Abstract
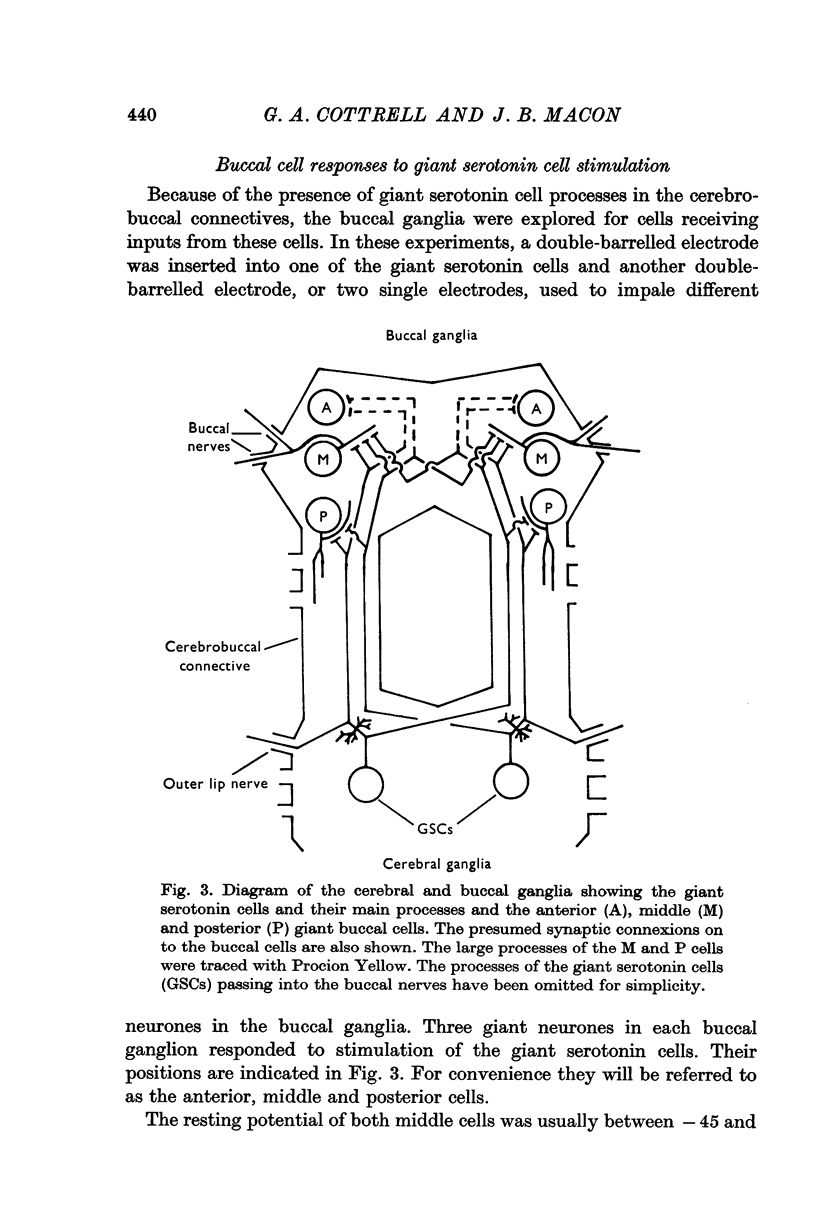
1. Each giant serotonin cell in Helix pomatia makes synaptic connexions with three non-amine-containing neurones: the anterior, middle and posterior buccal cells.
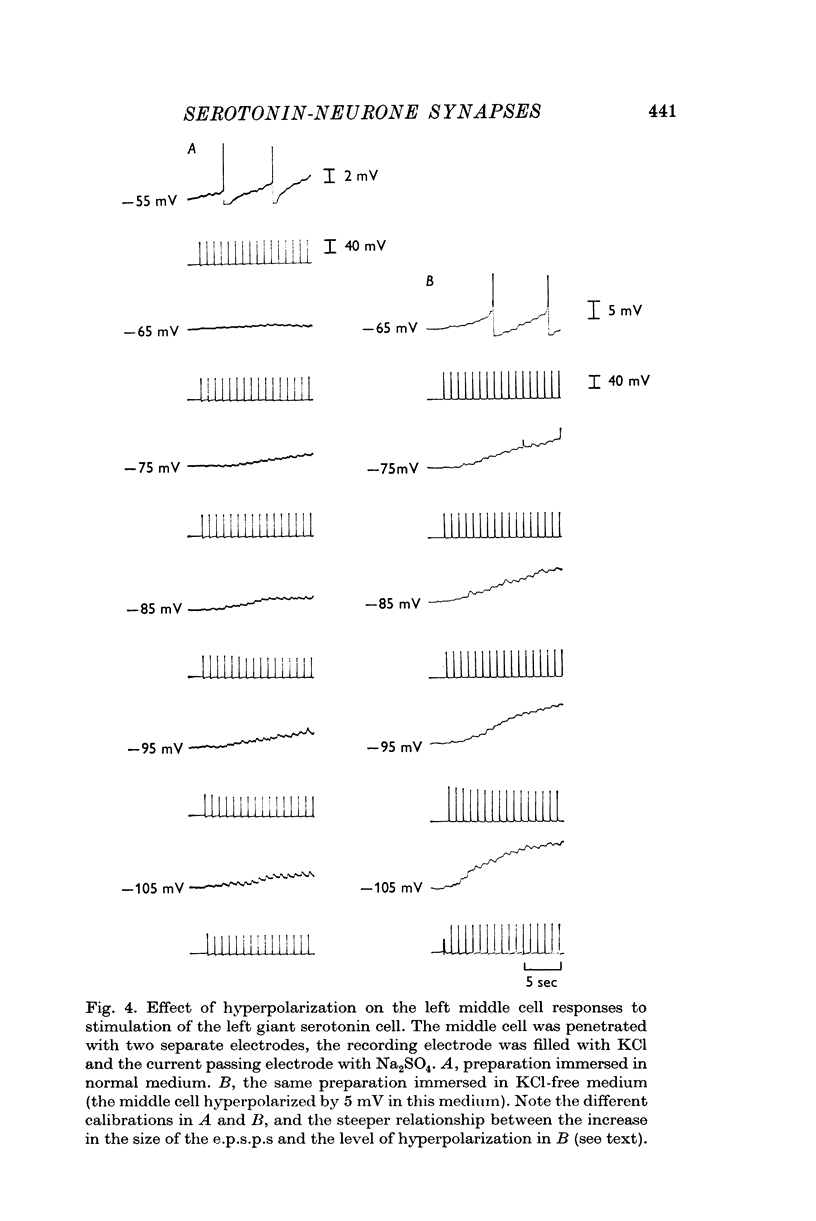
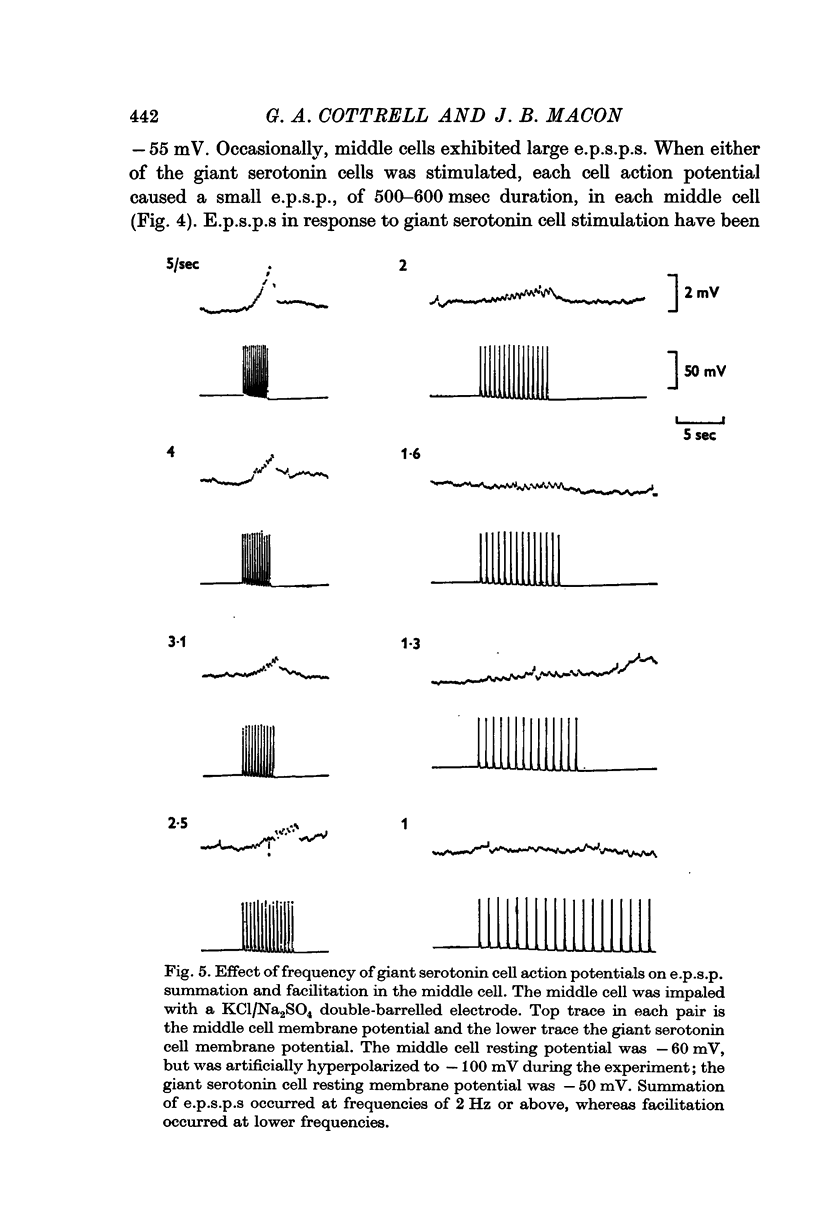
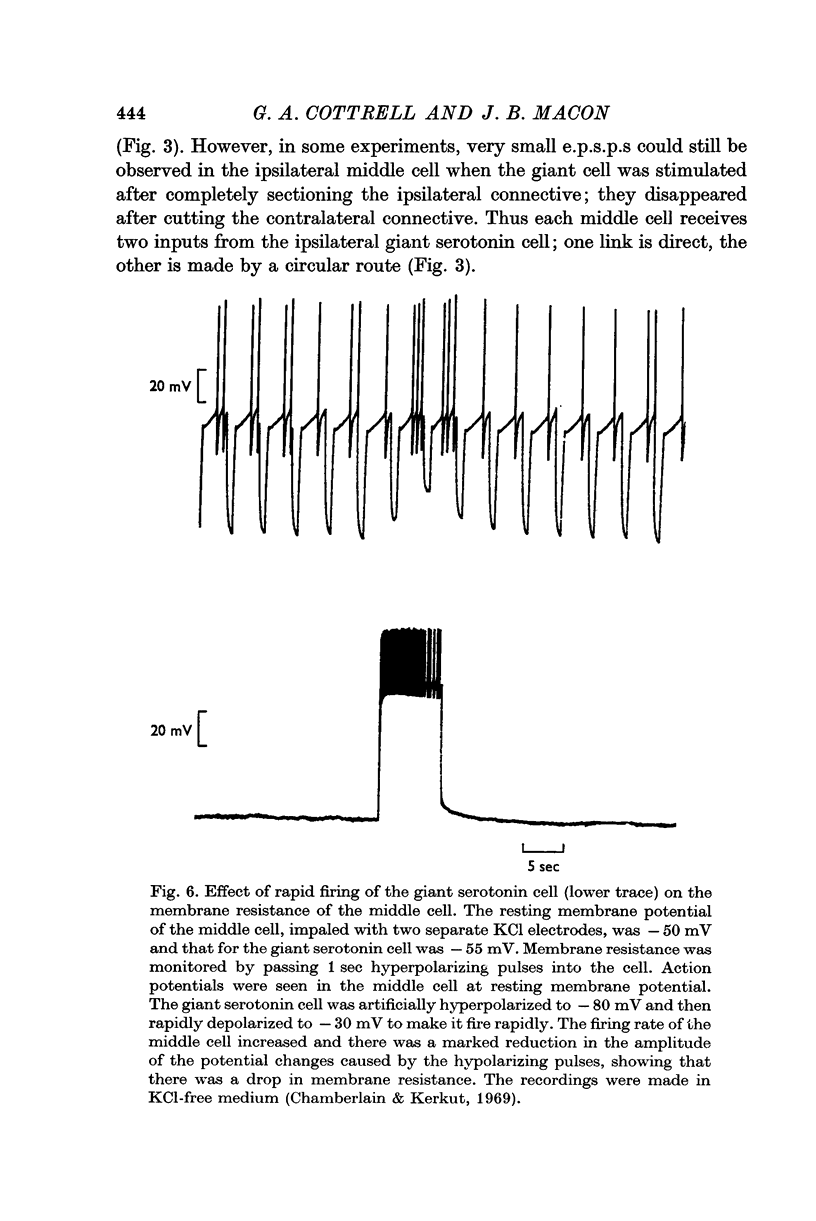
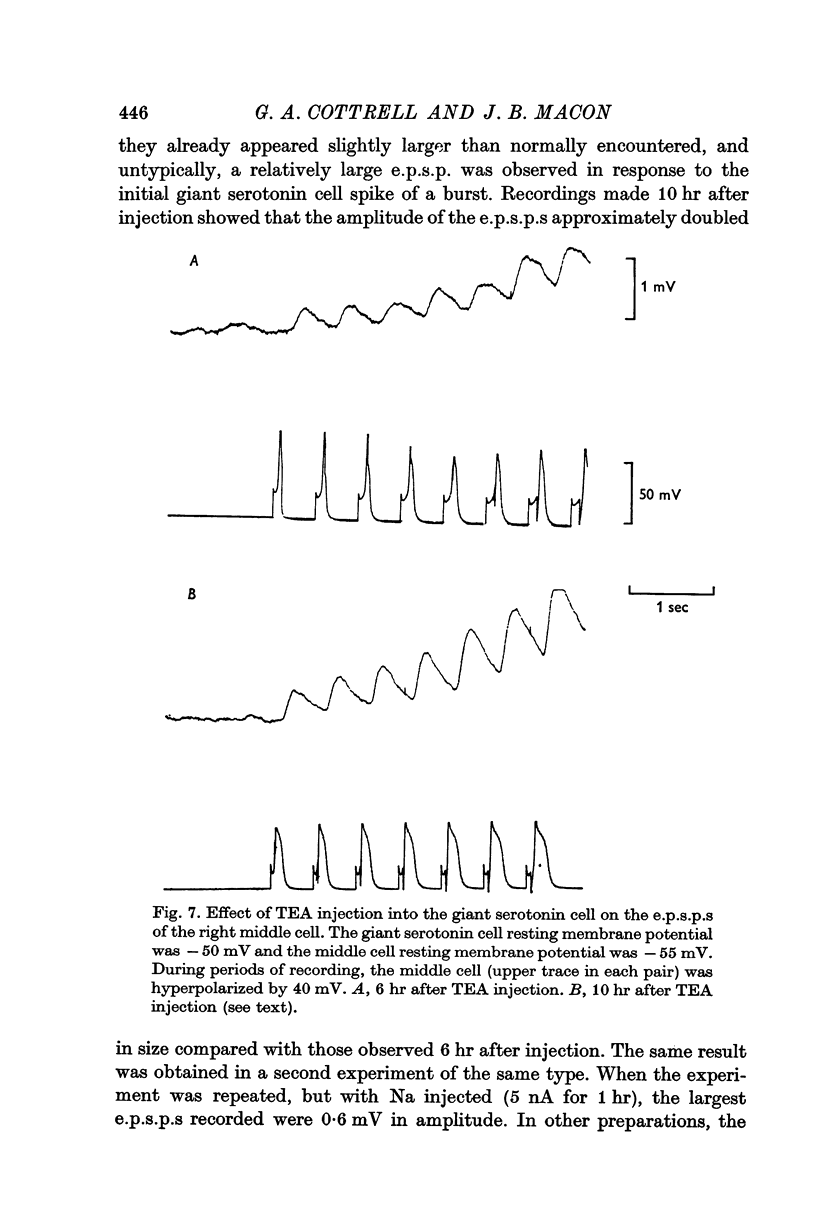
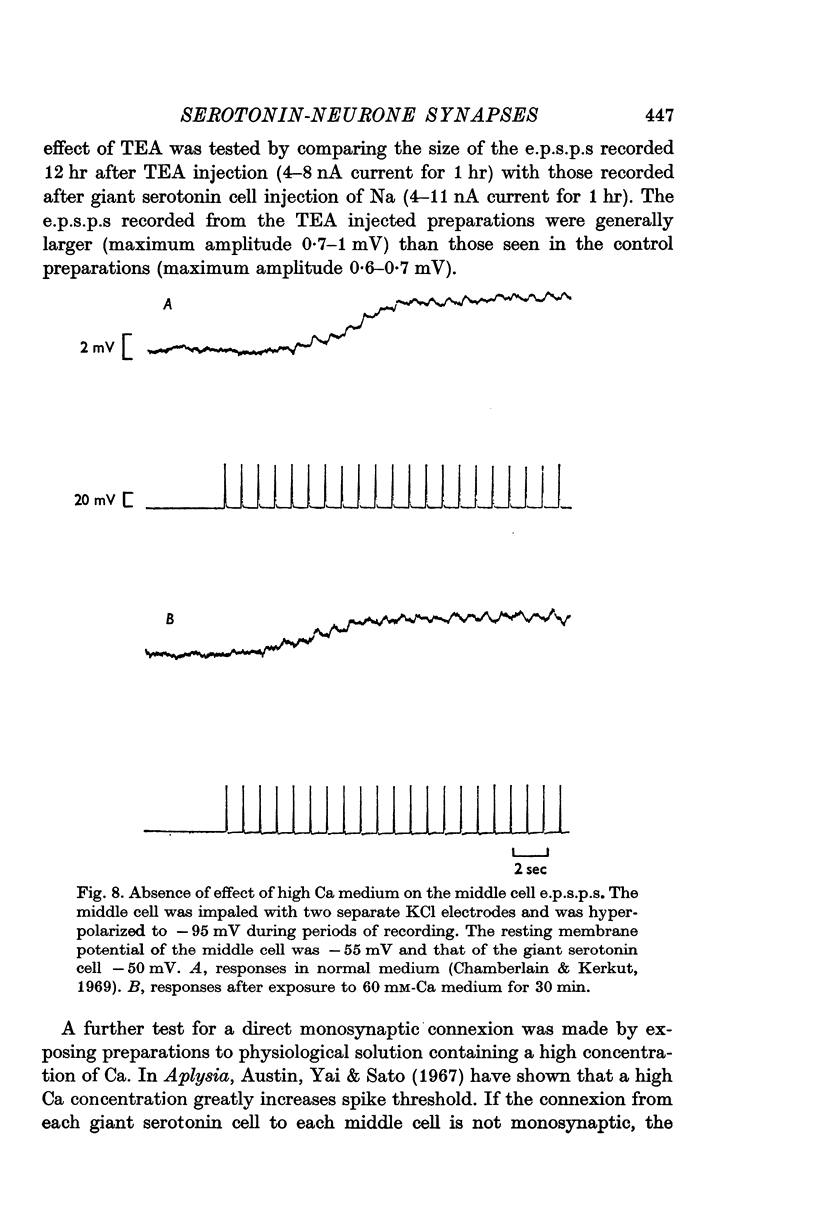
2. Individual e.p.s.p.s, of 500-600 msec duration, were observed in both left and right middle cells following each evoked giant serotonin cell action potential. They were facilitated with repetitive stimulation of the giant serotonin cells and summed to give rise to an action potential. The membrane resistance of the middle cells was reduced when the giant serotonin cells were stimulated to fire rapidly. Evidence is presented which suggests that the link between each giant serotonin cell and each middle cell is monosynaptic.
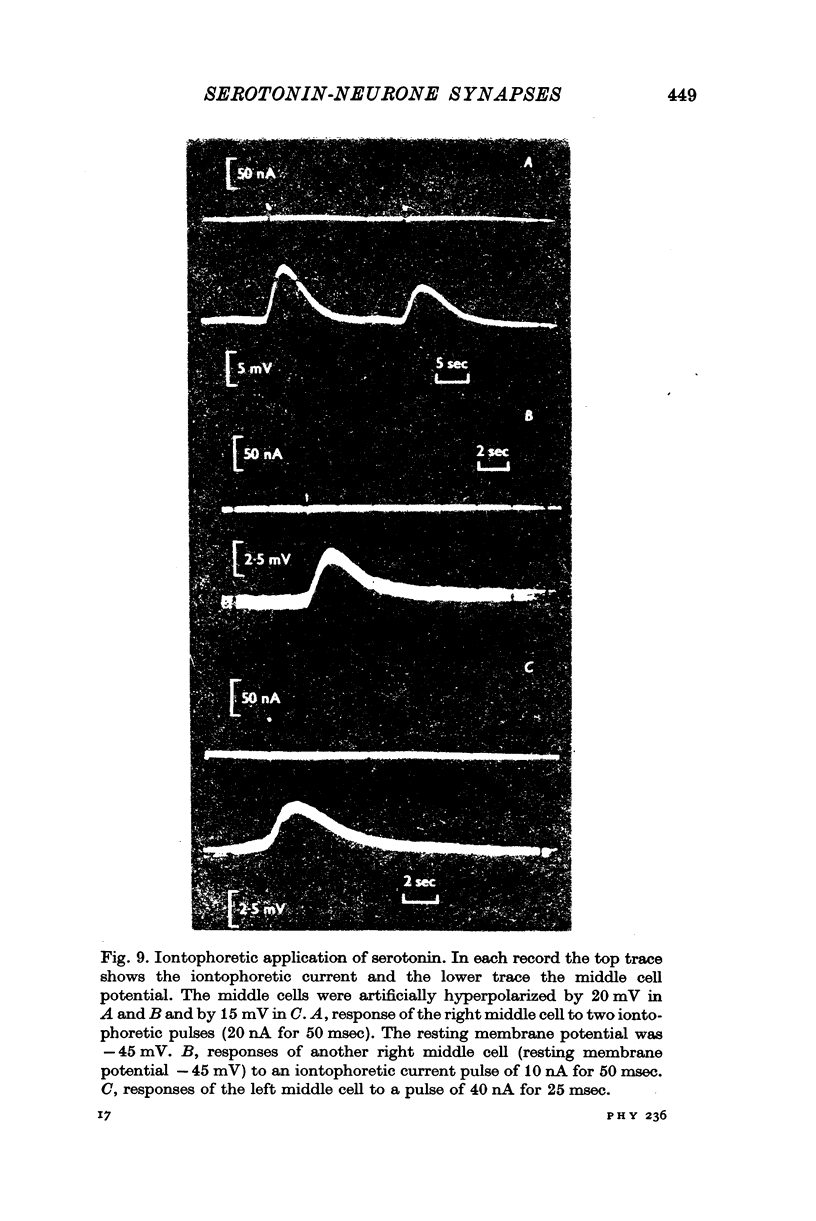
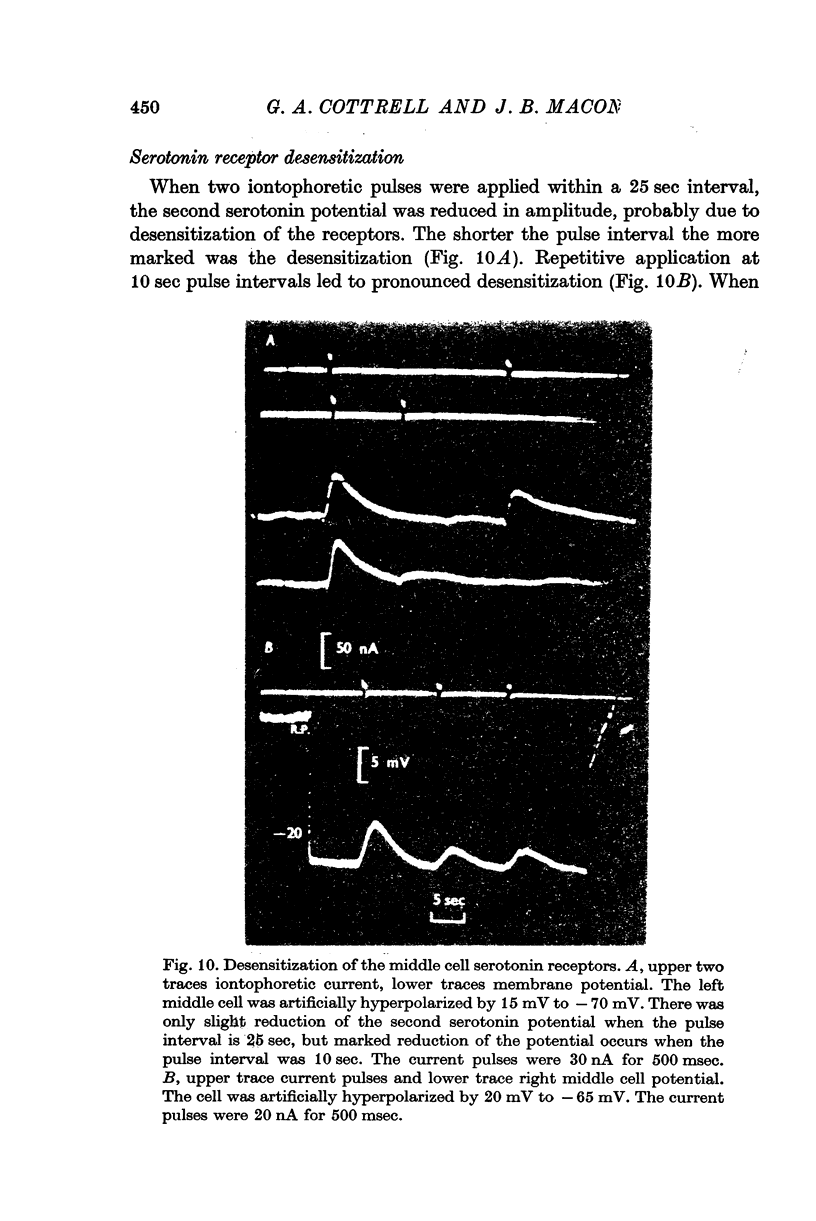
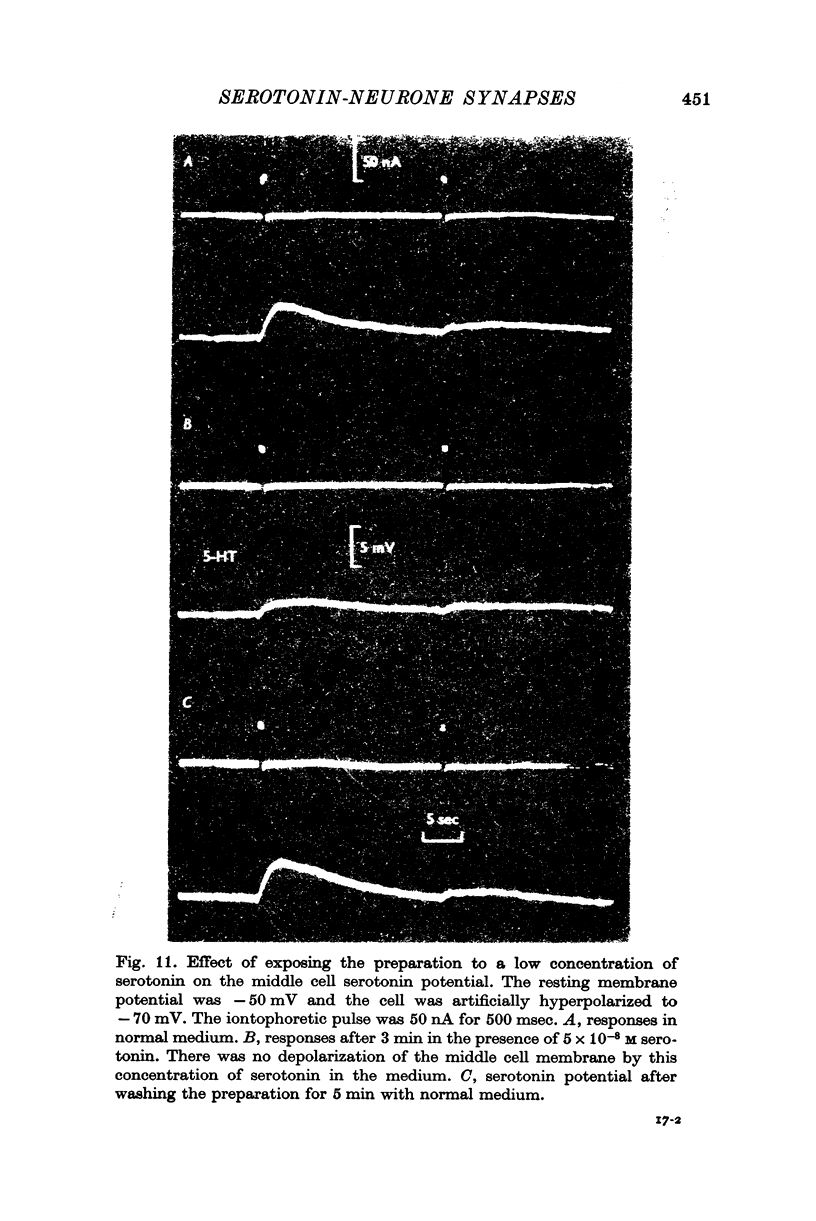
3. Iontophoretically applied serotonin produced a depolarizing potential change in the middle cell perikaryon; the response rapidly desensitized on repetitive application.
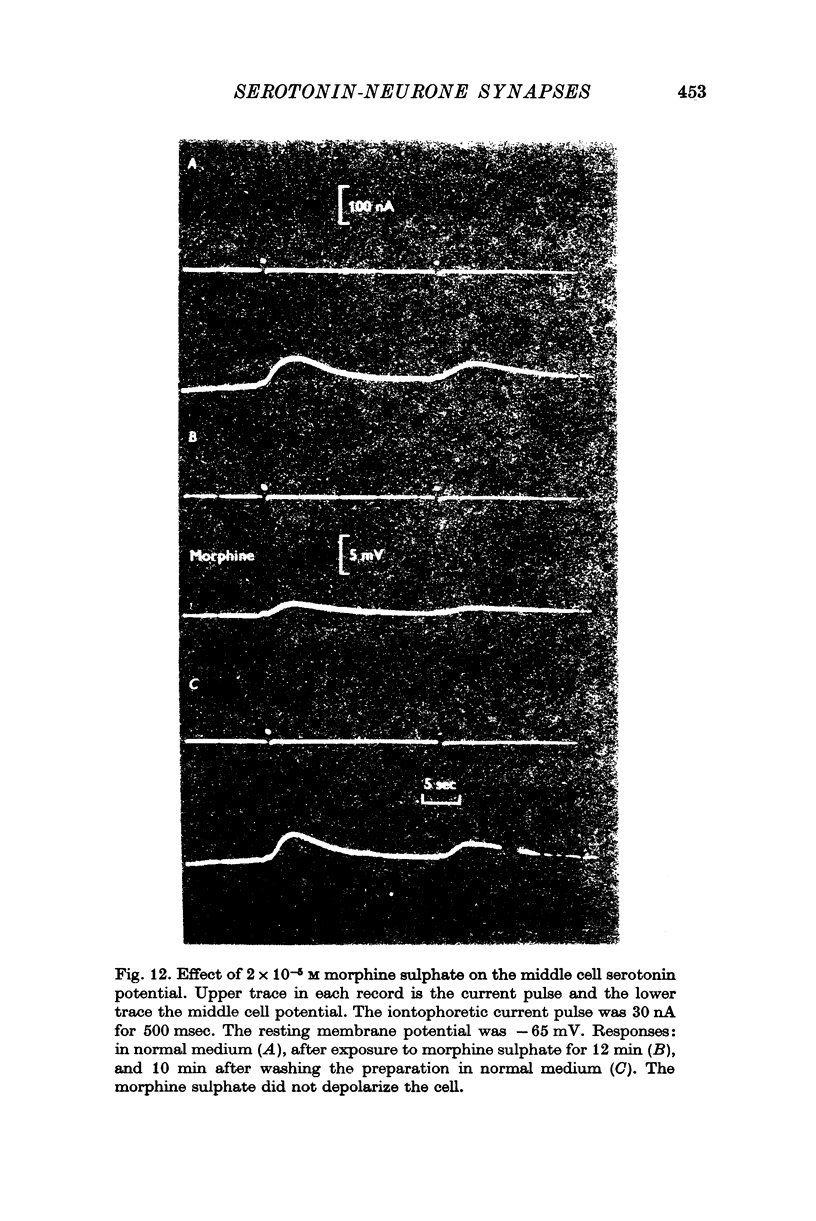
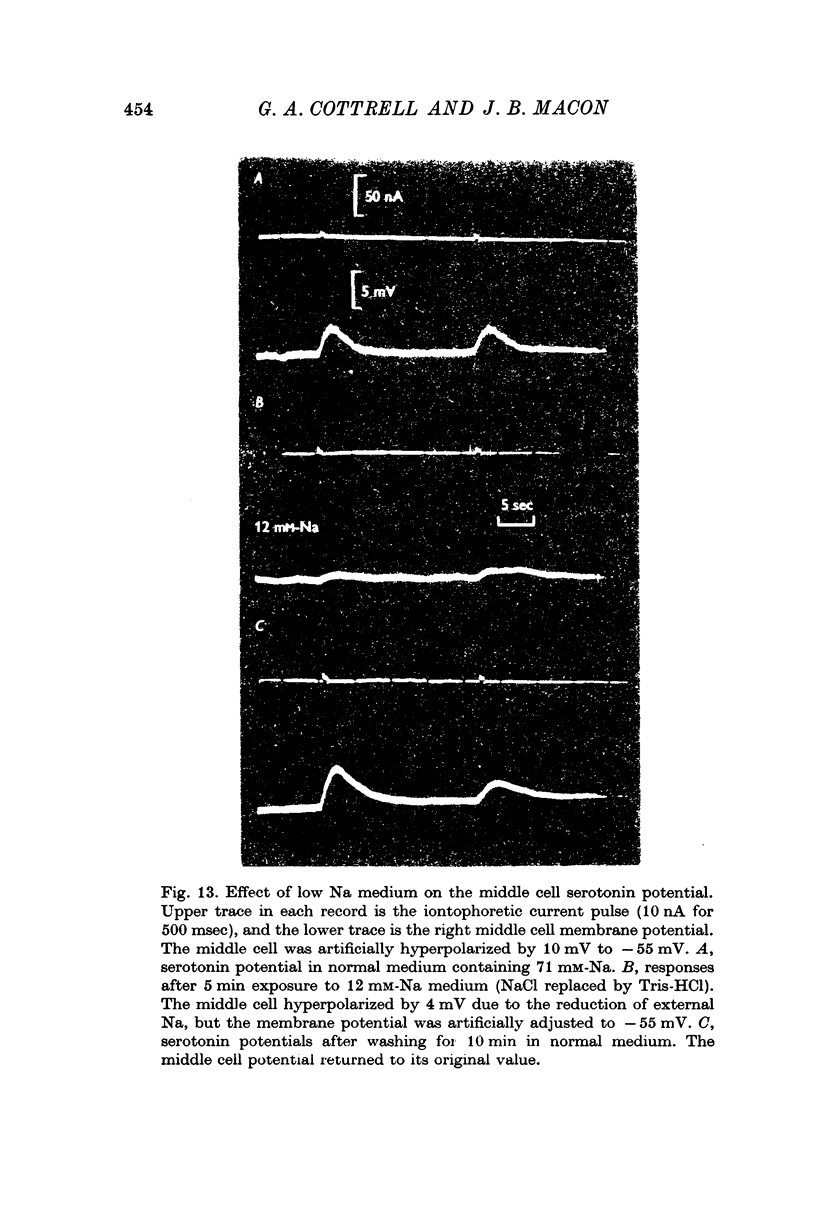
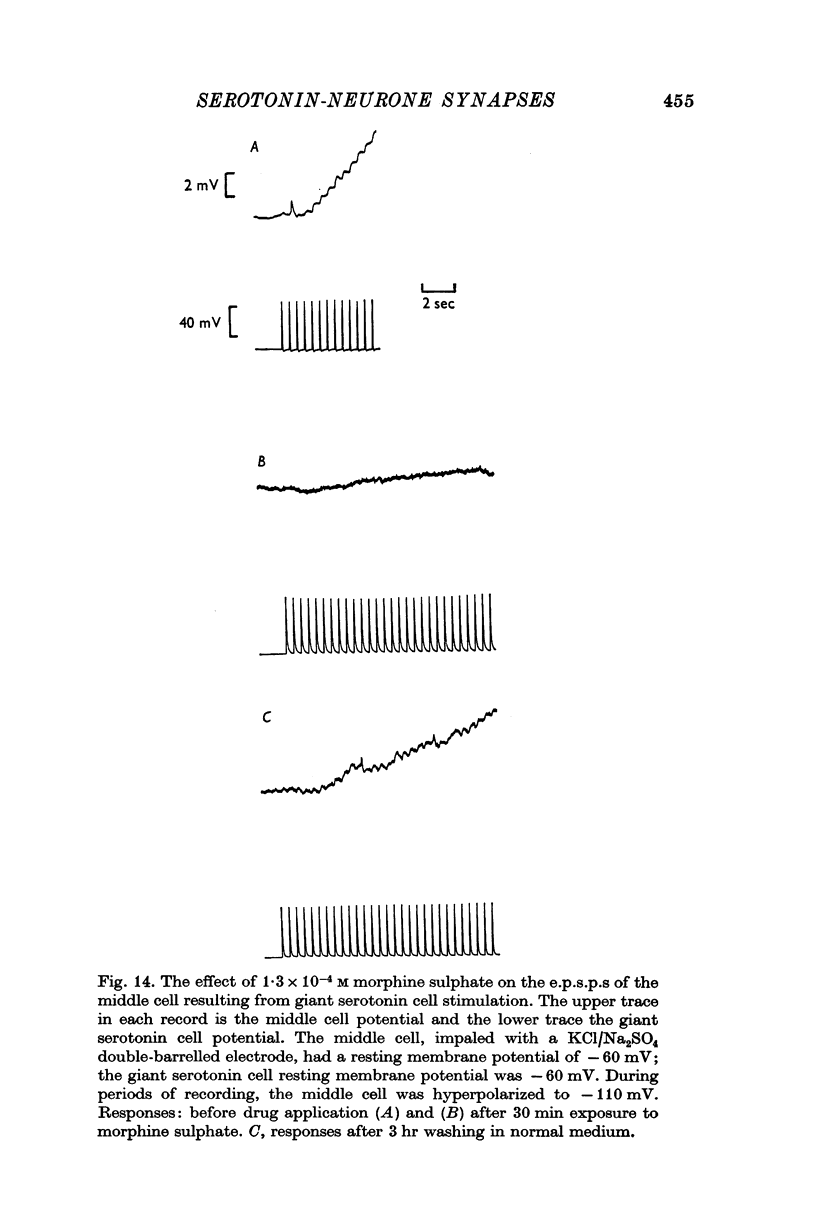
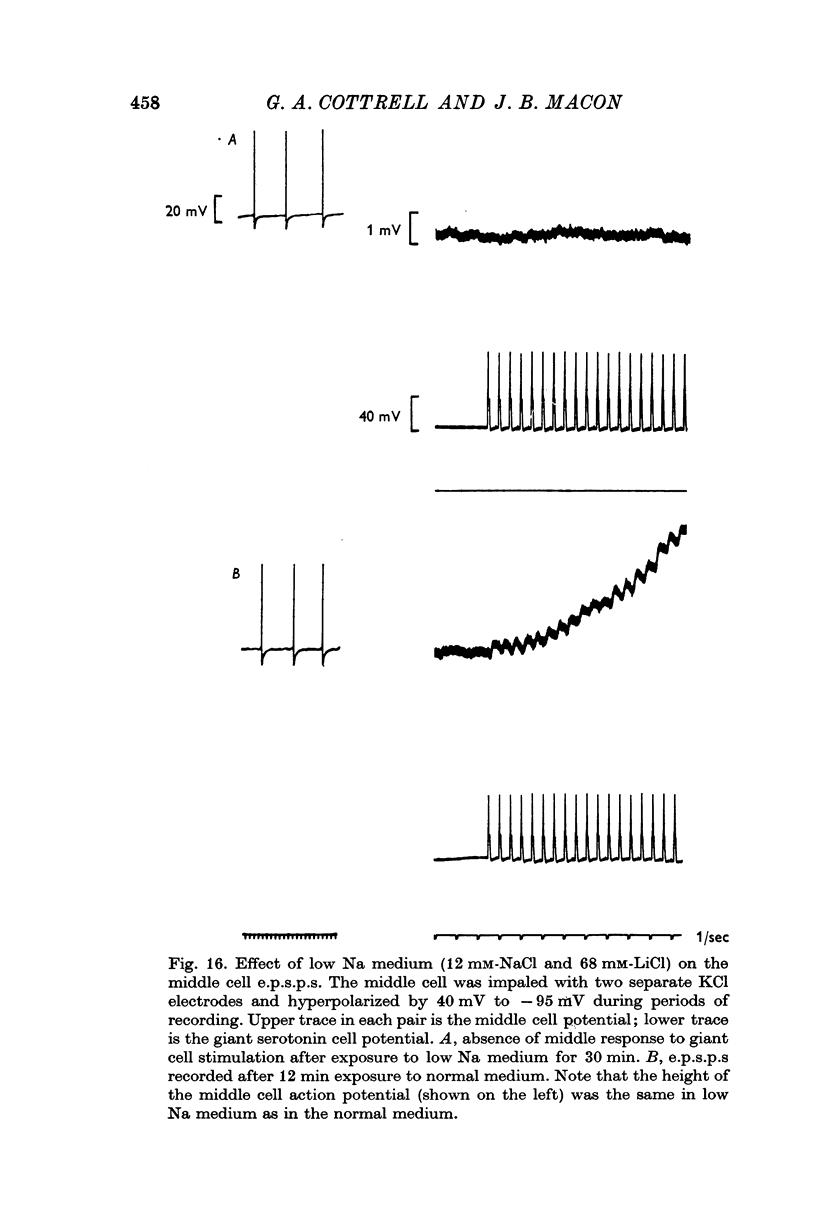
4. Morphine abolished reversibly the middle cell serotonin potential and antagonized transmission from the giant serotonin cells to the middle cells. Lowering the Na concentration of the medium reversibly diminished the size of the serotonin potential and the giant serotonin cell elicited e.p.s.p.s in the middle cells.
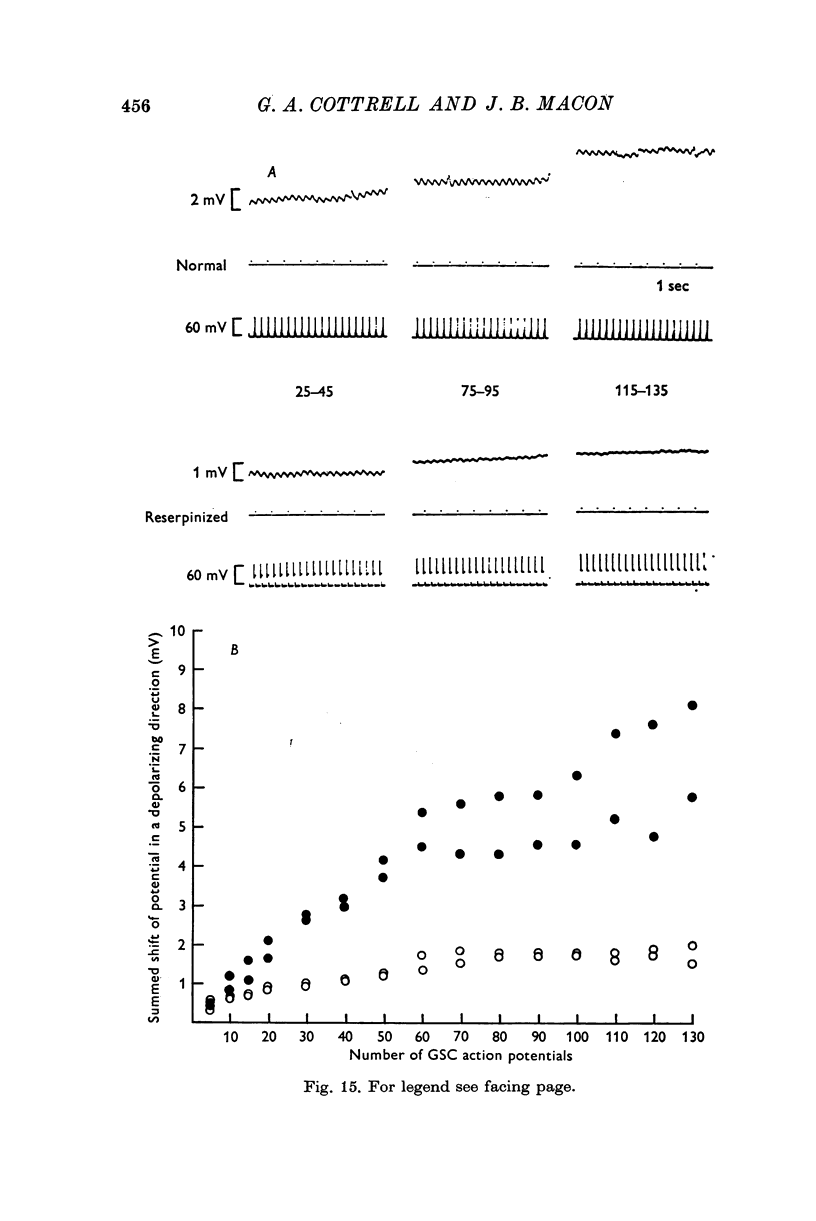
5. Reserpine, which depletes serotonin in the giant serotonin cell, impaired transmission from these cells to the middle cells.
6. The results suggest that serotonin is the synaptic transmitter released from the giant serotonin cells on to the middle cells and that this system is a suitable model for further analysis of the neuronal role of serotonin.
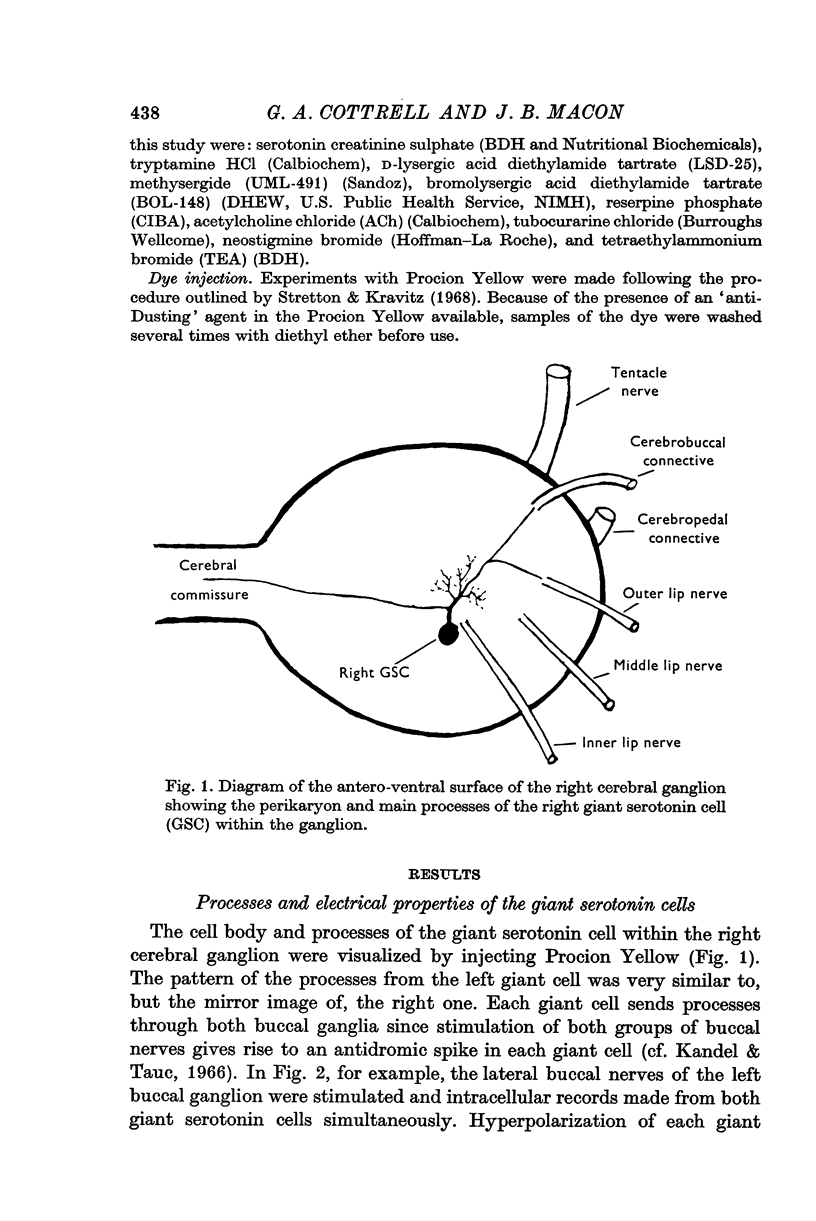
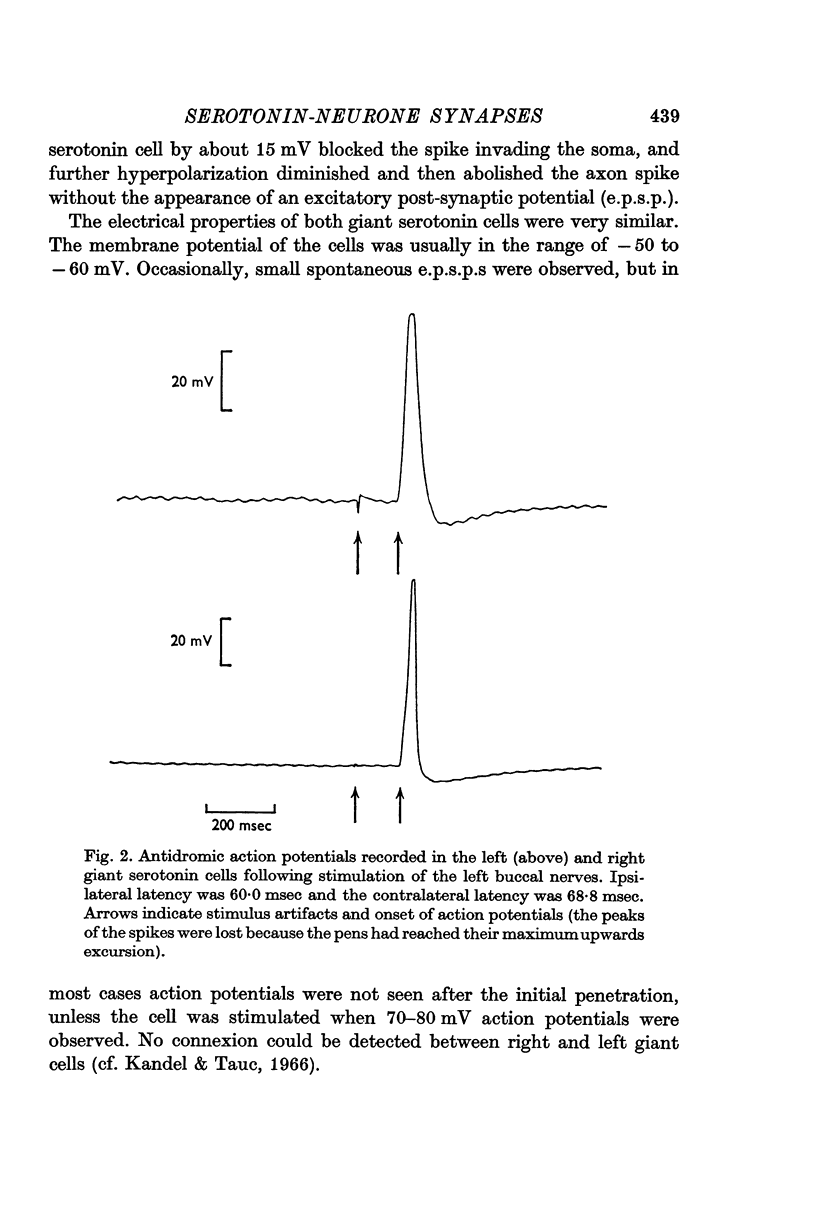
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P. Inhibitory and excitatory effects of dopamine on Aplysia neurones. J Physiol. 1972 Aug;225(1):173–209. doi: 10.1113/jphysiol.1972.sp009933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODIE B. B., SHORE P. A. A concept for a role of serotonin and norepinephrine as chemical mediators in the brain. Ann N Y Acad Sci. 1957 Mar 14;66(3):631–642. doi: 10.1111/j.1749-6632.1957.tb40753.x. [DOI] [PubMed] [Google Scholar]
- Cardot J. Variations saisonnières de la 5-hydroxytrptamine dans les tissus nerveux et cardiaque chez le mollusque Helix pomatia. C R Seances Soc Biol Fil. 1971;165(2):338–341. [PubMed] [Google Scholar]
- Cottrell G. A. Actions of LSD-25 and reserpine on a serotonergic synapse. J Physiol. 1970 May;208(1):28P–29P. [PubMed] [Google Scholar]
- Cottrell G. A. Direct postsynaptic responses to stimulation of serotonin-containing neurones. Nature. 1970 Mar 14;225(5237):1060–1062. doi: 10.1038/2251060a0. [DOI] [PubMed] [Google Scholar]
- Cottrell G. A., Osborne N. N. Subcellular localization of serotonin in an identified serotonin-containing neurone. Nature. 1970 Jan 31;225(5231):470–472. doi: 10.1038/225470a0. [DOI] [PubMed] [Google Scholar]
- GADDUM J. H., PICARELLI Z. P. Two kinds of tryptamine receptor. Br J Pharmacol Chemother. 1957 Sep;12(3):323–328. doi: 10.1111/j.1476-5381.1957.tb00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERASIMOV V. D. VLIIANIE IZMENENI I INONNOGO SOSTAVA SREDY NA PROTSESSY VOZBUZHDENIIA GIGANTSKIKH NERVNYKH KLETOK ULITKI. Fiziol Zh SSSR Im I M Sechenova. 1964 Apr;50:457–463. [PubMed] [Google Scholar]
- Gerschenfeld H. M. Chemical transmission in invertebrate central nervous systems and neuromuscular junctions. Physiol Rev. 1973 Jan;53(1):1–119. doi: 10.1152/physrev.1973.53.1.1. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M. Serotonin: two different inhibitory actions on snail neurons. Science. 1971 Mar 26;171(3977):1252–1254. doi: 10.1126/science.171.3977.1252. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Stefani E. An electrophysiological study of 5-hydroxytryptamine receptors of neurones in the molluscan nervous system. J Physiol. 1966 Aug;185(3):684–700. doi: 10.1113/jphysiol.1966.sp008010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenfeld H. M., Stefani E. Evidence for an excitatory transmitter role of serotonin in molluscan central synapses. Adv Pharmacol. 1968;6(Pt A):369–392. doi: 10.1016/s1054-3589(08)61193-x. [DOI] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E. R., Tauc L. Input organization of two symmetrical giant cells in the snail brain. J Physiol. 1966 Mar;183(2):269–286. doi: 10.1113/jphysiol.1966.sp007866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. The physiological role of three acetylcholine receptors in synaptic transmission in Aplysia. J Physiol. 1972 Aug;225(1):147–172. doi: 10.1113/jphysiol.1972.sp009932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K., Livengood D. R., Werman R. Correlation of transmitter release with membrane properties of the presynaptic fiber of the squid giant synapse. J Gen Physiol. 1967 Dec;50(11):2579–2601. doi: 10.1085/jgp.50.11.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmor M. F. The effects of temperature and ions on the current-voltage relation and electrical characteristics of a molluscan neurone. J Physiol. 1971 Nov;218(3):573–598. doi: 10.1113/jphysiol.1971.sp009634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. The ionic requirements for the production of action potentials in helix pomatia neurones. Pflugers Arch. 1968;304(3):215–241. doi: 10.1007/BF00592126. [DOI] [PubMed] [Google Scholar]
- Osbone N. N., Cottrell G. A. Amine and amino acid microanalysis of two identified snail neurons with known characteristics. Experientia. 1972 Jun 15;28(6):656–658. doi: 10.1007/BF01944960. [DOI] [PubMed] [Google Scholar]
- Osborne N. N., Cottrell G. A. Distribution of biogenic amines in the slug, Limax maximus. Z Zellforsch Mikrosk Anat. 1971;112(1):15–30. doi: 10.1007/BF00665618. [DOI] [PubMed] [Google Scholar]
- Pentreath V. W., Cottrell G. A. Selective uptake of 5-hydroxytryptamine by axonal processes in Helix pomatia. Nat New Biol. 1972 Oct 18;239(94):213–214. doi: 10.1038/newbio239213a0. [DOI] [PubMed] [Google Scholar]
- Roberts M. H., Straughan D. W. Excitation and depression of cortical neurones by 5-hydroxytryptamine. J Physiol. 1967 Nov;193(2):269–294. doi: 10.1113/jphysiol.1967.sp008357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELSH J. H. Serotonin as a possible neurohumoral agent; evidence obtained in lower animals. Ann N Y Acad Sci. 1957 Mar 14;66(3):618–630. doi: 10.1111/j.1749-6632.1957.tb40752.x. [DOI] [PubMed] [Google Scholar]


