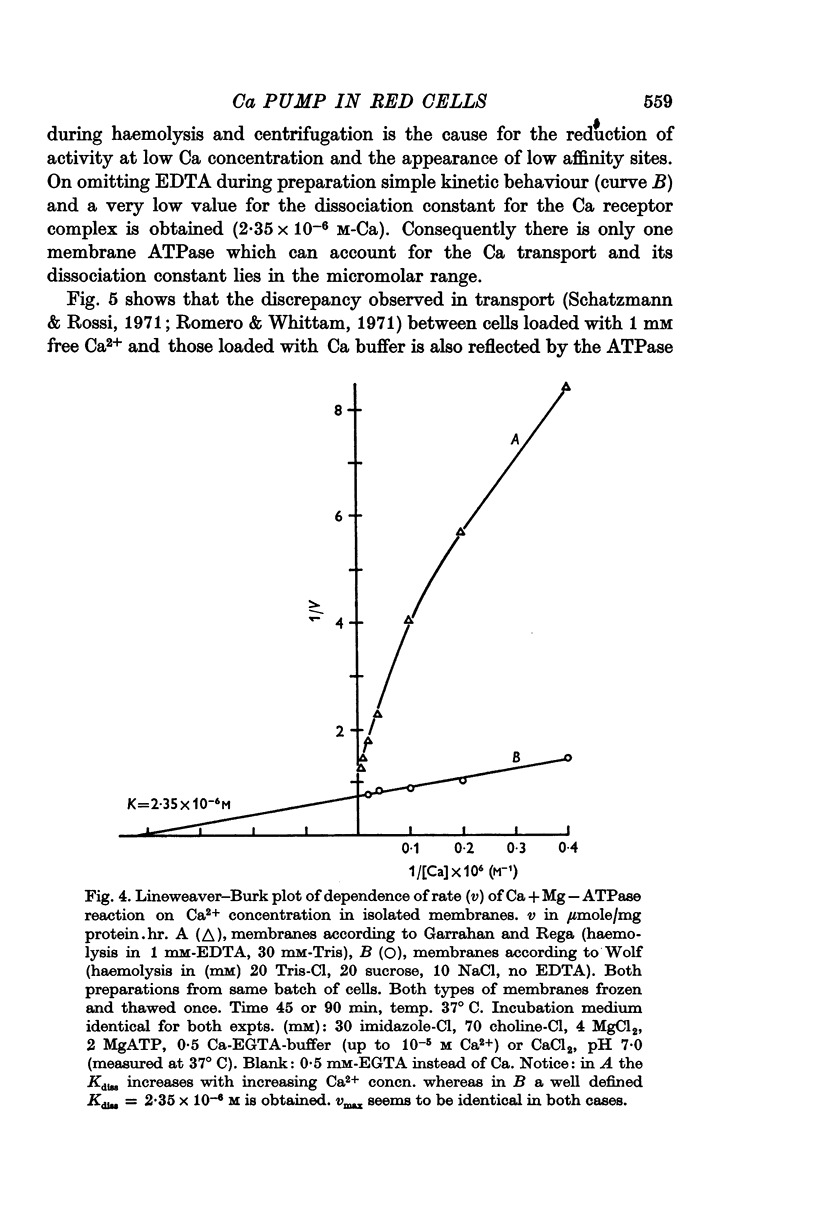
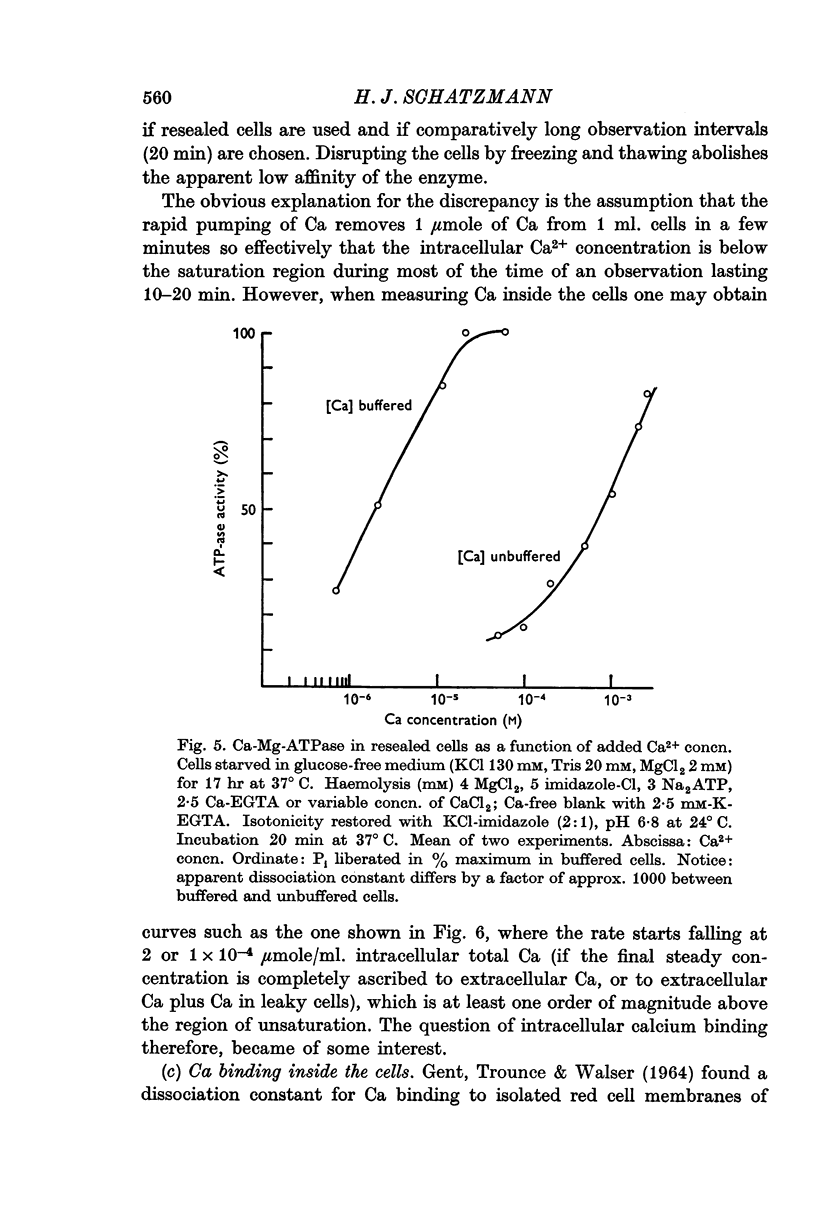
1. In resealed human red cells loaded with Ca-EGTA buffer solutions it was found that the intracellular free Ca2+ concentration for half saturation of the Ca transport system (which pumps Ca out of the cell) is equal to or smaller than 4 × 10-6 M and thus closely agrees with the dissociation constant of the Ca + Mg activated membrane ATPase.
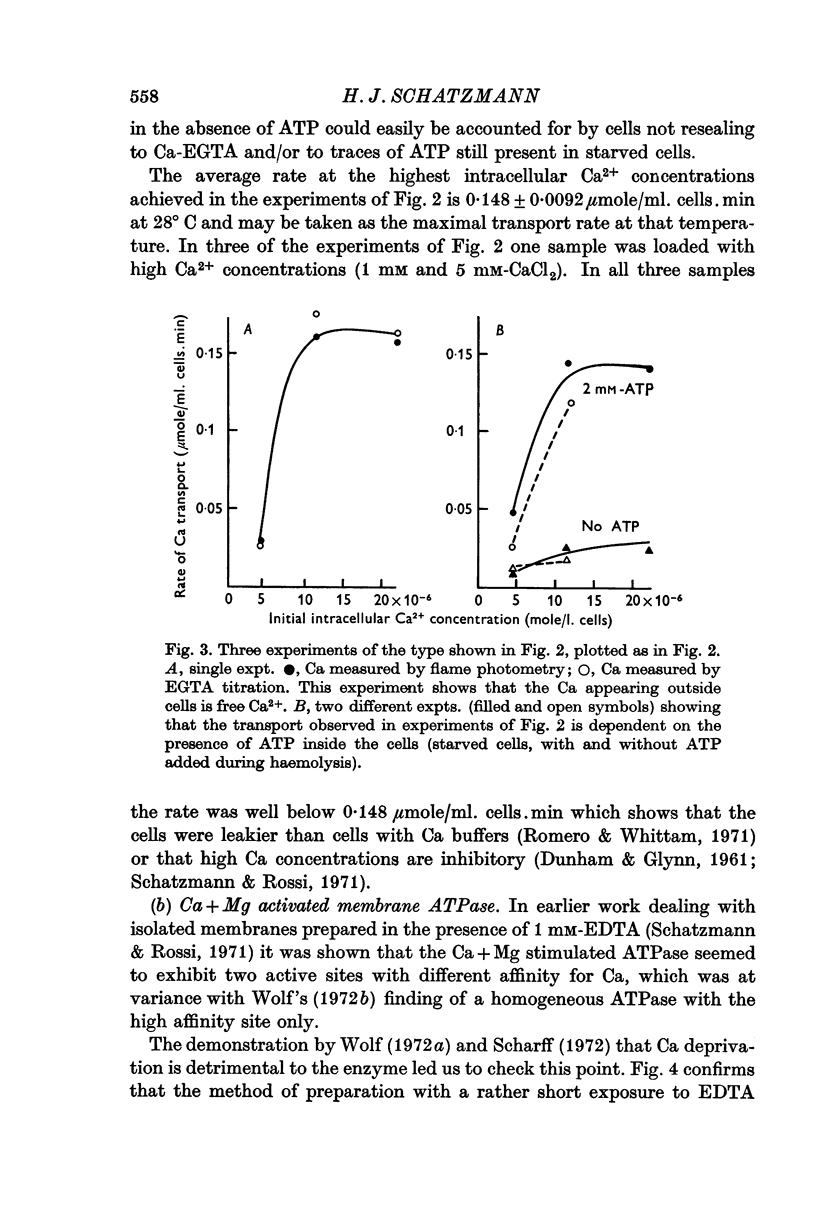
2. The maximal rate of Ca transport from resealed cells to medium was found to be 0·148 ± 0·009 μmole/ml. cells.min at 28° C.
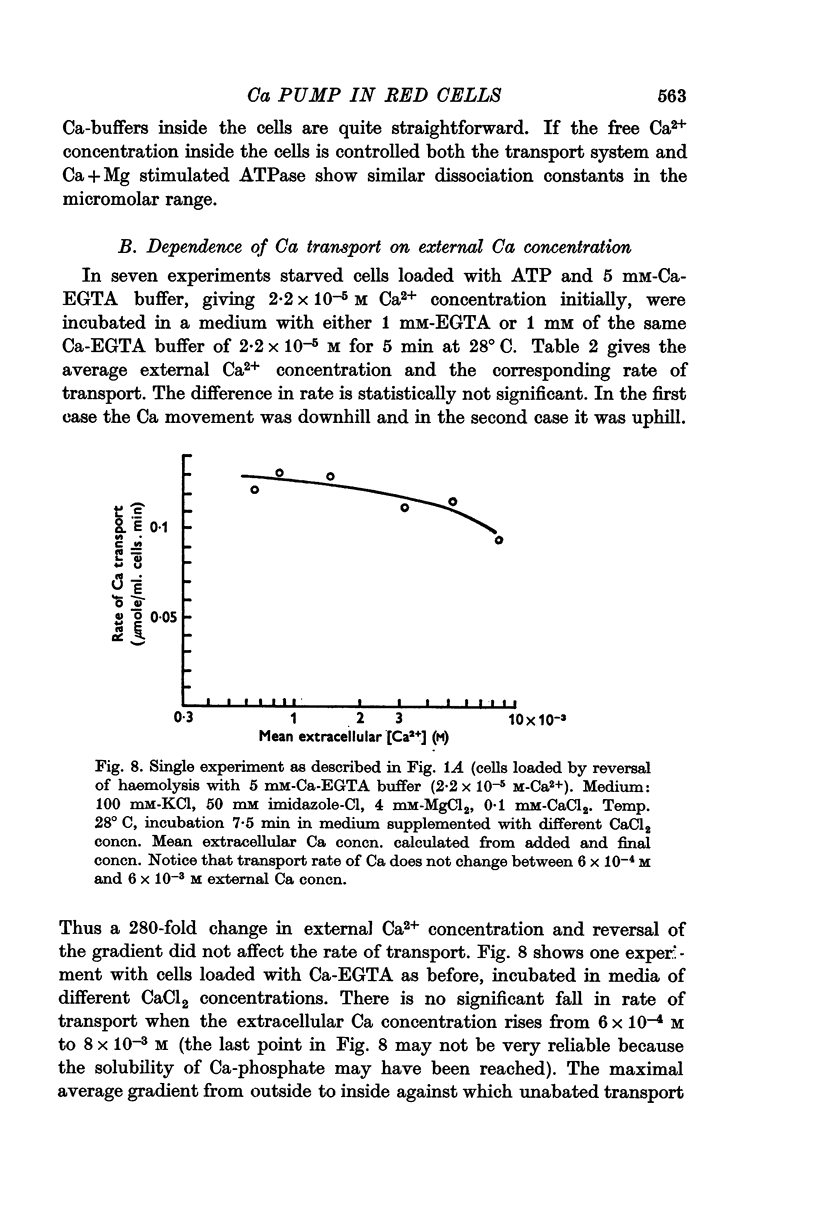
3. The rate of Ca transport was unaffected by a variation of the extracellular Ca2+ concentration from 3·10-7 to 5·10-3 M.
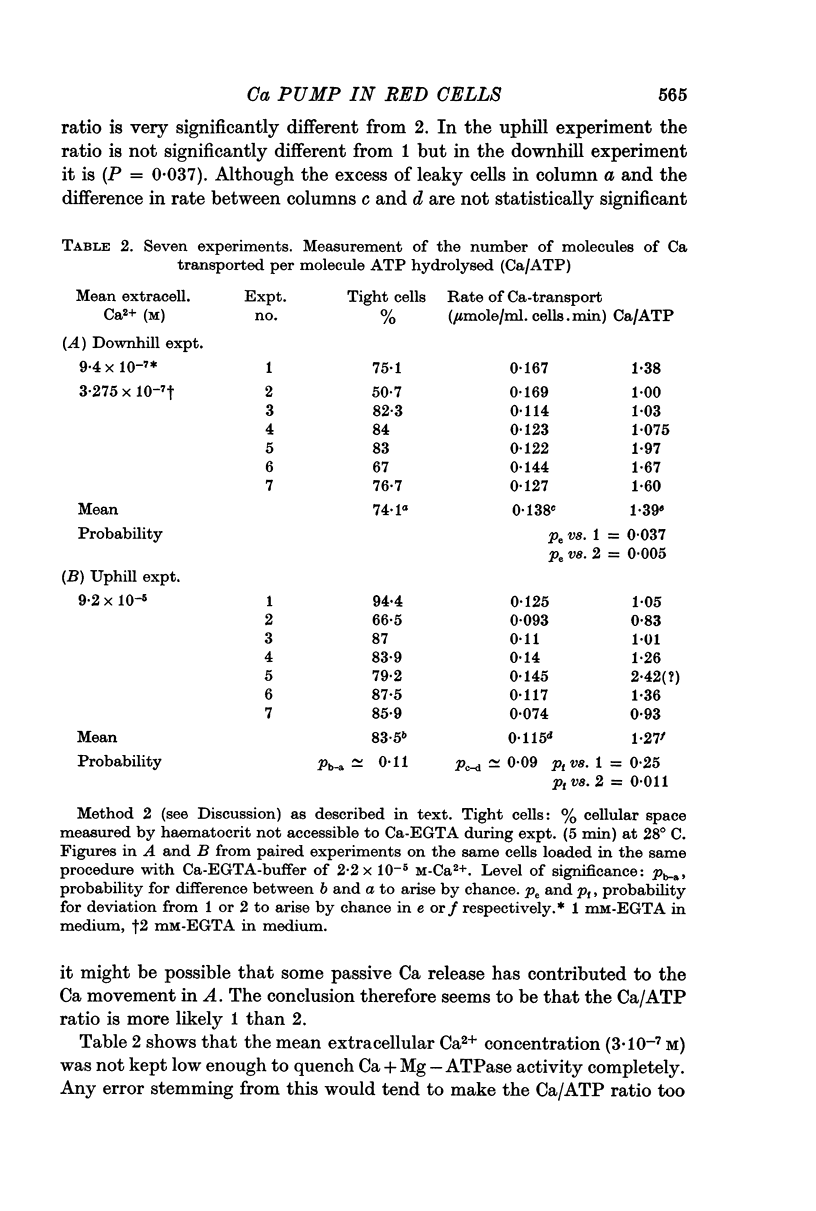
4. Evidence is presented making it probable that the stoichiometric relation between Ca transported and ATP hydrolysed is 1:1 rather than 2:1.
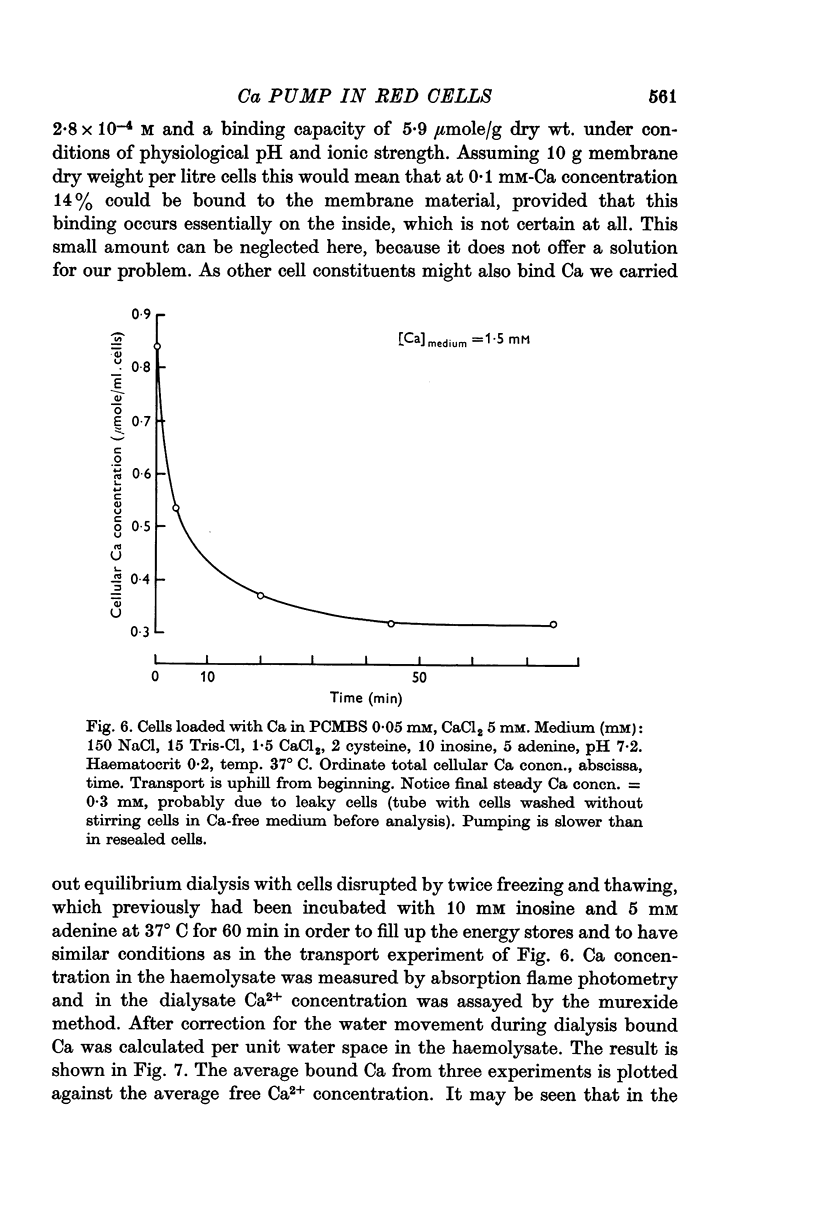
5. As the Ca transport is quite rapid even at half saturation and the passive leak for Ca negligible in intact cells it can be predicted that the steady-state cellular Ca2+ concentration must be low, most probably less than 10-6 μmole/ml. cells. Transport from cells containing 5·10-7 μmole/ml. into blood plasma is thermodynamically compatible with the normal plasma Ca2+ concentration and the normal cellular ATP, ADP and Pi content.
6. Treatment with the mercurial PCMBS in the cold for 15 hr allows to load red cells with 1 μmole Ca/ml. cells without destroying their ability to transport Ca after removal of the mercurial.
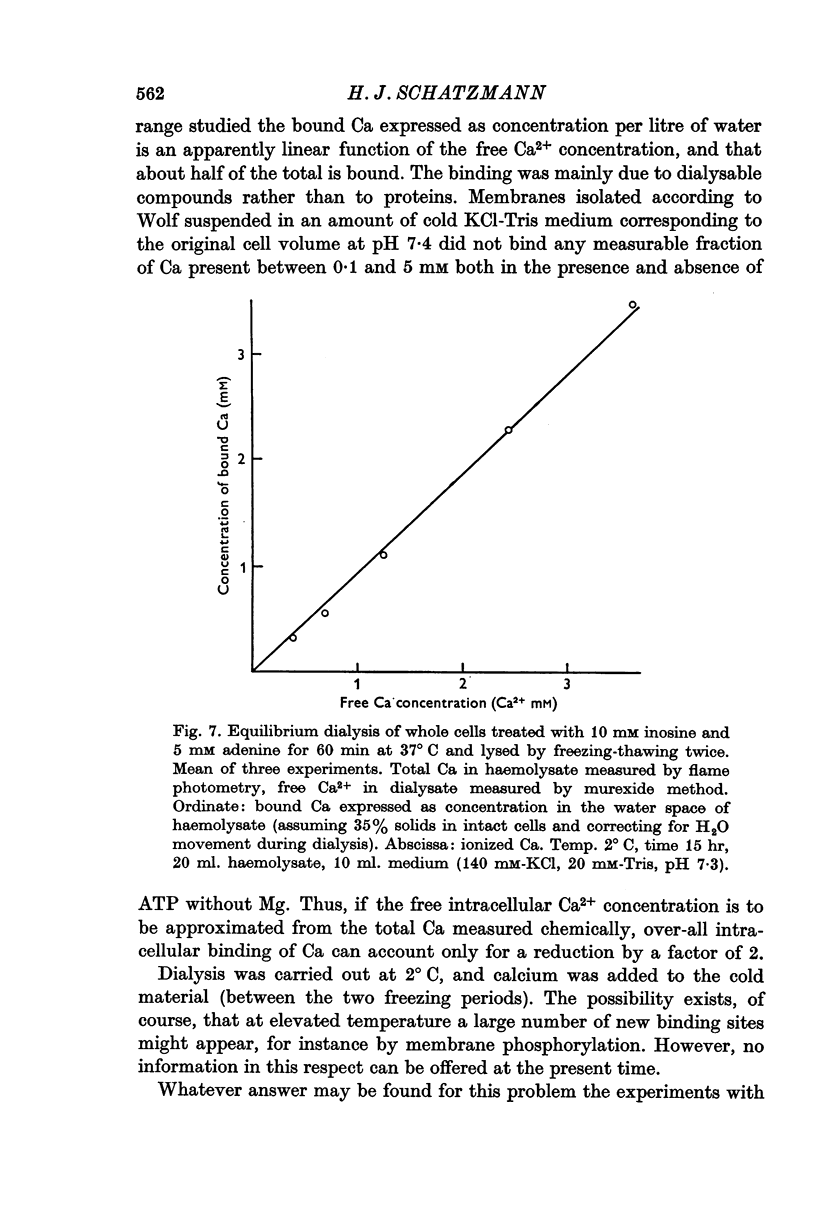
7. It is shown that at high cellular Ca concentrations (0·1-3 μmole/ml. cells) about 50% of the total is free Ca2+ on account of binding mainly to dialysable cell constituents.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R., SAVAGE E., HUGHES L., MARLOW A. A. Carbohydrate intermediates and related cofactors in the human erythrocyte. J Appl Physiol. 1953 Jul;6(1):51–56. doi: 10.1152/jappl.1953.6.1.51. [DOI] [PubMed] [Google Scholar]
- BENZINGER T., KITZINGER C., HEMS R., BURTON K. Free-energy changes of the glutaminase reaction and the hydrolysis of the terminal pyrophosphate bond of adenosine triphosphate. Biochem J. 1959 Feb;71(2):400–407. doi: 10.1042/bj0710400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond G. H., Green J. W. Effects of monovalent cations on the (Mg 2+ + Ca 2+ )-dependent ATPase of the red cell membrane. Biochim Biophys Acta. 1971 Aug 13;241(2):393–398. doi: 10.1016/0005-2736(71)90038-1. [DOI] [PubMed] [Google Scholar]
- Cha Y. N., Shin B. C., Lee K. S. Active uptake of Ca++ and Ca plus,plus-activated Mg++ ATPase in red cell membrane fragments. J Gen Physiol. 1971 Feb;57(2):202–215. doi: 10.1085/jgp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENT W. L., TROUNCE J. R., WALSER M. THE BINDING OF CALCIUM ION BY THE HUMAN ERYTHROCYTE MEMBRANE. Arch Biochem Biophys. 1964 Jun;105:582–589. doi: 10.1016/0003-9861(64)90054-2. [DOI] [PubMed] [Google Scholar]
- Garrahan P. J., Pouchan M. I., Rega A. F. Potassium activated phosphatase from human red blood cells. The mechanism of potassium activation. J Physiol. 1969 Jun;202(2):305–327. doi: 10.1113/jphysiol.1969.sp008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Rega A. F. Cation loading of red blood cells. J Physiol. 1967 Nov;193(2):459–466. doi: 10.1113/jphysiol.1967.sp008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Shin B. C. Studies on the active transport of calcium in human red cells. J Gen Physiol. 1969 Dec;54(6):713–729. doi: 10.1085/jgp.54.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L. Effect of intracellular calcium on the potassium permeability of human red cells. J Physiol. 1970 Feb;206(2):35P–36P. [PubMed] [Google Scholar]
- Makinose M., Hasselbach W. ATP synthesis by the reverse of the sarcoplasmic calcium pump. FEBS Lett. 1971 Jan 30;12(5):271–272. doi: 10.1016/0014-5793(71)80196-5. [DOI] [PubMed] [Google Scholar]
- Olson E. J., Cazort R. J. Active calcium and strontium transport in human erythrocyte ghosts. J Gen Physiol. 1969 Mar;53(3):311–322. doi: 10.1085/jgp.53.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Romero P. J., Whittam R. The control by internal calcium of membrane permeability to sodium and potassium. J Physiol. 1971 May;214(3):481–507. doi: 10.1113/jphysiol.1971.sp009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff O. The influence of calcium ions on the preparation of the (Ca 2+ +Mg 2+ )-activated membrane ATPase in human red cells. Scand J Clin Lab Invest. 1972 Nov;30(3):313–320. doi: 10.3109/00365517209084296. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J. ATP-dependent Ca++-extrusion from human red cells. Experientia. 1966 Jun 15;22(6):364–365. doi: 10.1007/BF01901136. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J., Rossi G. L. (Ca 2+ + Mg 2+ )-activated membrane ATPases in human red cells and their possible relations to cation transport. Biochim Biophys Acta. 1971 Aug 13;241(2):379–392. doi: 10.1016/0005-2736(71)90037-x. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H. U. Effects of ethylenediaminetetra-acetate and deoxycholate on kinetic constants of the calcium ion-dependent adenosine triphosphatase of human erythrocyte membranes. Biochem J. 1972 Nov;130(1):311–314. doi: 10.1042/bj1300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf H. U. Purification of the Ca2 plus-dependent ATPase of human erythrocyte membranes. Biochim Biophys Acta. 1970 Dec 1;219(2):521–524. doi: 10.1016/0005-2736(70)90238-5. [DOI] [PubMed] [Google Scholar]
- Wolf H. U. Studies on a Ca 2+ -dependent ATPase of human erythrocyte membranes. Effects of Ca 2+ and H + . Biochim Biophys Acta. 1972 May 9;266(2):361–375. doi: 10.1016/0005-2736(72)90094-6. [DOI] [PubMed] [Google Scholar]


