Abstract
1. The excitability of the geniculo-striate pathway during a saccadic eye movement was studied in alert cats with chronically implanted electrodes. Excitability was assessed by the amplitude of post-synaptic components of field responses in both lateral geniculate nucleus and visual cortex to electrical stimulation of the optic chiasm. Modifications in amplitude were evaluated during the period following eye movements, by triggering a stimulator from potential shifts in the electrooculograms and altering delays in the stimulus pulse.
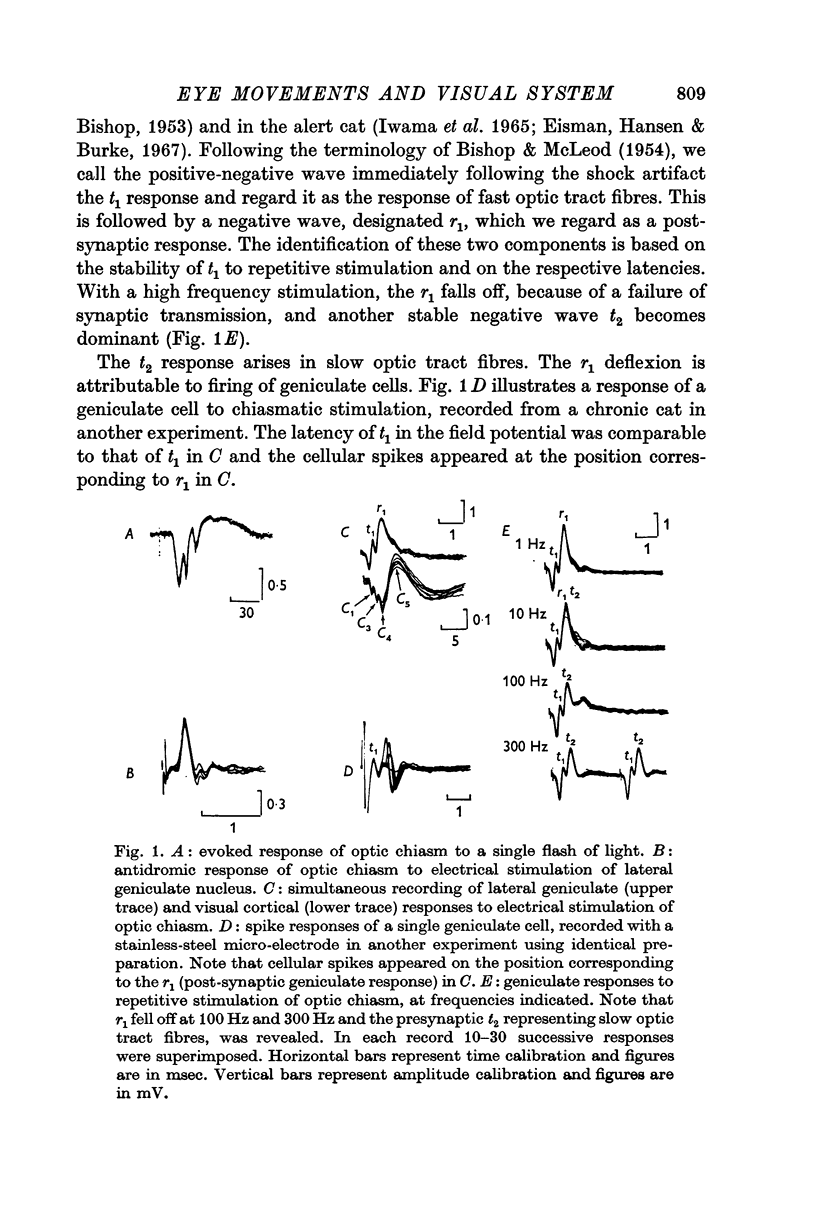
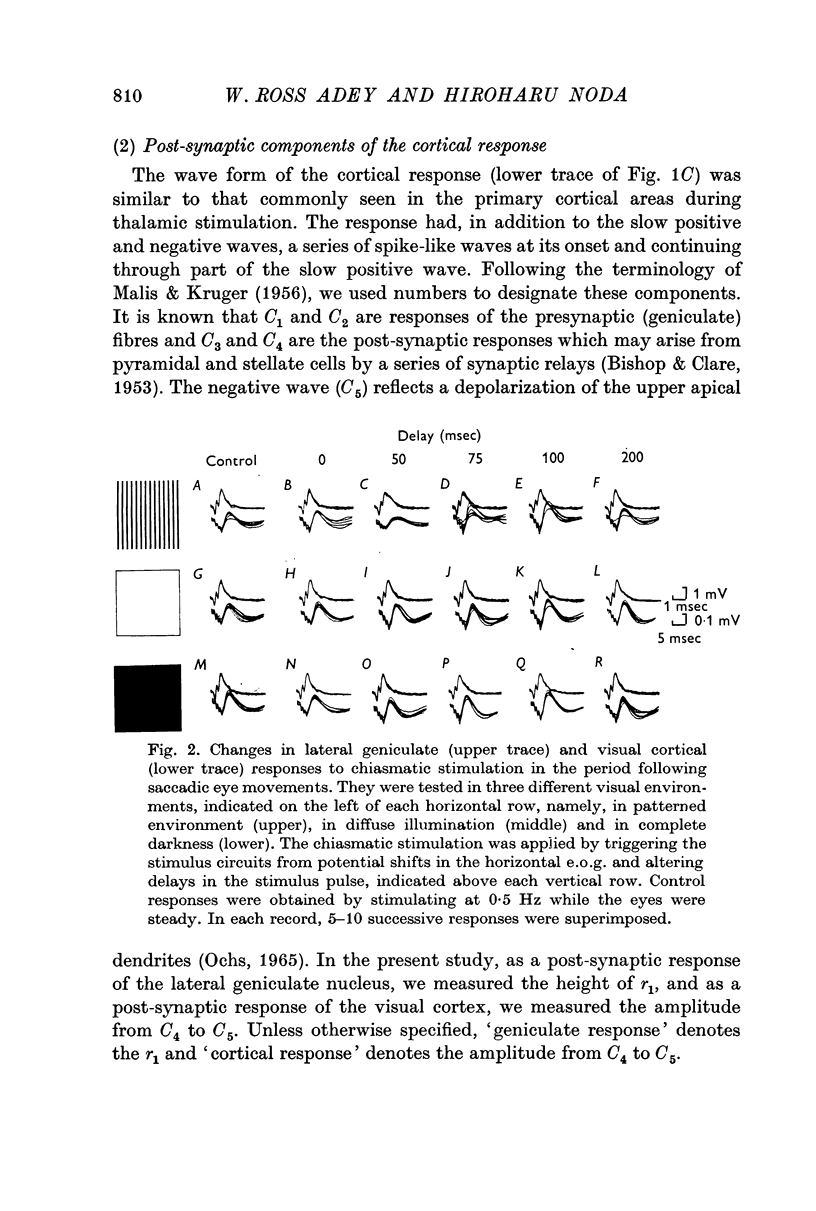
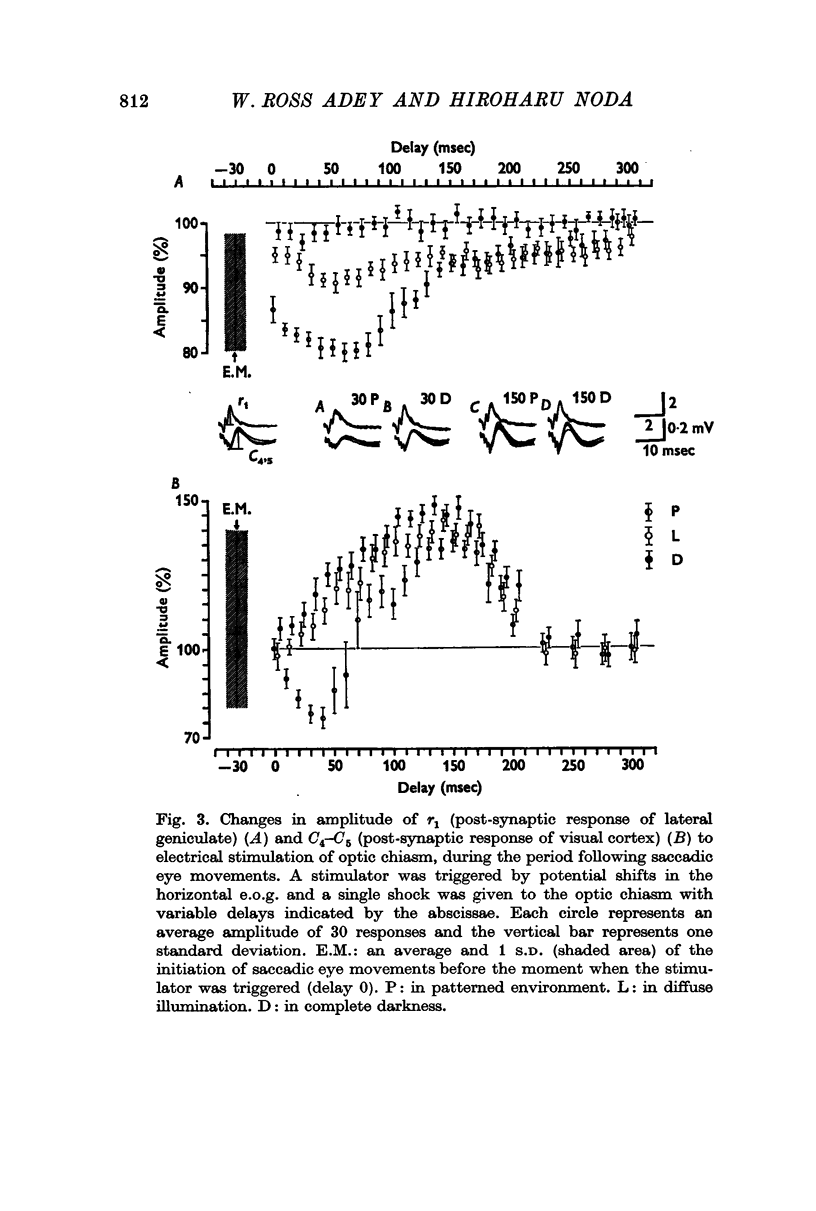
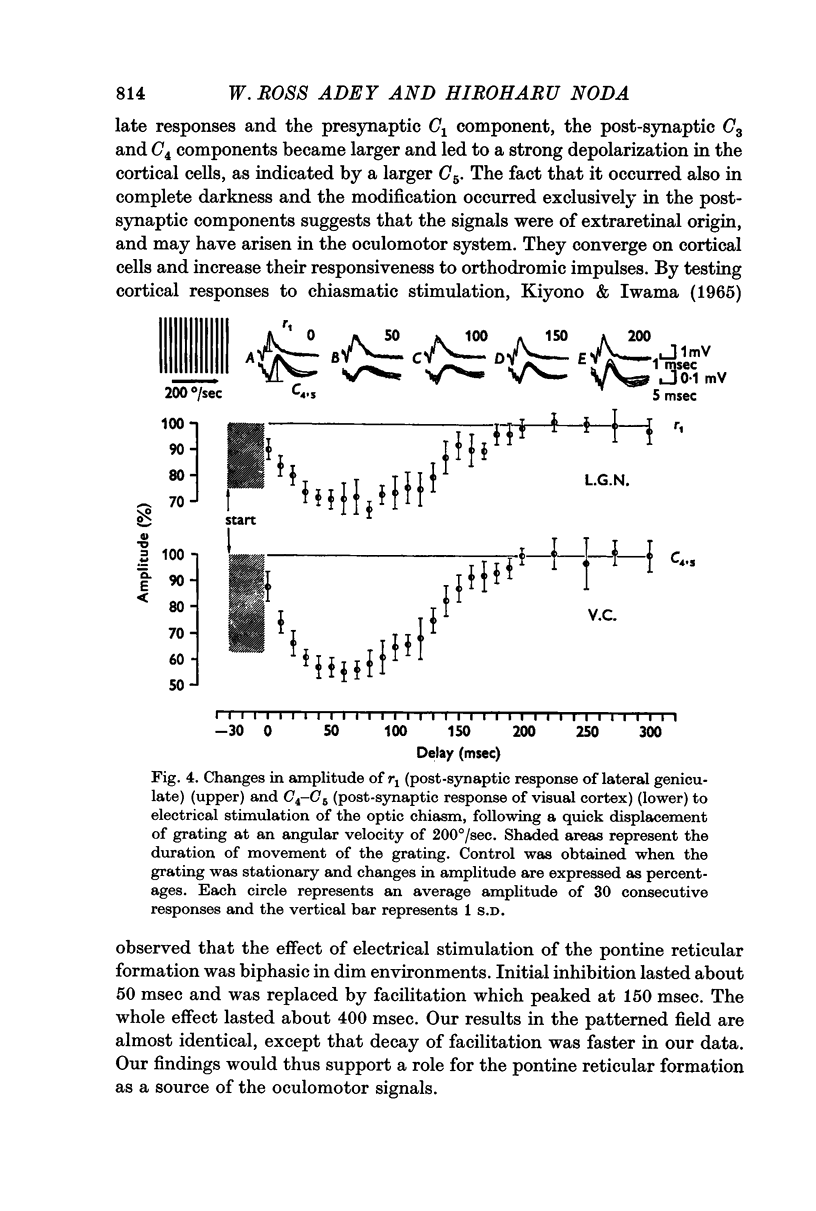
2. The post-synaptic component of the geniculate response was markedly depressed for about 150 msec, reaching a trough at approximately 100 msec after the initiation of an eye movement. This effect was dependent on the visual environment and was not observed in complete darkness. A similar depression occurred when the visual field was abruptly moved by retinal impulses generated by a quick displacement of the image of the visual world associated with an eye movement. The depression reflected a reduction of cellular discharge to the orthodromic volley and hence a suppression of the transmission of visual information through the lateral geniculate nucleus. This may be a mechanism for saccadic suppression.
3. The post-synaptic components of the cortical response were enhanced for about 200 msec, reaching a peak at approximately 150 msec after the initiation of an eye movement. Although this facilitation occurred also in complete darkness, it did not occur when the visual field was abruptly shifted while the eyes were stationary. The fact that it occurred with eye movements and exclusively in the post-synaptic components suggests that it was caused by signals from a system closely related to eye movements. This may be a manifestation of the corollary mechanism.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP G. H., CLARE M. Sequence of events in optic cortex response to volleys of impulses in the radiation. J Neurophysiol. 1953 Sep;16(5):490–498. doi: 10.1152/jn.1953.16.5.490. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O., McLEOD J. G. Nature of potentials associated with synaptic transmission in lateral geniculate of cat. J Neurophysiol. 1954 Jul;17(4):387–414. doi: 10.1152/jn.1954.17.4.387. [DOI] [PubMed] [Google Scholar]
- BISHOP P. O. Synaptic transmission; an analysis of the electrical activity of the lateral geniculate nucleus in the cat after optic nerve stimulation. Proc R Soc Lond B Biol Sci. 1953 Jul 15;141(904):362–392. doi: 10.1098/rspb.1953.0048. [DOI] [PubMed] [Google Scholar]
- BIZZI E., BROOKS D. C. FUNCTIONAL CONNECTIONS BETWEEN PONTINE RETICULAR FORMATION AND LATERAL GENICULATE NUCLEUS DURING DEEP SLEEP. Arch Ital Biol. 1963 Oct 5;101:666–680. [PubMed] [Google Scholar]
- Beeler G. W., Jr Visual threshold changes resulting from spontaneous saccadic eye movements. Vision Res. 1967 Sep;7(9):769–775. doi: 10.1016/0042-6989(67)90039-9. [DOI] [PubMed] [Google Scholar]
- Bizzi E. Changes in the orthodromic and antidromic response of optic tract during the eye movements of sleep. J Neurophysiol. 1966 Sep;29(5):861–870. doi: 10.1152/jn.1966.29.5.861. [DOI] [PubMed] [Google Scholar]
- Bizzi E. Discharge patterns of single geniculate neurons during the rapid eye movements of sleep. J Neurophysiol. 1966 Nov;29(6):1087–1095. doi: 10.1152/jn.1966.29.6.1087. [DOI] [PubMed] [Google Scholar]
- Büttner U., Fuchs A. F. Influence of saccadic eye movements on unit activity in simian lateral geniculate and pregeniculate nuclei. J Neurophysiol. 1973 Jan;36(1):127–141. doi: 10.1152/jn.1973.36.1.127. [DOI] [PubMed] [Google Scholar]
- CHANG H. T. Cortical response to stimulation of lateral geniculate body and the potentiation thereof by continuous illumination of retina. J Neurophysiol. 1952 Jan;15(1):5–26. doi: 10.1152/jn.1952.15.1.5. [DOI] [PubMed] [Google Scholar]
- Chase R., Kalil R. E. Suppression of visual evoked responses to flashes and pattern shifts during voluntary saccades. Vision Res. 1972 Feb;12(2):215–220. doi: 10.1016/0042-6989(72)90112-5. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H. Changes in visual evoked responses during the fast phase of optokinetic nystagmus in the rabbit. Vision Res. 1969 Jul;9(7):803–814. doi: 10.1016/0042-6989(69)90016-9. [DOI] [PubMed] [Google Scholar]
- Corazza R., Lombroso C. Phasic changes in level of the integrated dark discharge during spontaneous saccades. Electroencephalogr Clin Neurophysiol. 1970 Sep;29(3):323–324. [PubMed] [Google Scholar]
- Eisman J. A., Hansen S. M., Burke W. Synaptic responsiveness in the lateral geniculate nucleus of the alert cat. Vision Res. 1967 May;7(5):385–399. doi: 10.1016/0042-6989(67)90047-8. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E. G., Vaughan H. G., Jr, Valenstein E. Inhibition of visual evoked responses to patterned stimuli during voluntary eye movements. Electroencephalogr Clin Neurophysiol. 1967 Mar;22(3):204–209. doi: 10.1016/0013-4694(67)90222-2. [DOI] [PubMed] [Google Scholar]
- Hansen S. M., Bruce I. S., Burke W. The effect of retinal illumination and retinal blockade on synaptic transmission in the lateral geniculate nucleus of the cat. Vision Res. 1967 May;7(5):401–414. doi: 10.1016/0042-6989(67)90048-x. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Functional organization of the periphery effect in retinal ganglion cells. Vision Res. 1972 Nov;12(11):1857–1879. doi: 10.1016/0042-6989(72)90076-4. [DOI] [PubMed] [Google Scholar]
- JOUVET M., MICHEL F. Corrélations électromyographique du sommeil chez le chat décortiqué et mésencéphalique chronique. C R Seances Soc Biol Fil. 1959;153(3):422–425. [PubMed] [Google Scholar]
- Jeannerod M., Putkonen P. T. Lateral geniculate unit activity and eye movements: saccade-locked changes in dark and in light. Exp Brain Res. 1971 Nov 30;13(5):533–546. doi: 10.1007/BF00234284. [DOI] [PubMed] [Google Scholar]
- Kawamura H., Marchiafava P. L. Excitability changes along visual pathways during eye tracking movements. Arch Ital Biol. 1968 May;106(2):141–156. [PubMed] [Google Scholar]
- Kiyono S., Iwama K. Phasic activity of cat's cerebral cortex during paradoxical sleep. Med J Osaka Univ. 1965 Dec;16(3):149–159. [PubMed] [Google Scholar]
- MALIS L. I., KRUGER L. Multiple responses and excitability of cat's visual cortex. J Neurophysiol. 1956 Mar;19(2):172–186. doi: 10.1152/jn.1956.19.2.172. [DOI] [PubMed] [Google Scholar]
- MCILWAIN J. T. RECEPTIVE FIELDS OF OPTIC TRACT AXONS AND LATERAL GENICULATE CELLS: PERIPHERAL EXTENT AND BARBITURATE SENSITIVITY. J Neurophysiol. 1964 Nov;27:1154–1173. doi: 10.1152/jn.1964.27.6.1154. [DOI] [PubMed] [Google Scholar]
- MOURET J., JEANNEROD M., JOUVET M. [Electrical activity of the visual system during the paradoxical phase of sleep in the cat]. J Physiol (Paris) 1963;55:305–306. [PubMed] [Google Scholar]
- Mackay D. M. Elevation of visual threshold by displacement of retinal image. Nature. 1970 Jan 3;225(5227):90–92. doi: 10.1038/225090a0. [DOI] [PubMed] [Google Scholar]
- Malcolm L. J., Bruce I. S., Burke W. Excitability of the lateral geniculate nucleus in the alert, non-alert and sleeping cat. Exp Brain Res. 1970;10(3):283–297. doi: 10.1007/BF00235052. [DOI] [PubMed] [Google Scholar]
- Michael J. A., Stark L. Electrophysiological correlates of saccadic suppression. Exp Neurol. 1967 Feb;17(2):233–246. doi: 10.1016/0014-4886(67)90148-3. [DOI] [PubMed] [Google Scholar]
- Michael J. A., Stark L. Interactions between eye movements and the visually evoked response in the cat. Electroencephalogr Clin Neurophysiol. 1966 Nov;21(5):478–488. doi: 10.1016/0013-4694(66)90196-9. [DOI] [PubMed] [Google Scholar]
- Mitrani L., Yakimoff N., Mateeff S. Dependence of visual suppression on the angular size of voluntary saccadic eye movements. Vision Res. 1970 May;10(5):411–415. doi: 10.1016/0042-6989(70)90121-5. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Schwartz K. S. Lateral geniculate and occipital cortex spikes with eye movements in awake and sleeping cats: temporal and functional correlations. Exp Neurol. 1972 May;35(2):300–304. doi: 10.1016/0014-4886(72)90156-2. [DOI] [PubMed] [Google Scholar]
- Noda H., Freeman R. B., Jr, Creutzfeldt O. D. Neuronal correlates of eye movements in the visual cortex of the cat. Science. 1972 Feb 11;175(4022):661–664. doi: 10.1126/science.175.4022.661. [DOI] [PubMed] [Google Scholar]
- Ogawa T. Suppression of trans-geniculate transmission during the rapid phase of caloric nystagmus in the alert squirrel monkey. Brain Res. 1972 Mar 10;38(1):211–216. doi: 10.1016/0006-8993(72)90606-3. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. Neural basis of the spontaneous optokinetic response produced by visual inversion. J Comp Physiol Psychol. 1950 Dec;43(6):482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Sakakura H. Spontaneous and evoked unitary activities of cat lateral geniculate neurons in sleep and wakefulness. Jpn J Physiol. 1968 Feb 15;18(1):23–42. doi: 10.2170/jjphysiol.18.23. [DOI] [PubMed] [Google Scholar]
- Starr A., Angel R., Yeates H. Visual suppression during smooth following and saccadic eye movements. Vision Res. 1969 Jan;9(1):195–197. doi: 10.1016/0042-6989(69)90042-x. [DOI] [PubMed] [Google Scholar]
- Stryker M., Blakemore C. Saccadic and disjunctive eye movements in cats. Vision Res. 1972 Dec;12(12):2005–2013. doi: 10.1016/0042-6989(72)90054-5. [DOI] [PubMed] [Google Scholar]
- VOLKMANN F. C. Vision during voluntary saccadic eye movements. J Opt Soc Am. 1962 May;52:571–578. doi: 10.1364/josa.52.000571. [DOI] [PubMed] [Google Scholar]
- Volkmann F. C., Schick A. M., Riggs L. A. Time course of visual inhibition during voluntary saccades. J Opt Soc Am. 1968 Apr;58(4):562–569. doi: 10.1364/josa.58.000562. [DOI] [PubMed] [Google Scholar]
- Wurtz R. H., Goldberg M. E. Superior colliculus cell responses related to eye movements in awake monkeys. Science. 1971 Jan 8;171(3966):82–84. doi: 10.1126/science.171.3966.82. [DOI] [PubMed] [Google Scholar]
- Zuber B. L., Stark L. Saccadic suppression: elevation of visual threshold associated with saccadic eye movements. Exp Neurol. 1966 Sep;16(1):65–79. doi: 10.1016/0014-4886(66)90087-2. [DOI] [PubMed] [Google Scholar]


