Abstract
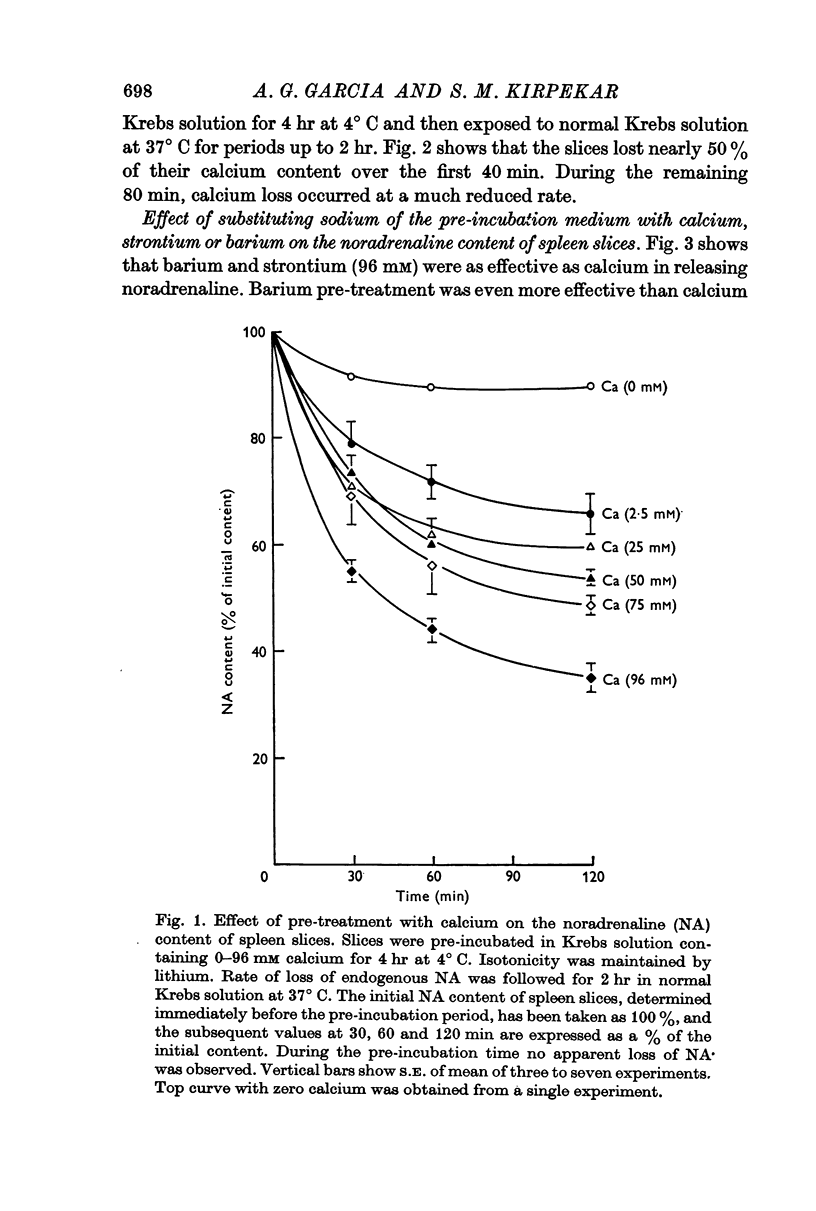
1. Spleen slices pre-incubated for different periods at 4° C in Krebs solution containing varying concentrations of calcium, up to 96 mM, lost their endogenous noradrenaline stores when reincubated in normal Krebs solution at 37° C for 2 hr. Rate of loss of noradrenaline was roughly related to the calcium concentration of the pre-incubation medium and the pre-exposure time.
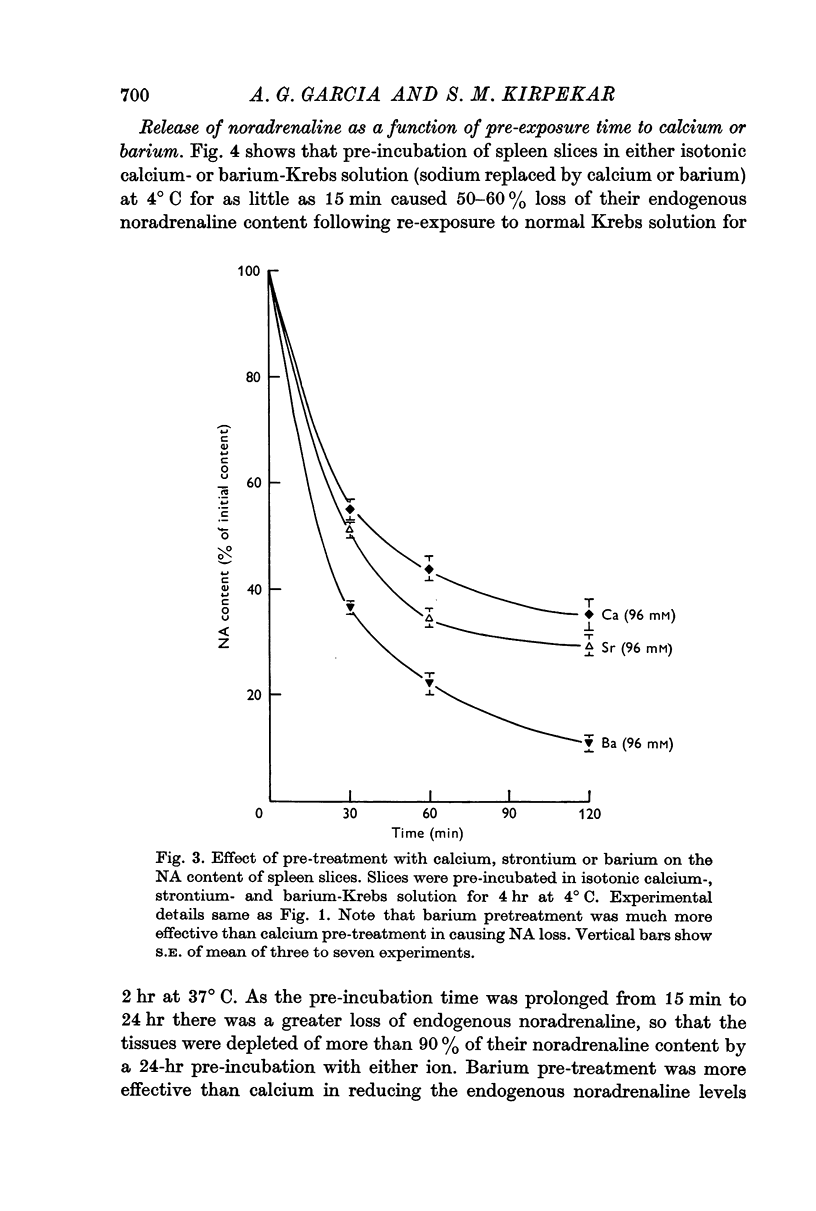
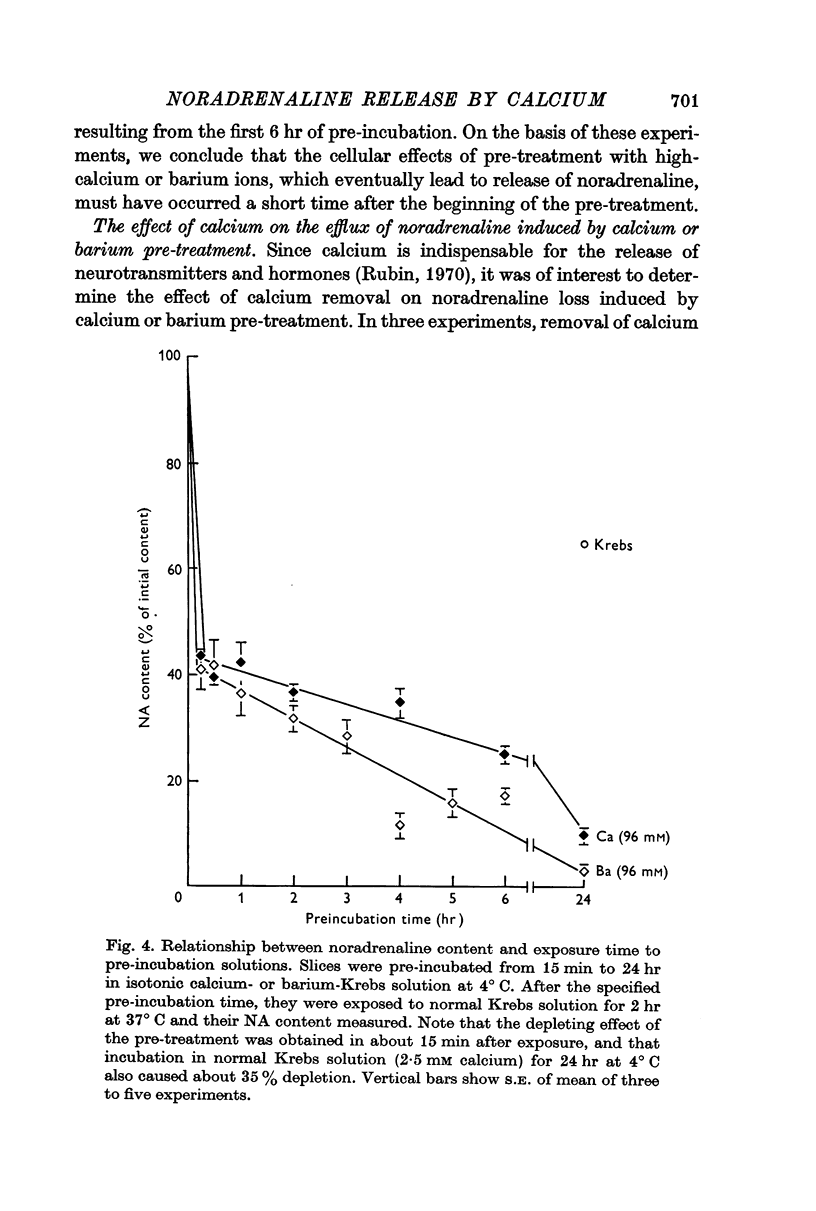
2. Pre-treatment with isotonic barium or strontium (96 mM) Krebs solution also induced release of noradrenaline from spleen slices when re-exposed to normal Krebs solution. Barium was more effective than either calcium or strontium.
3. The enhanced release induced by calcium pre-treatment occurred in the absence of calcium, with or without EGTA.
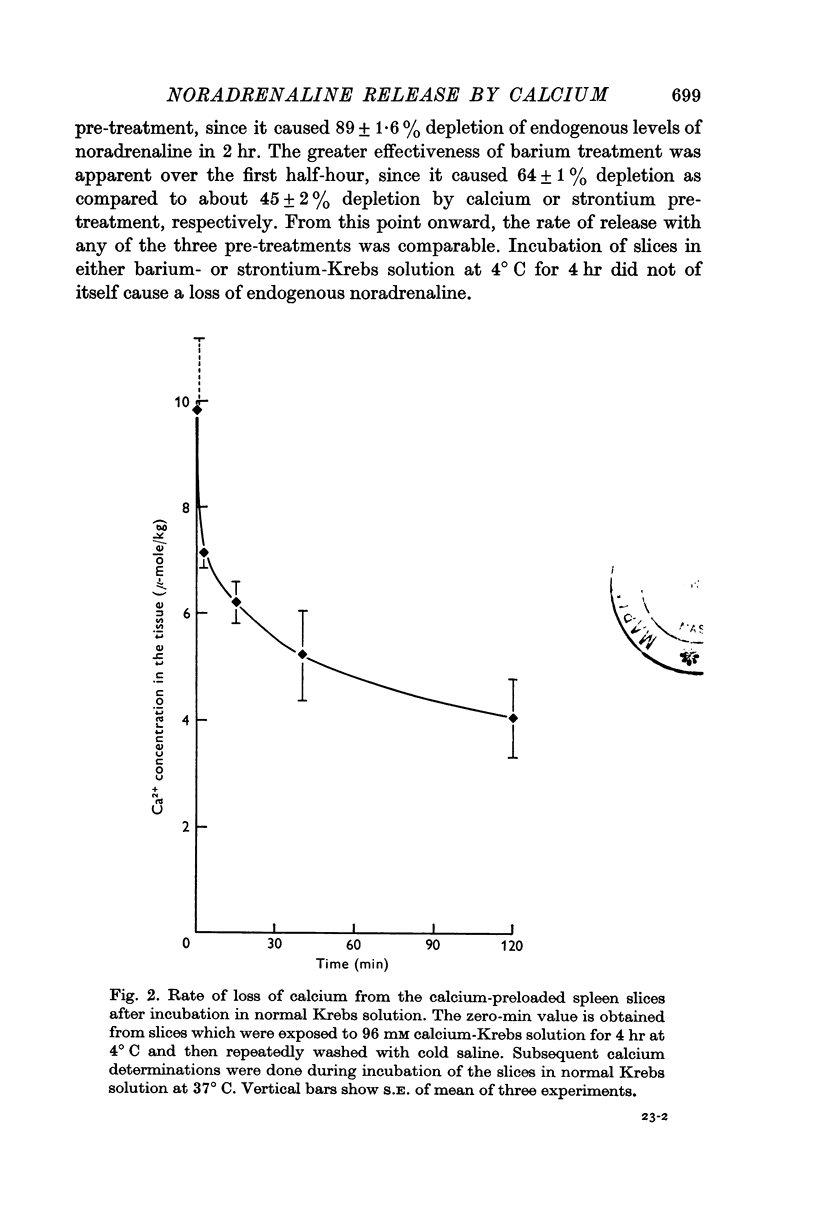
4. Tissue calcium concentration of spleen slices was 0·68 m-mole/kg. Pre-treatment of slices with normal or 96 mM calcium-Krebs solution for 4 hr at 4° C increased the calcium concentration to 2·57 and 9·9 m-mole/kg, respectively.
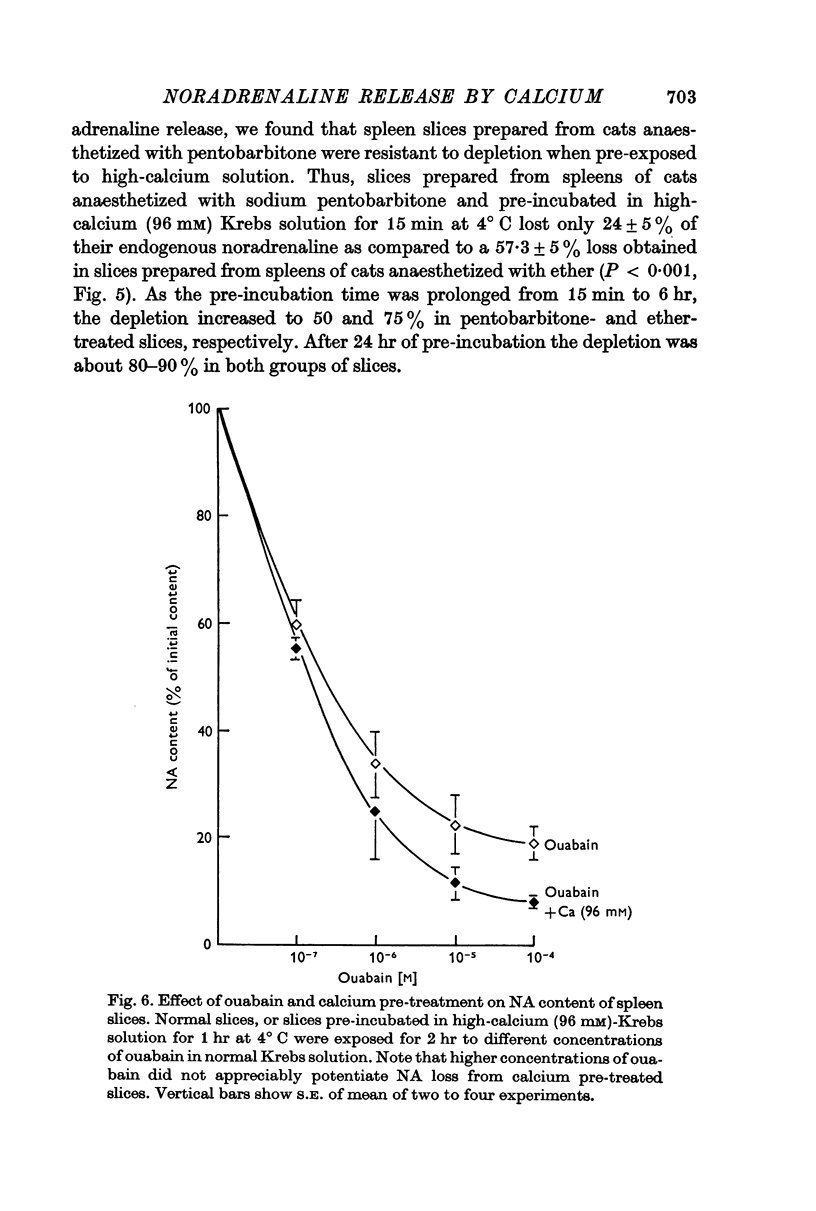
5. Ouabain, which caused a dose-dependent release of noradrenaline, did not modify the release induced by calcium pre-treatment.
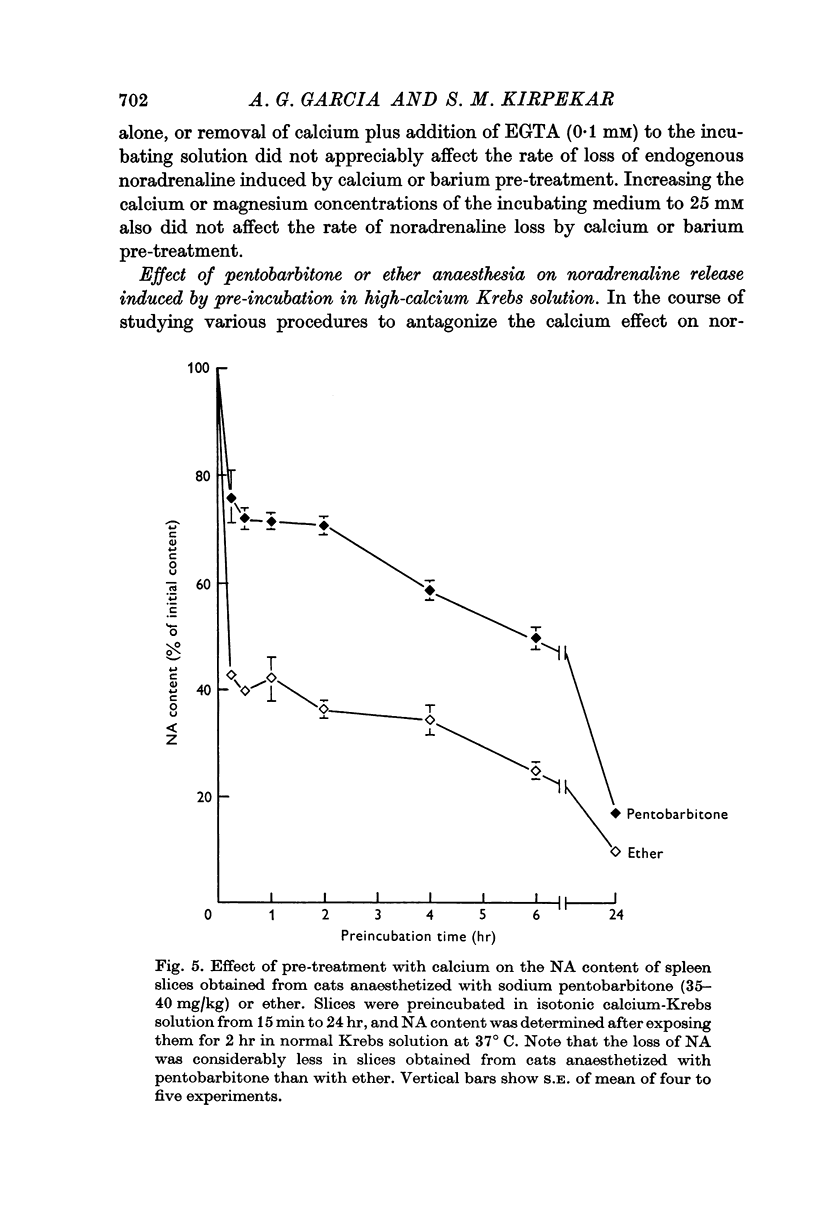
6. Spleen slices prepared from cats anaesthetized with sodium pentobarbitone instead of ether were resistant to noradrenaline depletion by calcium pre-treatment.
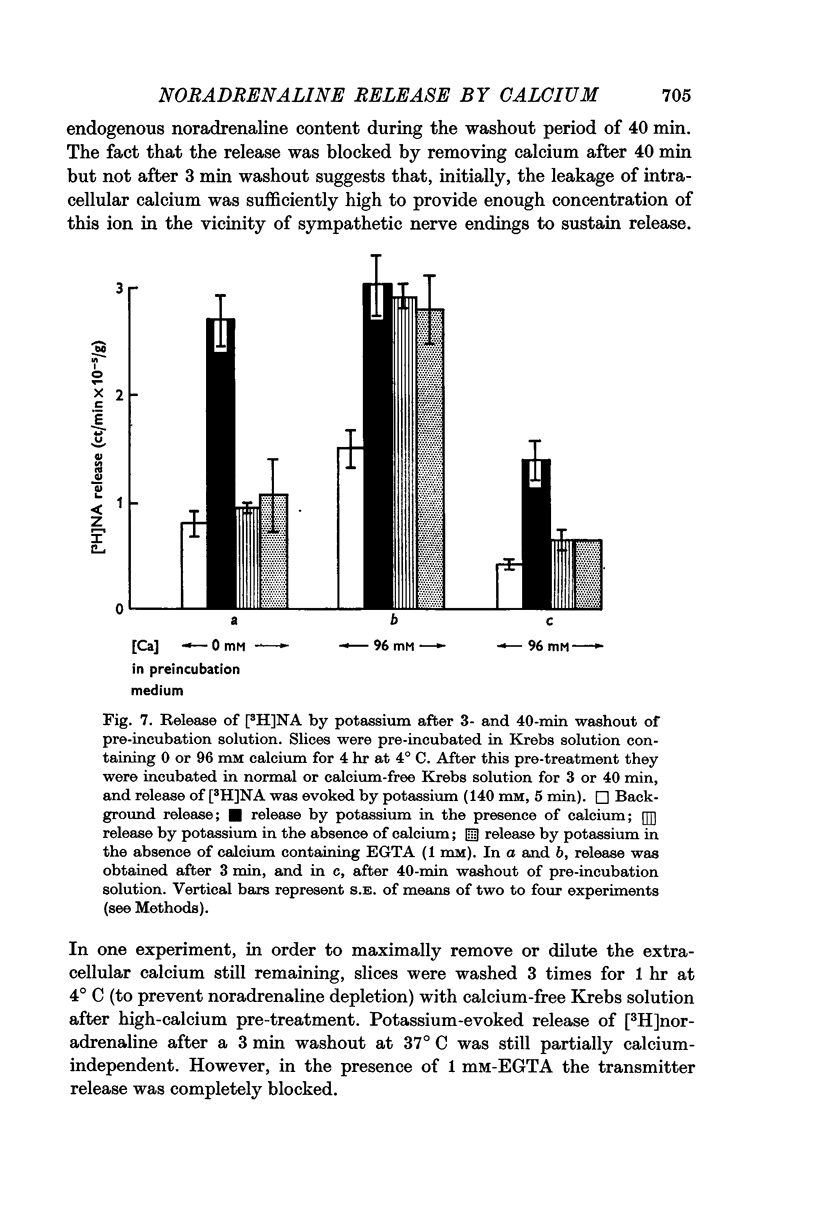
7. Evoked release of [3H]noradrenaline by high potassium from calcium-pre-treated slices did not occur in the absence of external calcium, even though the calcium pre-treatment enhanced the tissue concentration of this ion by nearly tenfold.
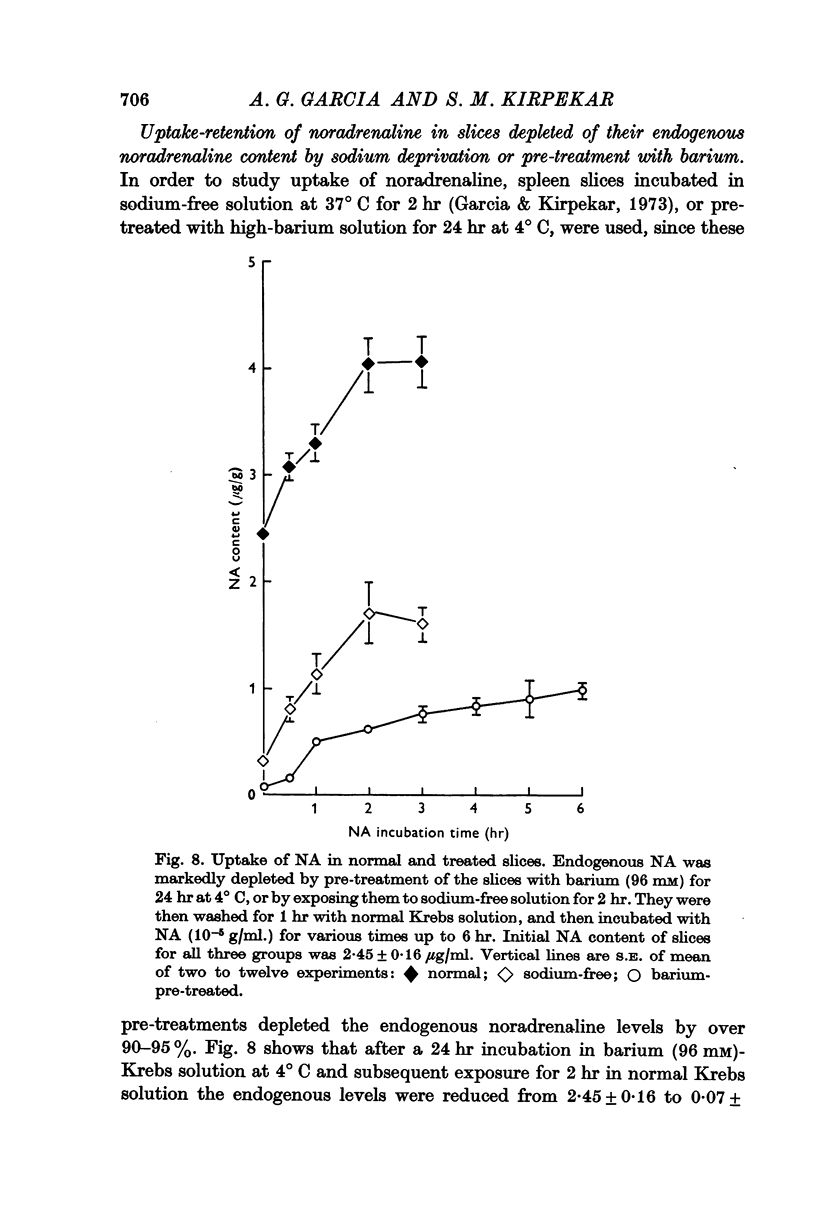
8. Net uptake of noradrenaline in normal and in treated slices whose noradrenaline content was severely reduced by barium pre-treatment or sodium withdrawal was comparable.
9. Specific activity of released and endogenous [3H]noradrenaline increased as the tissue stores of noradrenaline were reduced.
10. It is suggested that the spontaneous loss of tissue noradrenaline after pre-treatment with high-calcium solution was due to inhibition of sodium-potassium-activated ATPase by intracellular accumulation of calcium ions. Evidence is presented to suggest that vesicles depleted of their endogenous transmitter by pre-treatment with calcium, strontium or barium, or by sodium withdrawal, are re-used for the storage and release of exogenous noradrenaline.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DESMEDT J. E. Electrical activity and intracellular sodium concentration in frog muscle. J Physiol. 1953 Jul;121(1):191–205. doi: 10.1113/jphysiol.1953.sp004940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSMAN A., FURCHGOTT R. F. THE EFFECTS OF EXTERNAL CALCIUM CONCENTRATION ON THE DISTRIBUTION AND EXCHANGE OF CALCIUM IN RESTING AND BEATING GUINEA-PIG AURICLES. J Pharmacol Exp Ther. 1964 Jan;143:107–119. [PubMed] [Google Scholar]
- Garcia A. G., Kirpekar S. M. Release of noradrenaline from the cat spleen by sodium deprivation. Br J Pharmacol. 1973 Apr;47(4):729–747. doi: 10.1111/j.1476-5381.1973.tb08200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINGSLEY G. R., ROBNETT O. Further studies on a new dye method for the direct photometric determination of calcium. Am J Clin Pathol. 1958 Feb;29(2):171–175. doi: 10.1093/ajcp/29.2_ts.171. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Puig M., Wakade A. R. Modification of the evoked release of noradrenaline from the perfused cat spleen by various ions and agents. J Physiol. 1972 Mar;221(3):601–615. doi: 10.1113/jphysiol.1972.sp009770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Yamamoto H. Release of noradrenaline and dopamine by nerve stimulation in the cat spleen perfused with 3 H-dopamine. Br J Pharmacol. 1971 Sep;43(1):86–96. doi: 10.1111/j.1476-5381.1971.tb07159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G., Szeto J. Effect of sodium pentobarbital on calcium in mammalian heart muscle. Am J Physiol. 1972 Feb;222(2):339–344. doi: 10.1152/ajplegacy.1972.222.2.339. [DOI] [PubMed] [Google Scholar]
- Rubin R. P. The role of calcium in the release of neurotransmitter substances and hormones. Pharmacol Rev. 1970 Sep;22(3):389–428. [PubMed] [Google Scholar]
- SKOU J. C. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957 Feb;23(2):394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- Shellenberger M. K., Gordon J. H. A rapid, simplified procedure for simultaneous assay of norepinephrine, dopamine, and 5-hydroxytryptamine from discrete brain areas. Anal Biochem. 1971 Feb;39(2):356–372. doi: 10.1016/0003-2697(71)90426-x. [DOI] [PubMed] [Google Scholar]


