Abstract
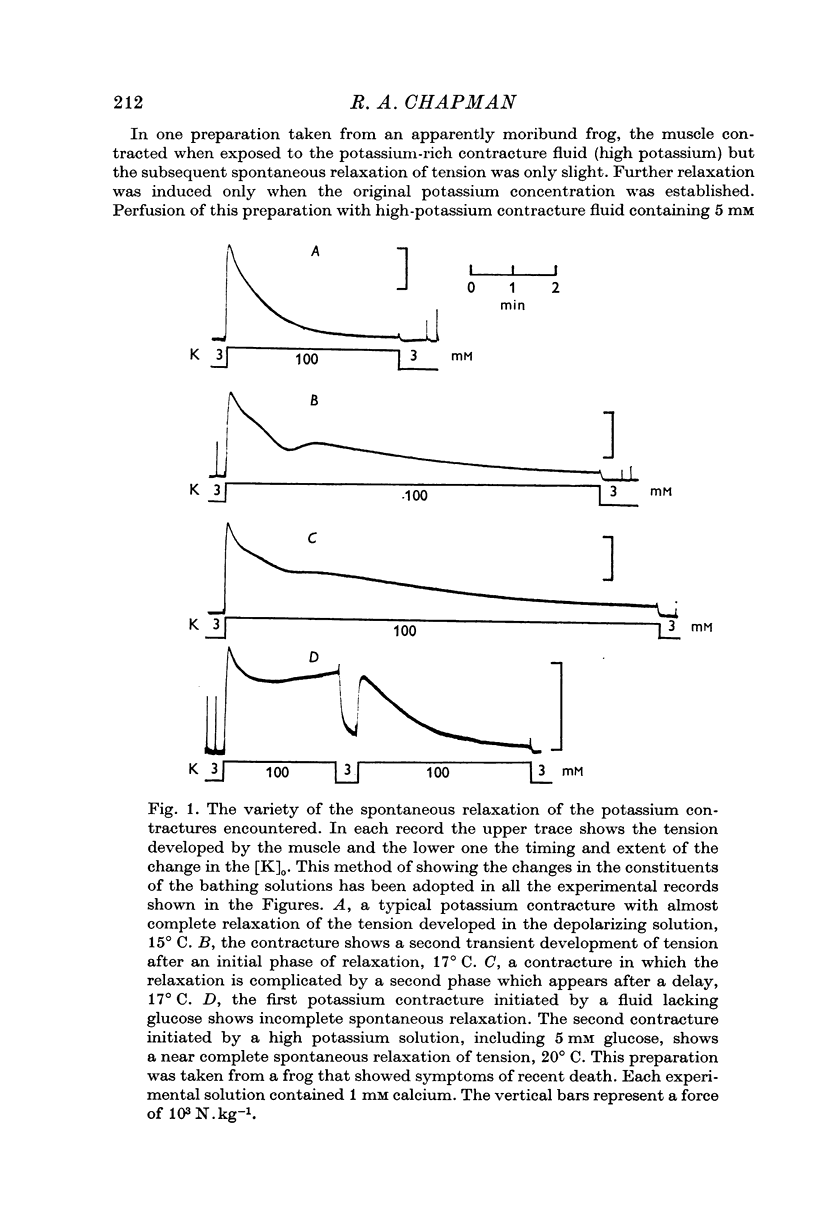
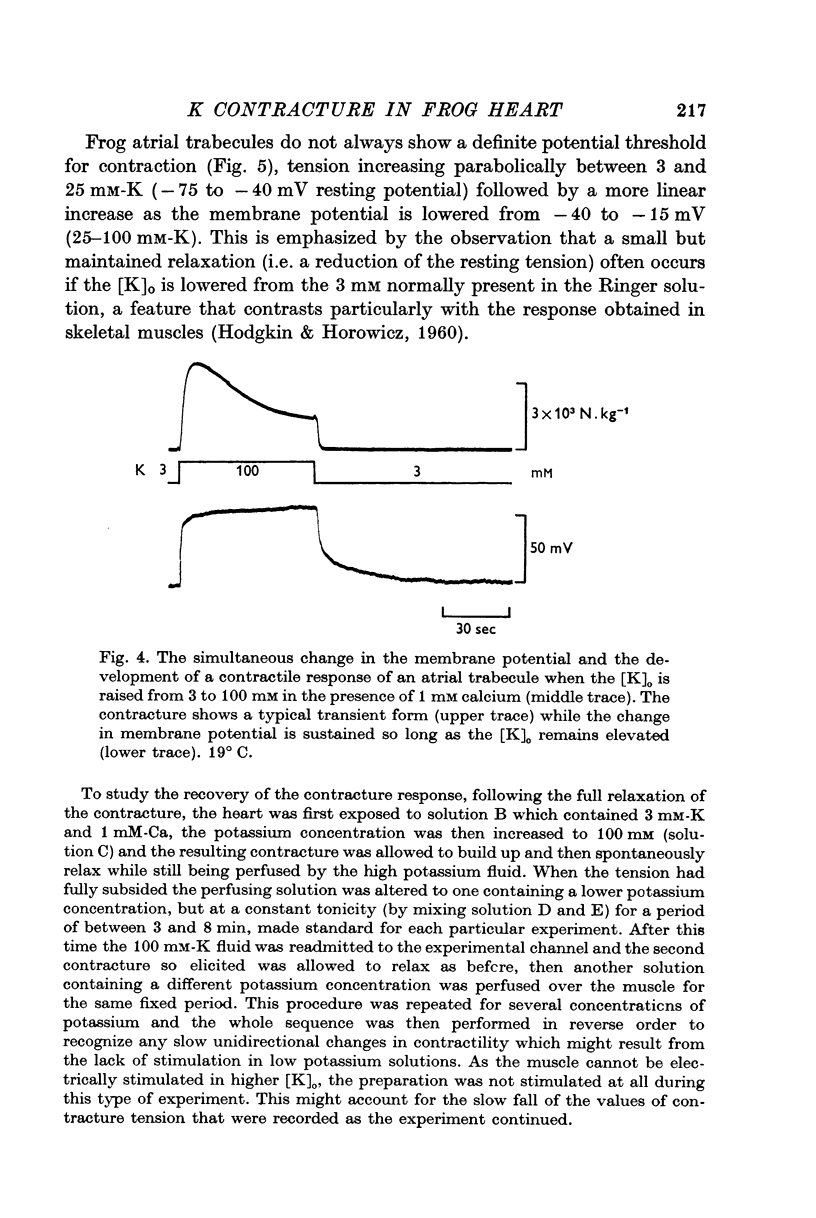
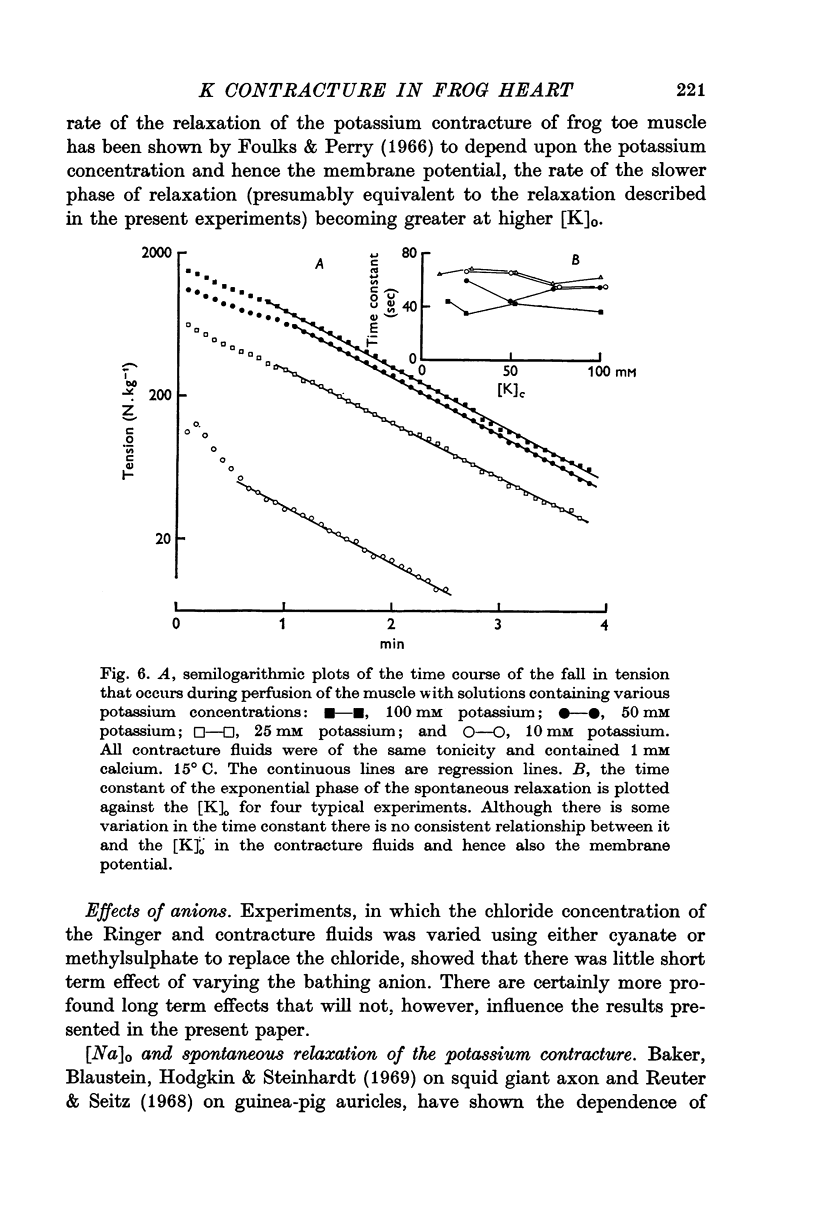
1. The tension generated by isolated frog atrial trabecules, during exposure to solutions containing a high potassium concentration, is not maintained but spontaneously relaxes. The final part of this relaxation can be fitted by a single exponential function.
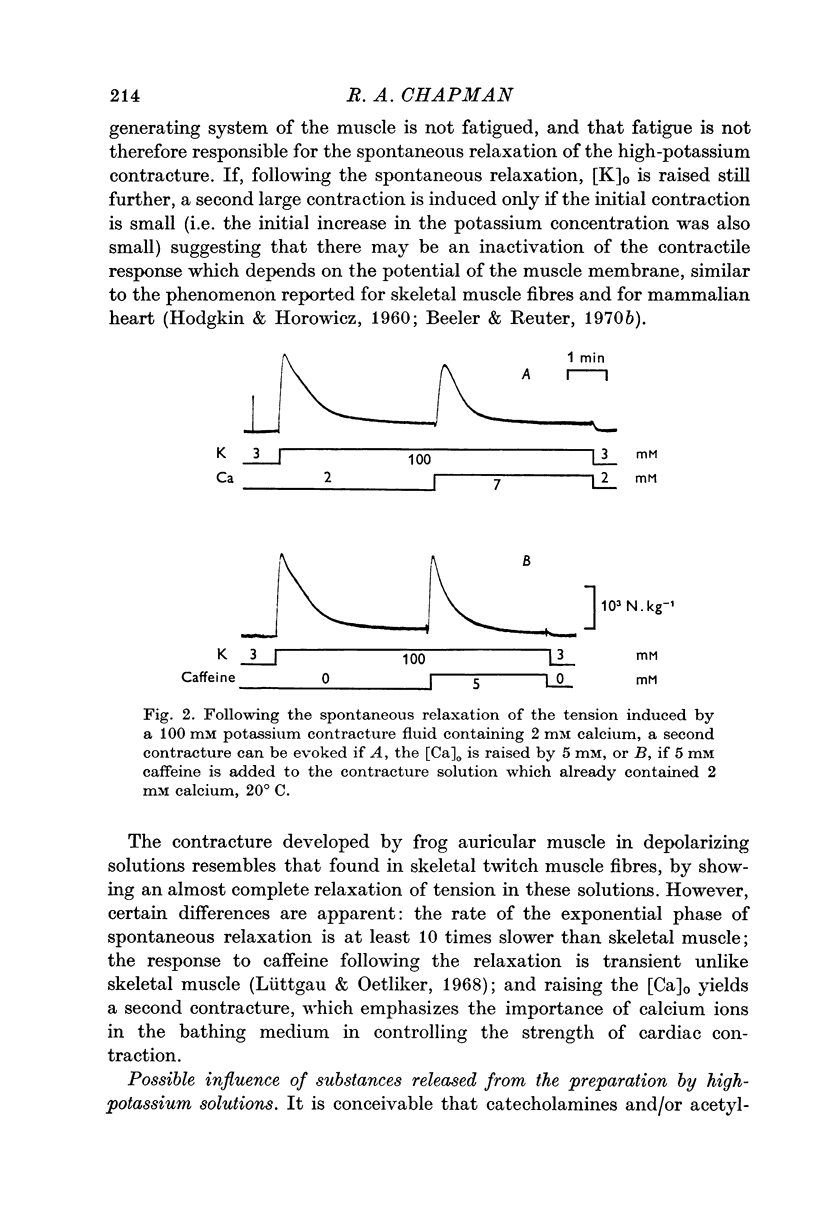
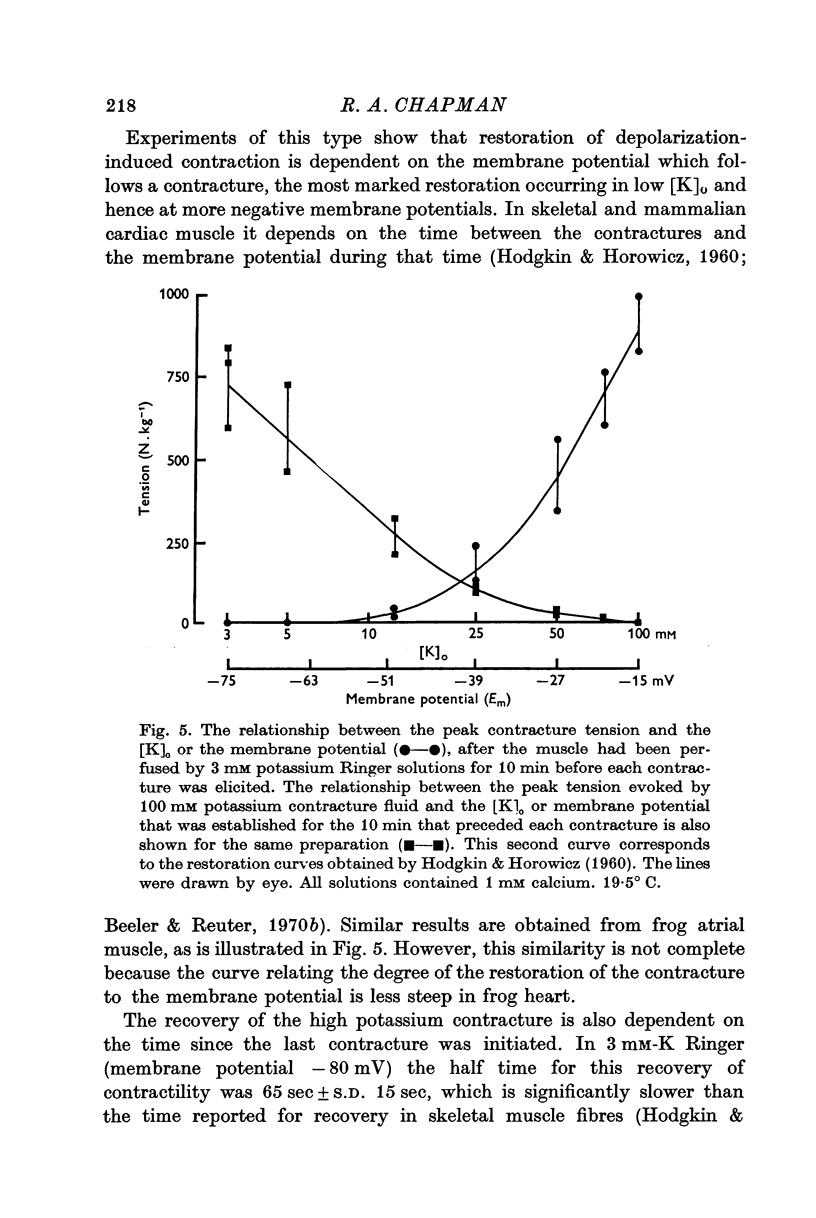
2. The recovery of the tension generating mechanisms following the spontaneous relaxation of a potassium contracture depends on the preceding membrane potential and the time since the last contracture.
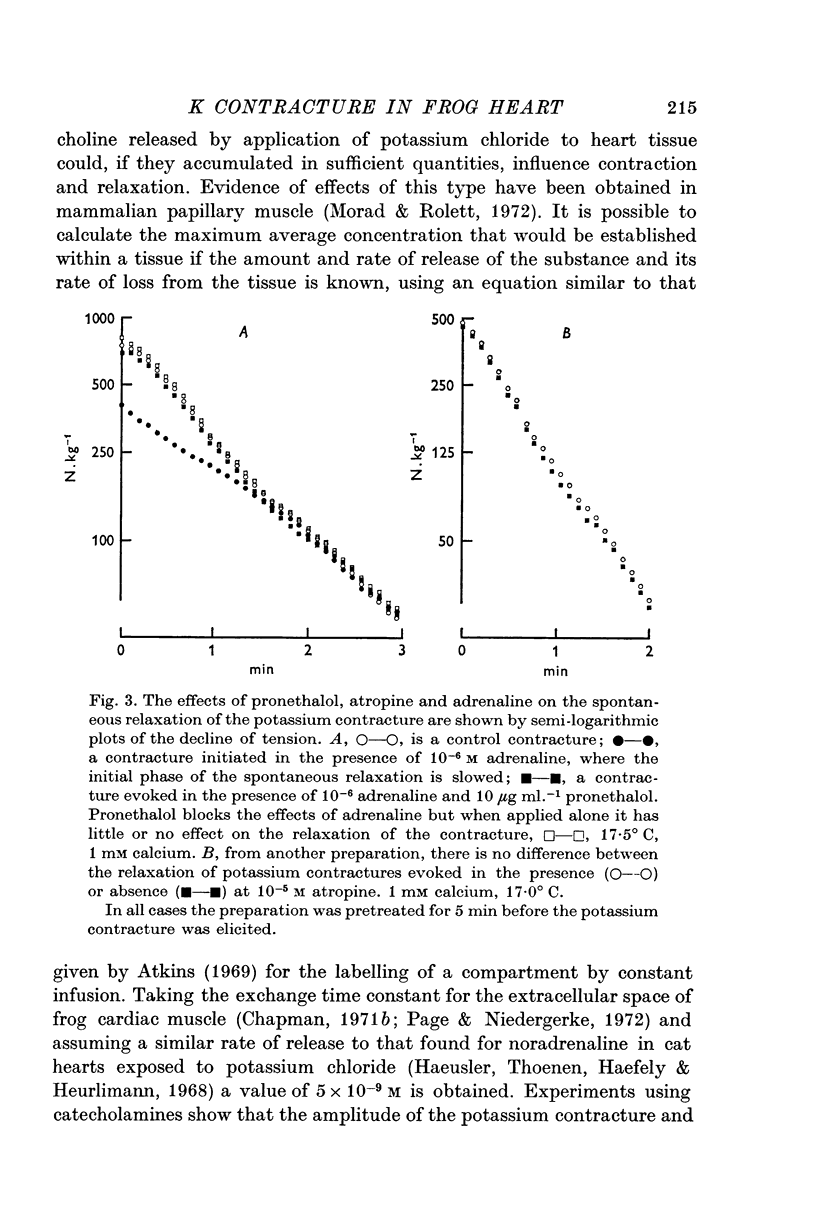
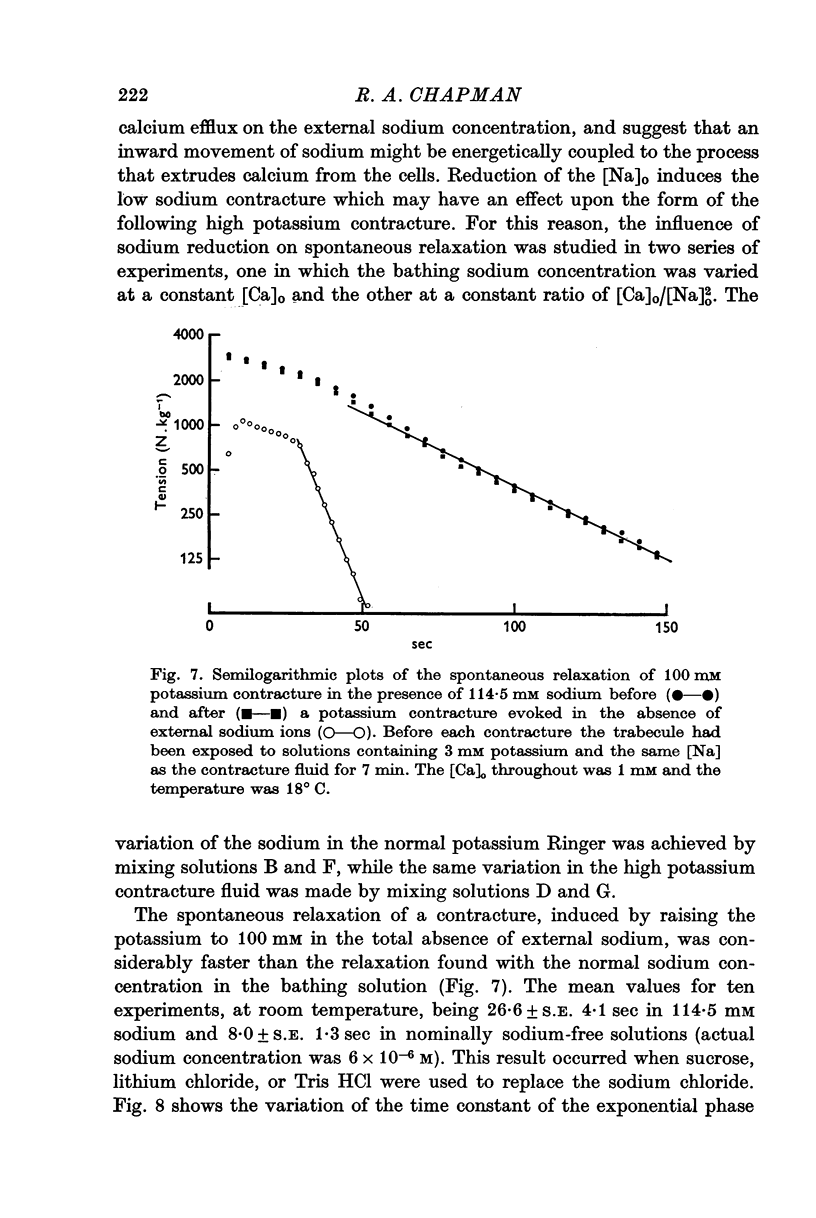
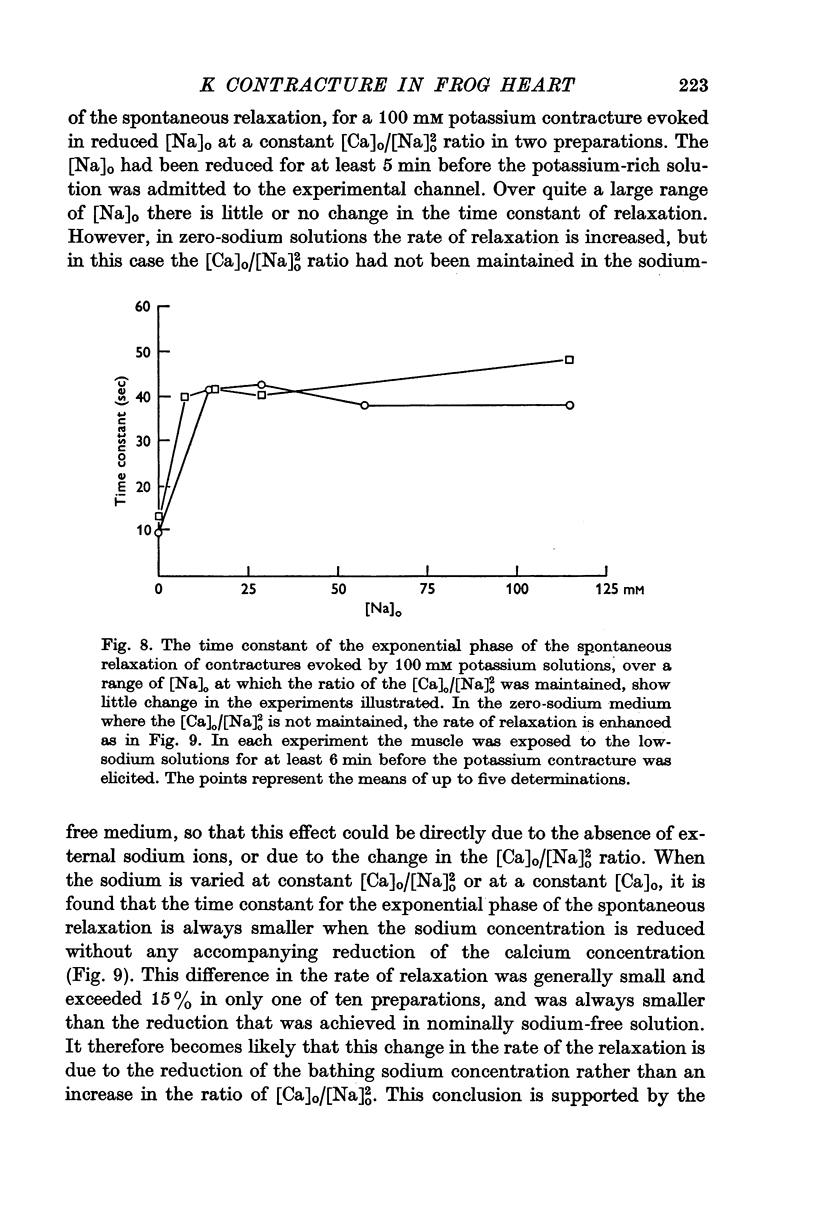
3. The rate of the exponential phase of the spontaneous relaxation is independent of the [K]o and hence the membrane potential, the [Ca]o; and when the [Ca]o/[Na]o2 ratio is maintained it is also independent of the [Na]o. This relaxation is not influenced by atropine or pronethalol.
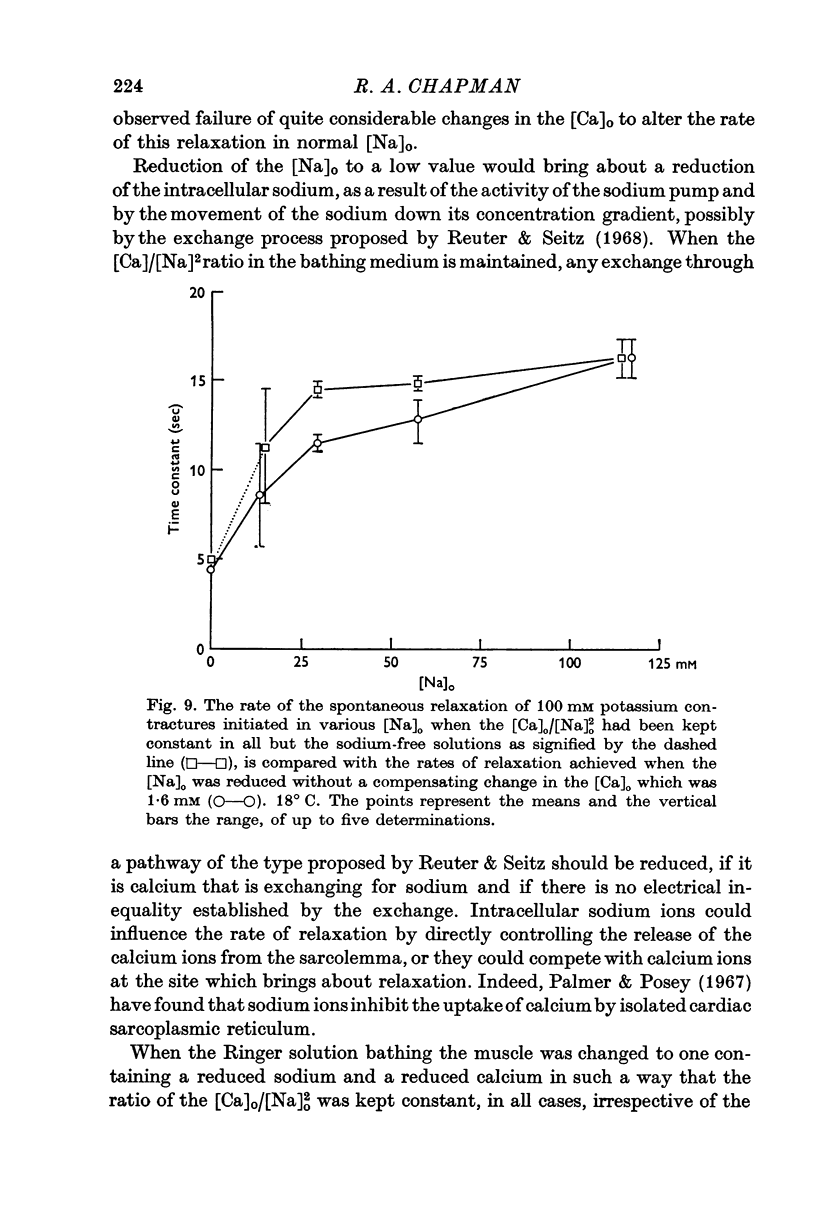
4. When sodium is totally excluded from the bathing medium the rate of relaxation of a later potassium contracture is much increased. It is argued that this change is due to a fall in the intracellular sodium concentration.
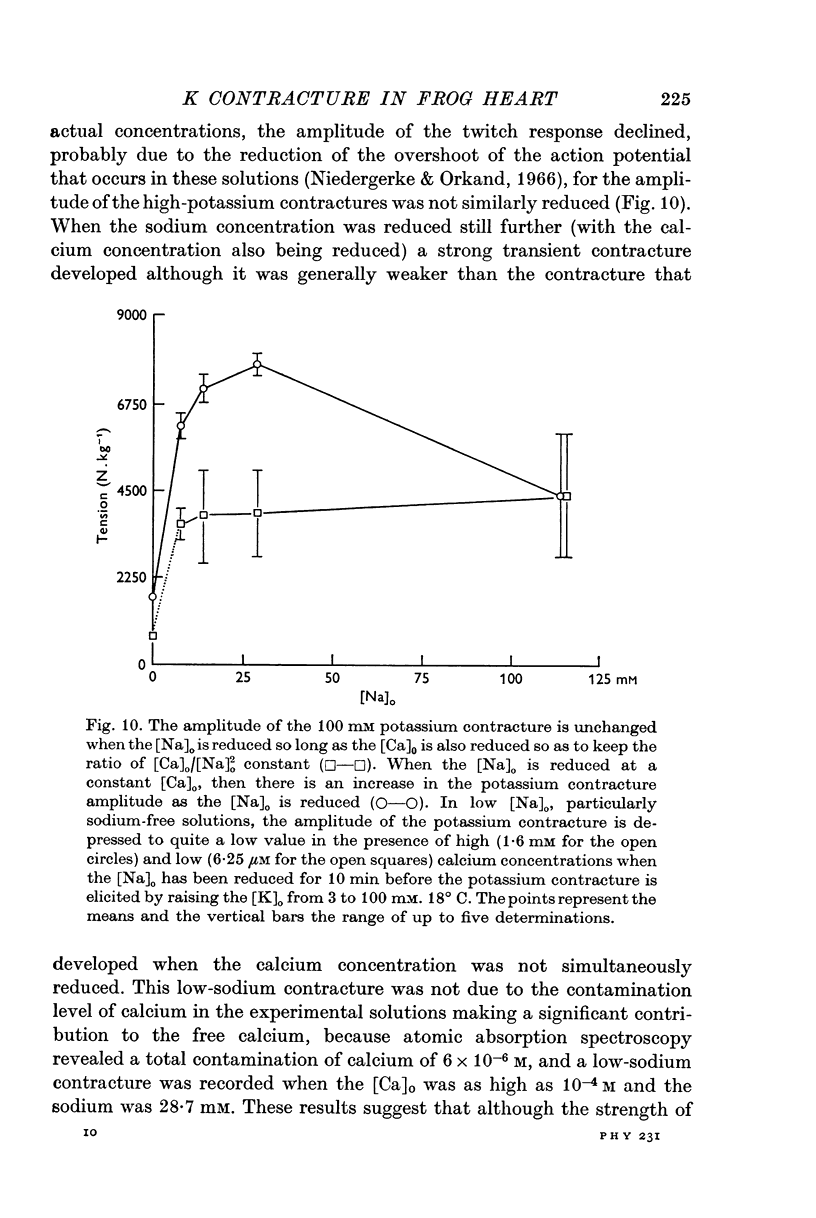
5. The consequences of these results are discussed, and the hypothesis that is favoured would require that contraction is induced by a transient release of calcium into the sarcoplasm, probably triggered by a potential dependent, and probably also transient, influx of calcium through the cell membrane. Relaxation is supposed to occur when this activator-calcium is then removed by an intracellular relaxing system that resembles the sarcoplasmic reticulum of other muscles. What this intracellular structure might be, is also discussed.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. The relation between membrane potential, membrane currents and activation of contraction in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):211–229. doi: 10.1113/jphysiol.1970.sp009057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozler E. Responses and Ca uptake of cardiac muscle in Na-free high-Ca solutions. Am J Physiol. 1971 Aug;221(2):618–622. doi: 10.1152/ajplegacy.1971.221.2.618. [DOI] [PubMed] [Google Scholar]
- Busselen P., Verdonck F., Carmeliet E. Contractions and potassium-contractures of heart muscle in sodium-free solutions. Arch Int Physiol Biochim. 1972 Jan;80(1):169–171. [PubMed] [Google Scholar]
- CHANCE B. THE ENERGY-LINKED REACTION OF CALCIUM WITH MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2729–2748. [PubMed] [Google Scholar]
- Caputo C. The time course of potassium contractures of single muscle fibres. J Physiol. 1972 Jun;223(2):483–505. doi: 10.1113/jphysiol.1972.sp009859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. Experimental alteration of the relationship between the external calcium concentration and the contractile force generated by auricular trabeculae isolated from the heart of the frog, Rana pipiens. J Physiol. 1971 Oct;218(1):147–161. doi: 10.1113/jphysiol.1971.sp009608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Miller D. J. Caffeine contractures induced in frog auricular trabeculae in the absence of external calcium. J Physiol. 1972 Sep;225(2):52P–54P. [PubMed] [Google Scholar]
- Chapman R. A., Miller D. J. The action of caffeine on frog myocardial contractility. J Physiol. 1971;217 (Suppl):64P–66P. [PubMed] [Google Scholar]
- Chapman R. A., Niedergerke R. Effects of calcium on the contraction of the hypodynamic frog heart. J Physiol. 1970 Dec;211(2):389–421. doi: 10.1113/jphysiol.1970.sp009284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Niedergerke R. Interaction between heart rate and calcium concentration in the control of contractile strength of the frog heart. J Physiol. 1970 Dec;211(2):423–443. doi: 10.1113/jphysiol.1970.sp009285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. The effects of temperature and metabolic inhibitors on the spontaneous relaxation of the potassium contracture of the heart of the frog Rana pipiens. J Physiol. 1973 Jun;231(2):233–249. doi: 10.1113/jphysiol.1973.sp010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The dependence of the contractile force generated by frog auricular trabeculae upon the external calcium concentration. J Physiol. 1971 May;215(1):139–162. doi: 10.1113/jphysiol.1971.sp009462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAHOTA Z., CARAFOLI E., ROSSI C. S., GAMBLE R. L., LEHNINGER A. L. THE STEADY STATE MAINTENANCE OF ACCUMULATED CA++ IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1965 Jun;240:2712–2720. [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Foulks J. G., Perry F. A. The relation between external potassium concentration and the relaxation rate of potassium-induced contractures in frog skeletal muscle. J Physiol. 1966 Oct;186(2):243–260. doi: 10.1113/jphysiol.1966.sp008032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs F., Briggs F. N. The site of calcium binding in relation to the activation of myofibrillar contraction. J Gen Physiol. 1968 May;51(5):655–676. doi: 10.1085/jgp.51.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. High potassium and low sodium contractures in sheep cardiac muscle. J Gen Physiol. 1971 Nov;58(5):483–510. doi: 10.1085/jgp.58.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. Voltage dependence and time dependence of contraction in sheep cardiac Purkinje fibers. Circ Res. 1971 Apr;28(4):446–460. doi: 10.1161/01.res.28.4.446. [DOI] [PubMed] [Google Scholar]
- Graham J. A., Lamb J. F. The effect of adrenaline on the tension developed in contractures and twitches of the ventricle of the frog. J Physiol. 1968 Jul;197(2):479–509. doi: 10.1113/jphysiol.1968.sp008571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. Potassium contractures in single muscle fibres. J Physiol. 1960 Sep;153:386–403. doi: 10.1113/jphysiol.1960.sp006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian F. J. The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres. J Physiol. 1971 Oct;218(1):117–145. doi: 10.1113/jphysiol.1971.sp009607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A. M. Contractile proteins of the heart. Physiol Rev. 1970 Jan;50(1):63–158. doi: 10.1152/physrev.1970.50.1.63. [DOI] [PubMed] [Google Scholar]
- Lamb J. F., McGuigan J. A. Contractures in a superfused frog's ventricle. J Physiol. 1966 Oct;186(2):261–283. doi: 10.1113/jphysiol.1966.sp008033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau H. C., Oetliker H. The action of caffeine on the activation of the contractile mechanism in straited muscle fibres. J Physiol. 1968 Jan;194(1):51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Orkand R. K. Excitation-concentration coupling in frog ventricle: evidence from voltage clamp studies. J Physiol. 1971 Dec;219(1):167–189. doi: 10.1113/jphysiol.1971.sp009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morad M., Rolett E. L. Relaxing effects of catecholamines on mammalian heart. J Physiol. 1972 Aug;224(3):537–558. doi: 10.1113/jphysiol.1972.sp009912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. The potassium chloride contracture of the heart and its modification by calcium. J Physiol. 1956 Dec 28;134(3):584–599. doi: 10.1113/jphysiol.1956.sp005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G., Hasker J. R. Effect of caffeine on calcium in subcellular fractions of cardiac muscle. Am J Physiol. 1966 Oct;211(4):950–954. doi: 10.1152/ajplegacy.1966.211.4.950. [DOI] [PubMed] [Google Scholar]
- Niedergerke R., Orkand R. K. The dependence of the action potential of the frog's heart on the external and intracellular sodium concentration. J Physiol. 1966 May;184(2):312–334. doi: 10.1113/jphysiol.1966.sp007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi R., Trautwein W. The dependence of cardiac contraction on depolarization and slow inward current. Pflugers Arch. 1971;323(3):187–203. doi: 10.1007/BF00586383. [DOI] [PubMed] [Google Scholar]
- Page S. G., Niedergerke R. Structures of physiological interest in the frog heart ventricle. J Cell Sci. 1972 Jul;11(1):179–203. doi: 10.1242/jcs.11.1.179. [DOI] [PubMed] [Google Scholar]
- Palmer R. F., Posey V. A. Ion effects on calcium accumulation by cardiac sarcoplasmic reticulum. J Gen Physiol. 1967 Sep;50(8):2085–2095. doi: 10.1085/jgp.50.8.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Garnier D., Gargouil Y. M., Coraboeuf E. Existence and role of a slow inward current during the frog atrial action potential. Pflugers Arch. 1969;308(2):91–110. doi: 10.1007/BF00587018. [DOI] [PubMed] [Google Scholar]
- Sommer J. R., Johnson E. A. Cardiac muscle. A comparative ultrastructural study with special reference to frog and chicken hearts. Z Zellforsch Mikrosk Anat. 1969;98(3):437–468. [PubMed] [Google Scholar]
- Staley N. A., Benson E. S. The ultrastructure of frog ventricular cardiac muscle and its relationship to mechanism of excitation-contraction coupling. J Cell Biol. 1968 Jul;38(1):99–114. doi: 10.1083/jcb.38.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe W. R., Seeman P. The site of action of caffeine and procaine in skeletal muscle. J Pharmacol Exp Ther. 1971 Nov;179(2):324–330. [PubMed] [Google Scholar]
- Vassort G., Rougier O. Membrane potential and slow inward current dependence of frog cardiac mechanical activity. Pflugers Arch. 1972;331(3):191–203. doi: 10.1007/BF00589126. [DOI] [PubMed] [Google Scholar]
- Weber A., Herz R. The relationship between caffeine contracture of intact muscle and the effect of caffeine on reticulum. J Gen Physiol. 1968 Nov;52(5):750–759. doi: 10.1085/jgp.52.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. The mechanism of the action of caffeine on sarcoplasmic reticulum. J Gen Physiol. 1968 Nov;52(5):760–772. doi: 10.1085/jgp.52.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. H., Heppner R. L., Weidmann S. Inotropic effects of electric currents. I. Positive and negative effects of constant electric currents or current pulses applied during cardiac action potentials. II. Hypotheses: calcium movements, excitation-contraction coupling and inotropic effects. Circ Res. 1969 Mar;24(3):409–445. doi: 10.1161/01.res.24.3.409. [DOI] [PubMed] [Google Scholar]


