Abstract
1. The movement of sodium ions across the membrane of the squid giant axon was measured by the use of radioactive tracers. Unidirectional fluxes were measured at rest and when the nerve was stimulated. The difference was considered the extra flux association with nerve impulses.
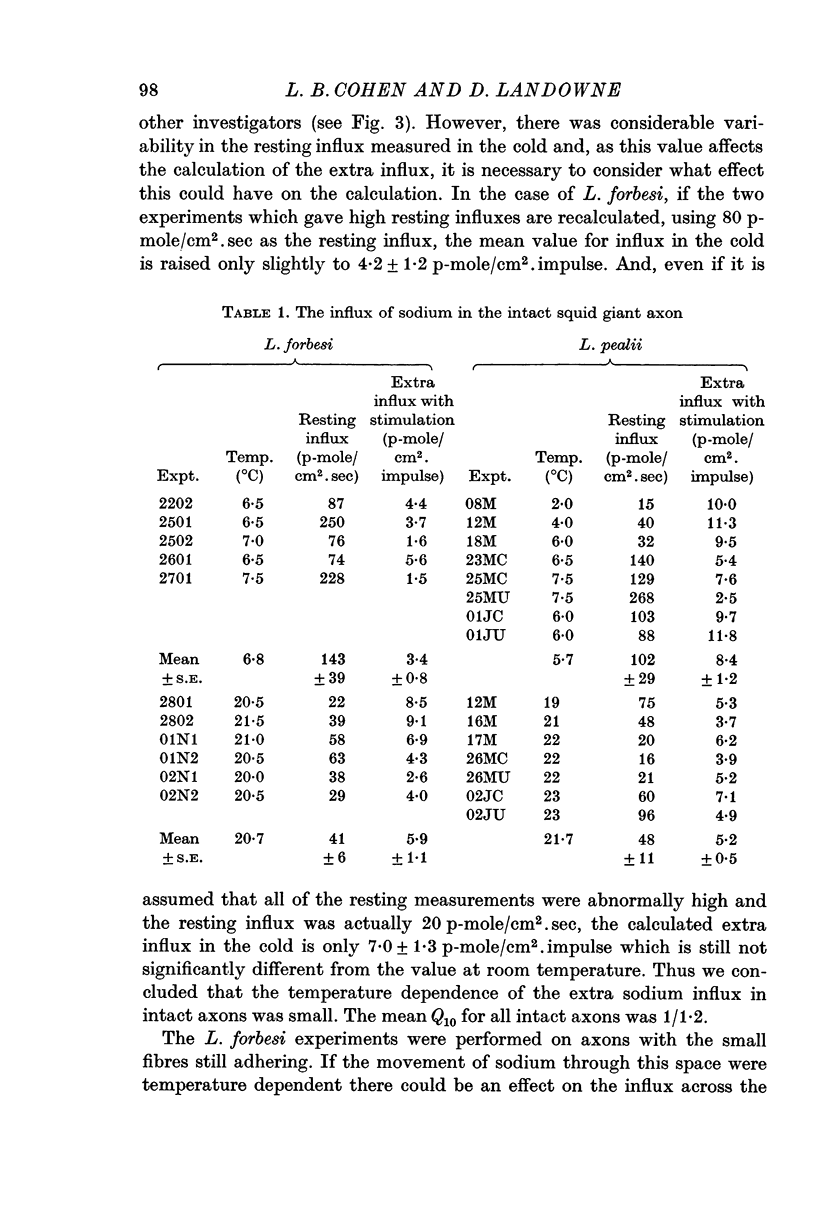
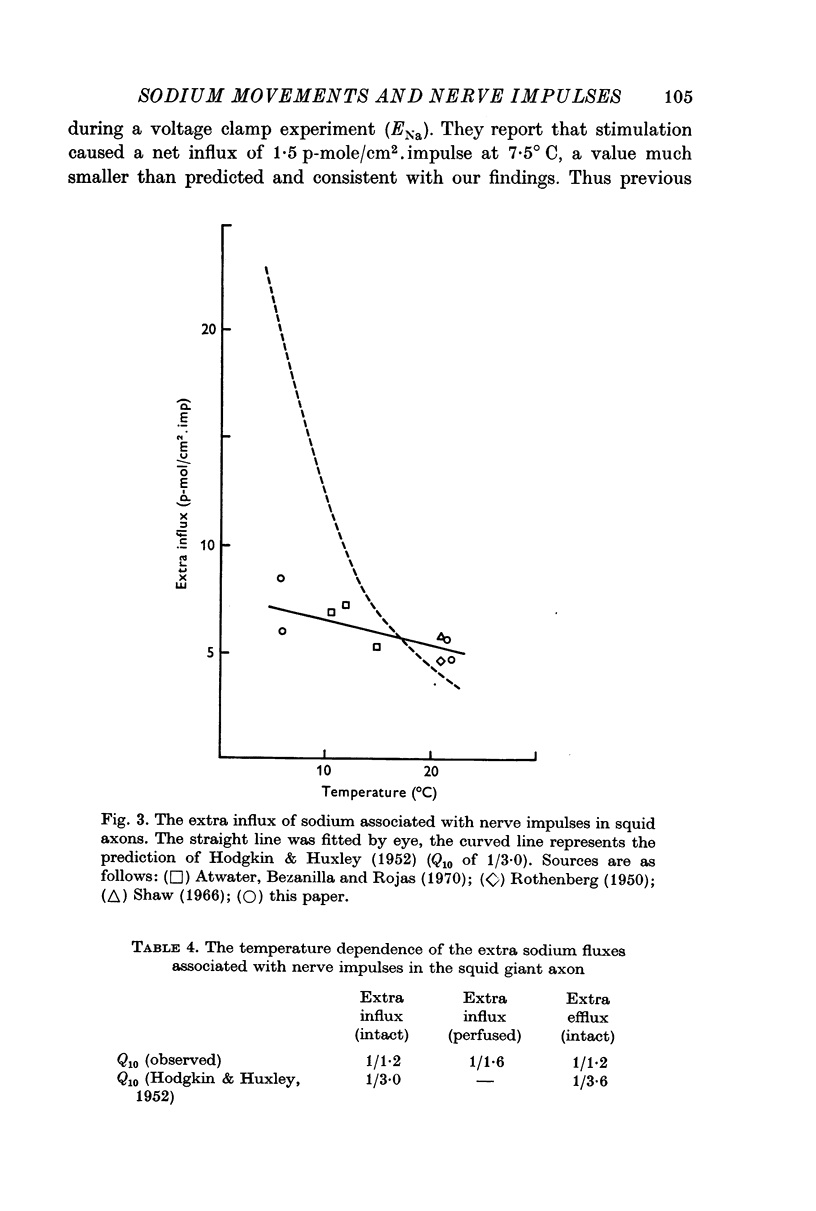
2. The extra influx in intact axons at room temperature was 5·5 p-mole/cm2. impulse. At 6° C the extra influx was 6·5 p-mole/cm2. impulse giving a Q10 of 1/1·2.
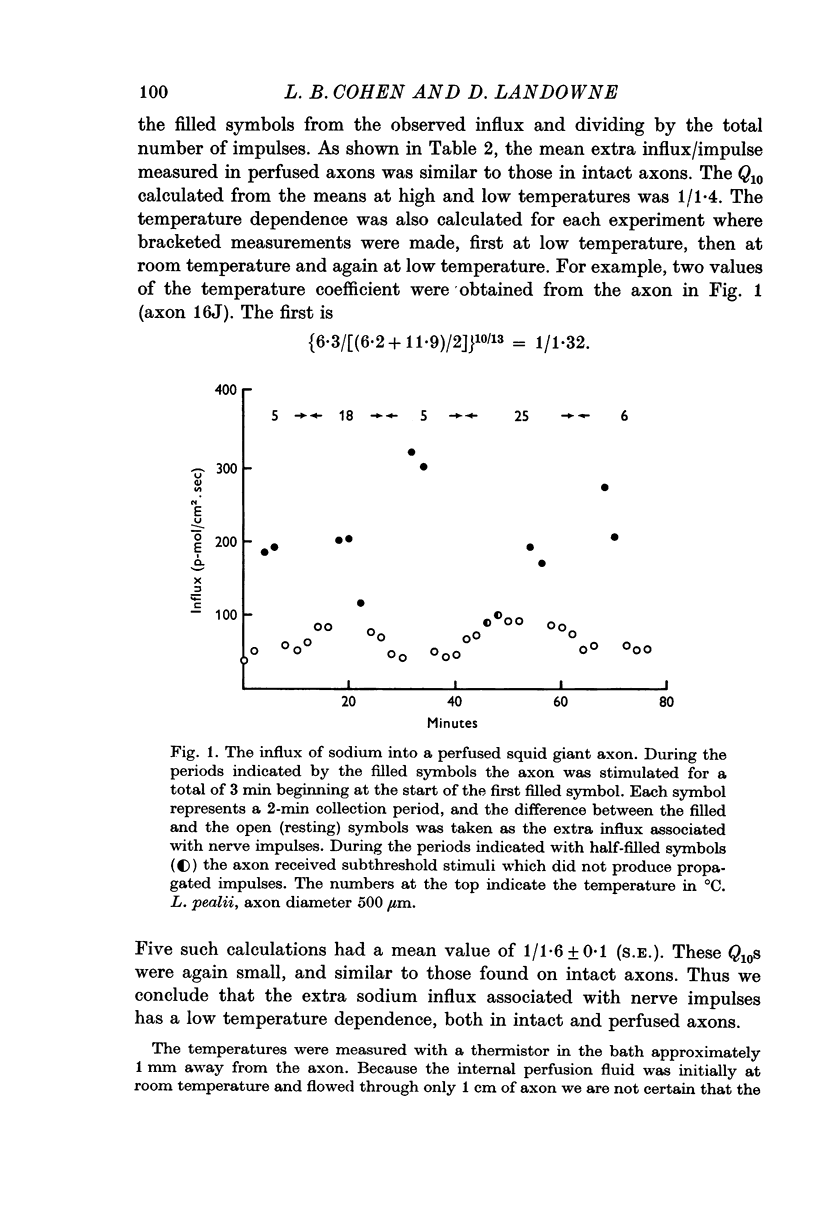
3. In perfused axons a Q10 of 1/1·6 was obtained for the extra sodium influx in bracketed experiments on individual axons.
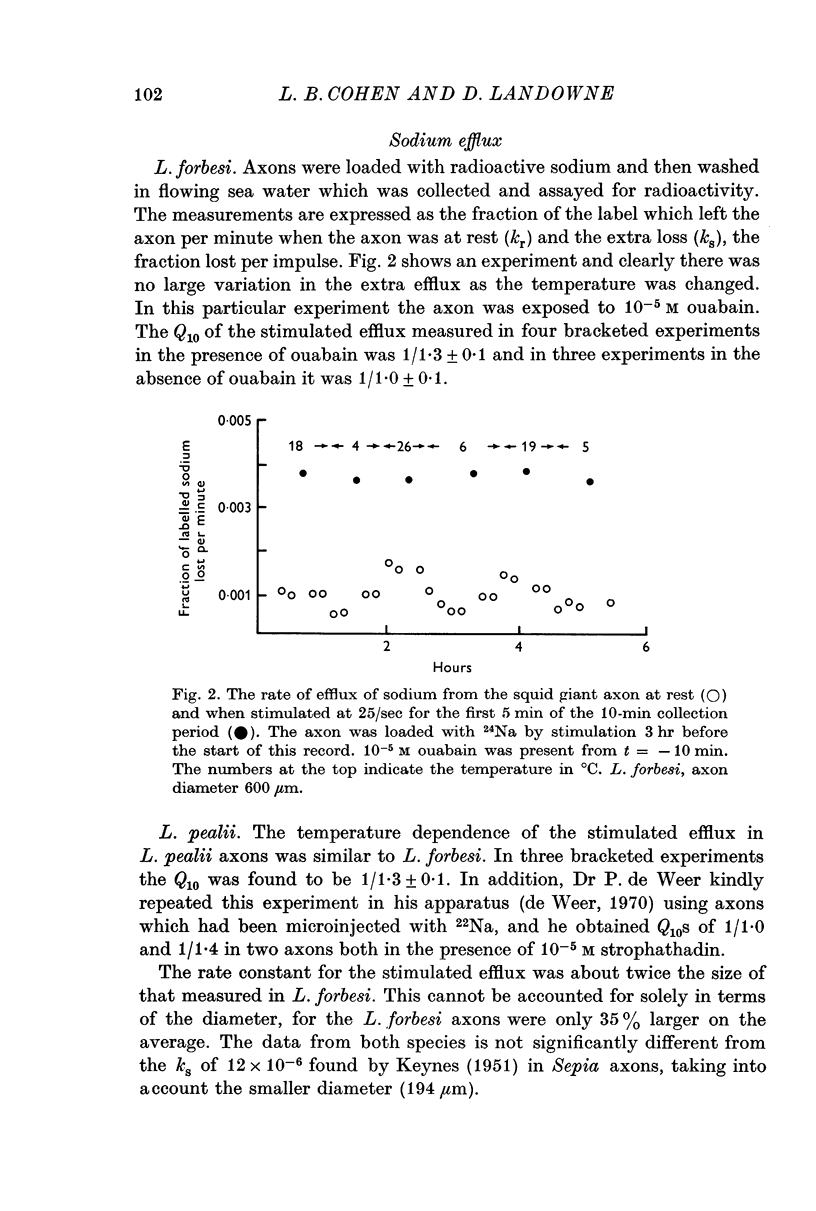
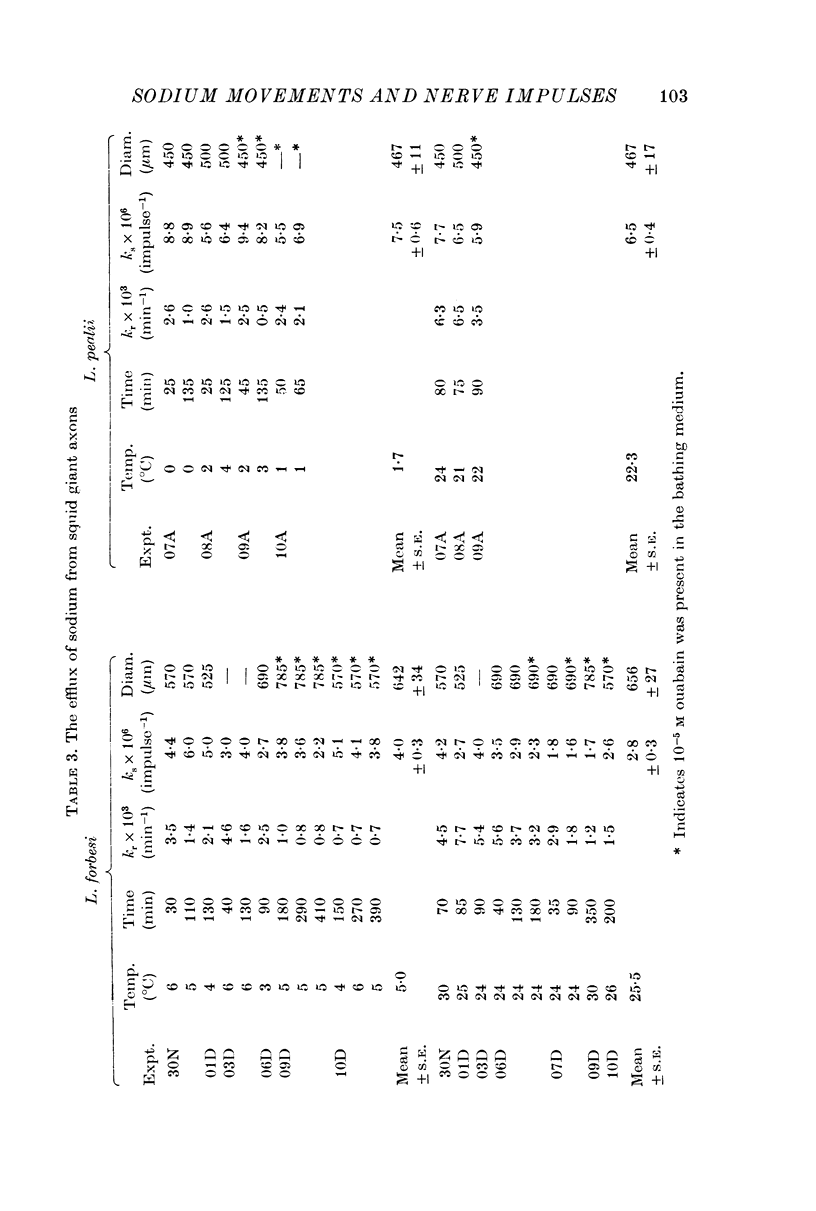
4. The Q10 of the extra sodium efflux associated with nerve impulses was found to be 1/1·2 in intact axons.
5. Hodgkin & Huxley had predicted a much larger temperature dependence for the extra fluxes. If this difference between prediction and experiment does not result from some experimental error, then the class of models for the ion fluxes suggested by Hodgkin & Huxley may be inapplicable.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwater I., Bezanilla F., Rojas E. Sodium influxes in internally perfused squid giant axon during voltage clamp. J Physiol. 1969 May;201(3):657–664. doi: 10.1113/jphysiol.1969.sp008778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwater I., Bezanilla F., Rojas E. Time course of the sodium permeability change during a single membrane action potential. J Physiol. 1970 Dec;211(3):753–765. doi: 10.1113/jphysiol.1970.sp009302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Keynes R. D., Manil J., Shaw T. I., Steinhardt R. A. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969 Feb;200(2):459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Time course of the sodium influx in squid giant axon during a single voltage clamp pulse. J Physiol. 1970 Mar;207(1):151–164. doi: 10.1113/jphysiol.1970.sp009054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S. Some physical aspects of bioelectric phenomena. Proc Natl Acad Sci U S A. 1949 Oct;35(10):558–566. doi: 10.1073/pnas.35.10.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. B., Keynes R. D., Landowne D. Changes in axon light scattering that accompany the action potential: current-dependent components. J Physiol. 1972 Aug;224(3):727–752. doi: 10.1113/jphysiol.1972.sp009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P. Effects of intracellular adenosine-5'-diphosphate and orthophosphate on the sensitivity of sodium efflux from squid axon to external sodium and potassium. J Gen Physiol. 1970 Nov;56(5):583–620. doi: 10.1085/jgp.56.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weer P., Geduldig D. Electrogenic sodium pump in squid giant axon. Science. 1973 Mar 30;179(4080):1326–1328. doi: 10.1126/science.179.4080.1326. [DOI] [PubMed] [Google Scholar]
- FITZHUGH R., COLE K. S. THEORETICAL POTASSIUM LOSS FROM SQUID AXONS AS A FUNCTION OF TEMPERATURE. Biophys J. 1964 Jul;4:257–265. doi: 10.1016/s0006-3495(64)86781-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KATZ B. The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol. 1949 Aug;109(1-2):240–249. doi: 10.1113/jphysiol.1949.sp004388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M. The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J Physiol. 1968 Feb;194(3):745–793. doi: 10.1113/jphysiol.1968.sp008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt R. C., Strieb J. D. A stored charge model for the sodium channel. Biophys J. 1971 Nov;11(11):868–885. doi: 10.1016/S0006-3495(71)86261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic movements during nervous activity. J Physiol. 1951 Jun;114(1-2):119–150. doi: 10.1113/jphysiol.1951.sp004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowne D. A new explanation of the ionic currents which flow during the nerve impulse. J Physiol. 1972 Apr;222(1):46P–47P. [PubMed] [Google Scholar]
- Landowne D. Movement of sodium ions associated with the nerve impulse. Nature. 1973 Apr 13;242(5398):457–459. doi: 10.1038/242457b0. [DOI] [PubMed] [Google Scholar]
- MOORE J. W., ADELMAN W. J., Jr Electronic measurement of the intracellular concentration and net flux of sodium in the squid axon. J Gen Physiol. 1961 Sep;45:77–92. doi: 10.1085/jgp.45.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHENBERG M. A. Studies on permeability in relation to nerve function, ionic movements across exonal membranes. Biochim Biophys Acta. 1950 Jan;4(1-3):96–114. doi: 10.1016/0006-3002(50)90012-6. [DOI] [PubMed] [Google Scholar]
- SHANES A. M. Effect of temperature on potassium liberation during nerve activity. Am J Physiol. 1954 Jun;177(3):377–382. doi: 10.1152/ajplegacy.1954.177.3.377. [DOI] [PubMed] [Google Scholar]
- Shaw T. I. Cation movements in perfused giant axons. J Physiol. 1966 Jan;182(1):209–216. doi: 10.1113/jphysiol.1966.sp007819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hertog A., Ritchie J. M. A comparison of the effect of temperature, metabolic inhibitors and of ouabain on the electrogenic componen of the sodium pump in mammalian non-myelinated nerve fibres. J Physiol. 1969 Oct;204(3):523–538. doi: 10.1113/jphysiol.1969.sp008929. [DOI] [PMC free article] [PubMed] [Google Scholar]


