Abstract
The responses of bipolar cells in the retina of the turtle have been studied by intracellular recording. Two types of bipolar cell have been identified: one gave graded depolarizing and the other graded hyperpolarizing responses to small circles of light (100 μm diameter). The responses of both types of cell were similar in the following respects.
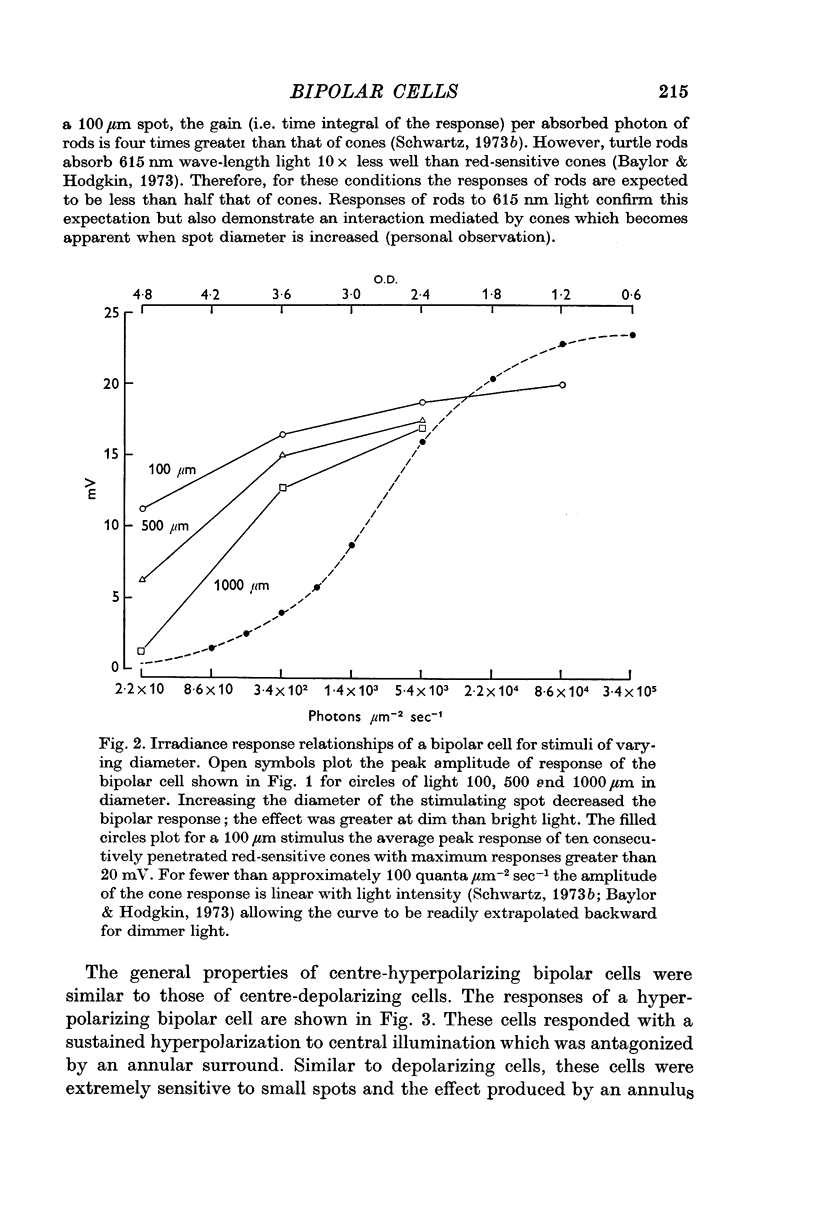
1. Both were extremely sensitive to dim light; the amplitude of response to a small circle of light increased with light intensity more steeply than the cone response.
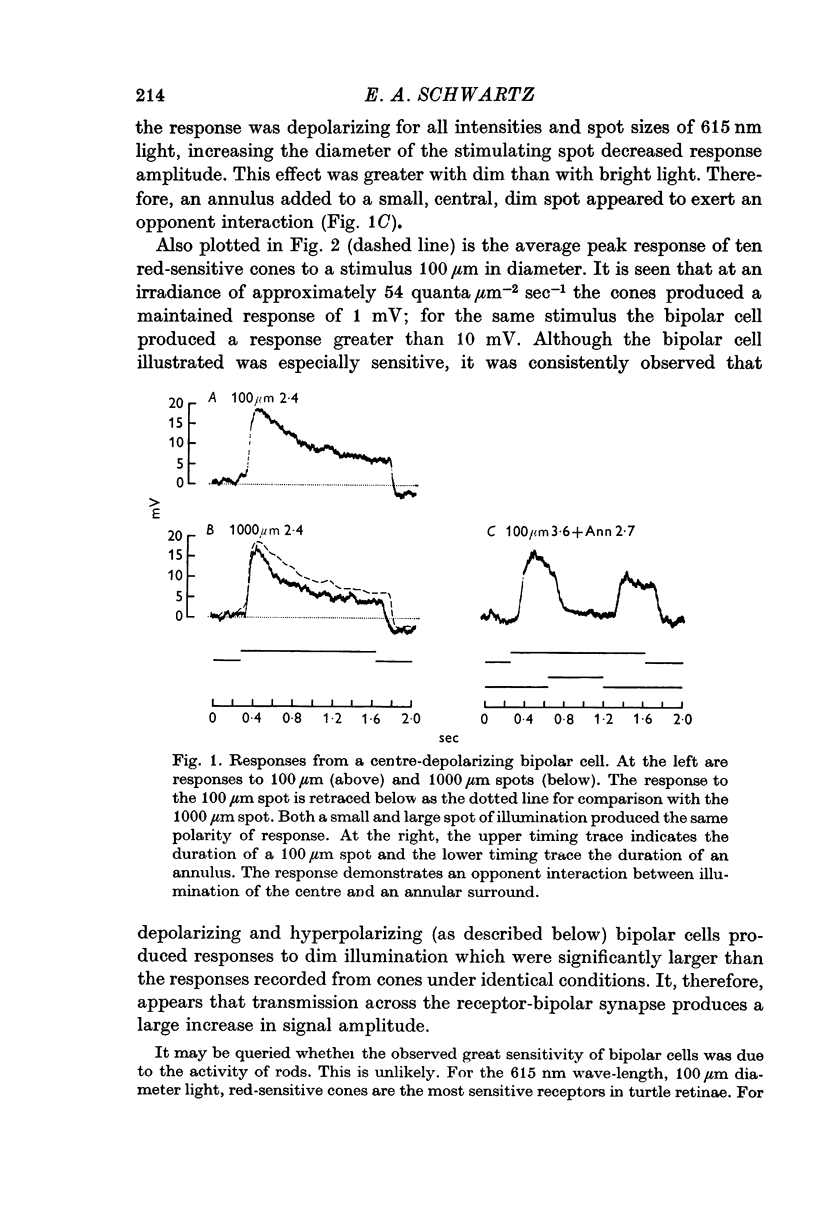
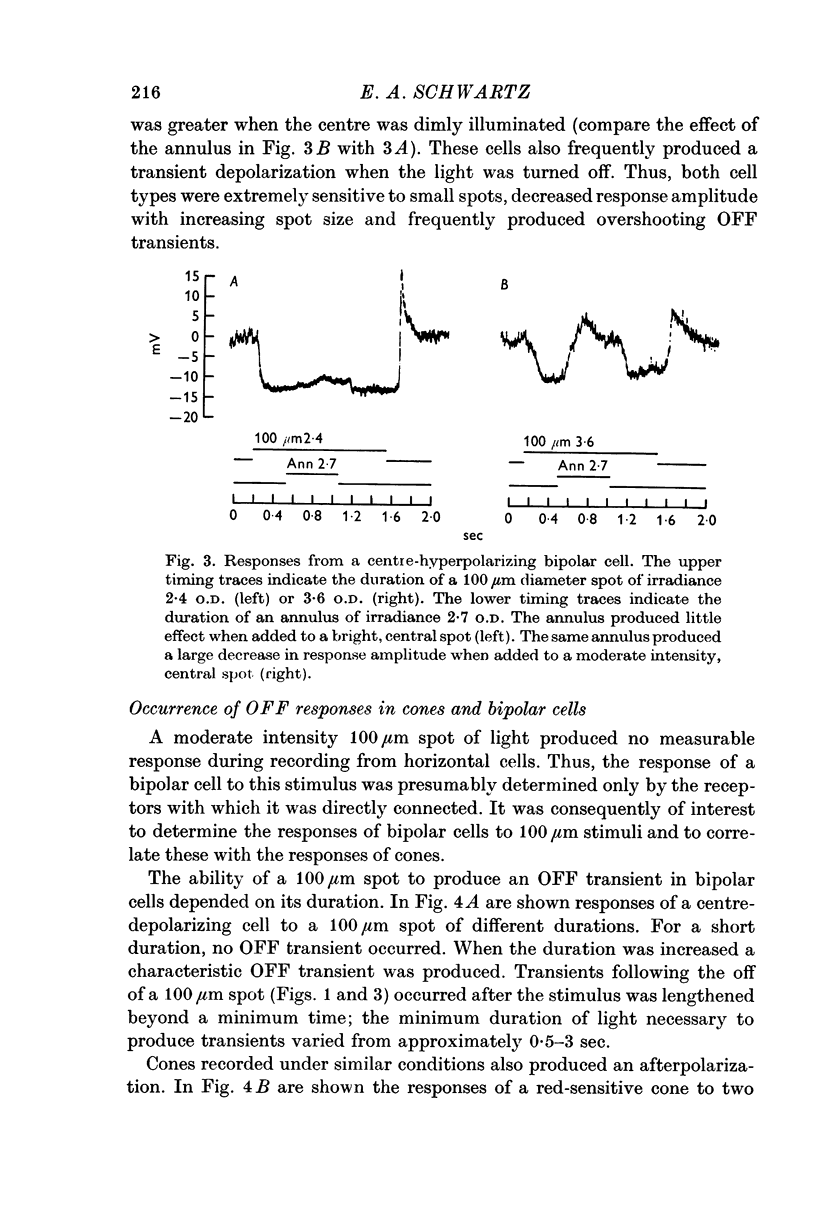
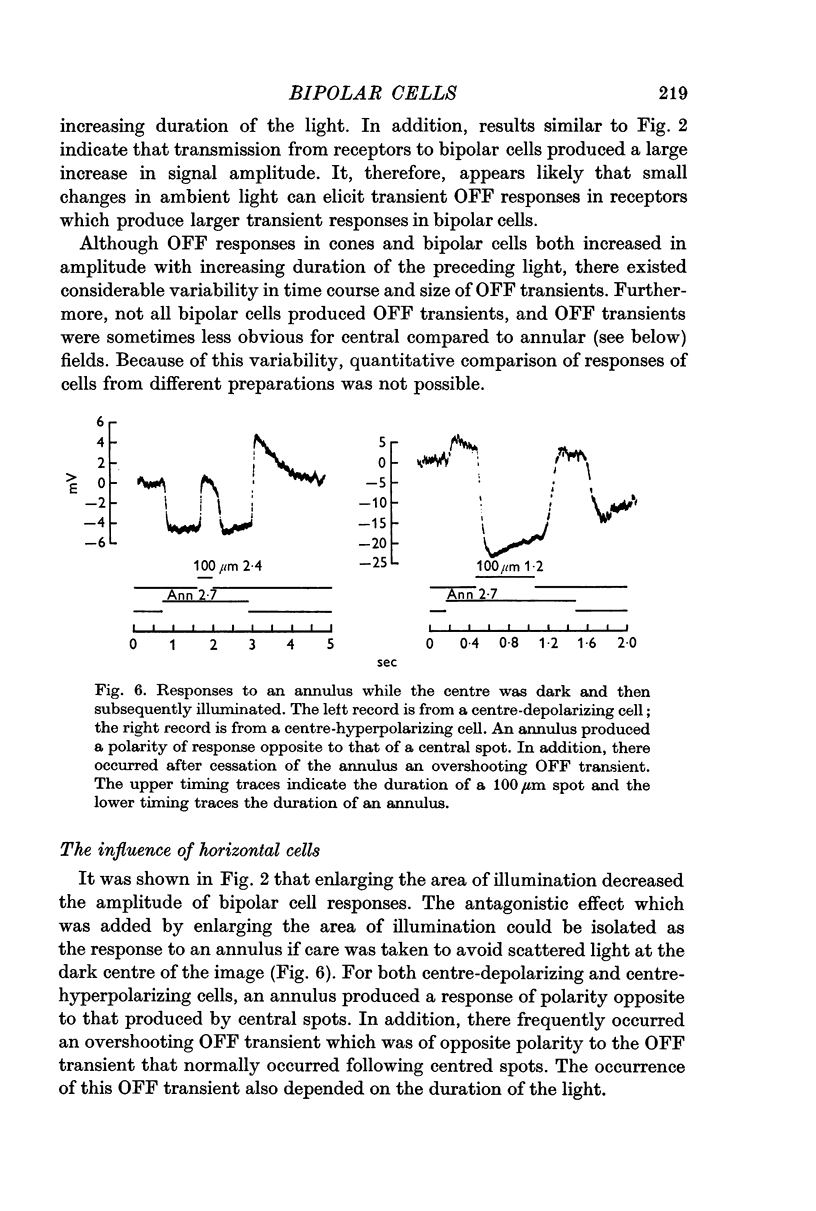
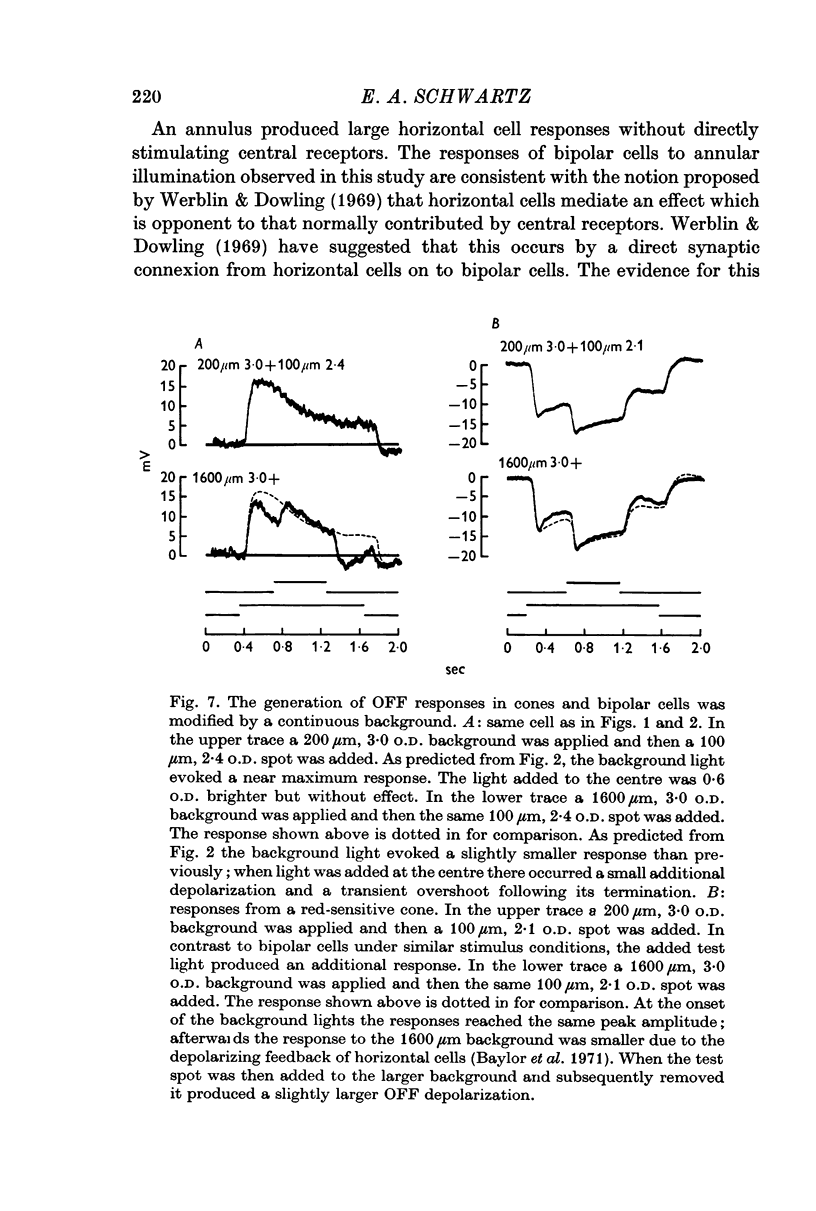
2. Enlarging the diameter of a spot added an antagonistic effect which decreased response amplitude. This decrease in response amplitude was more apparent at dim than at bright light. Stimulating only distant areas of retina with an annulus produced a response of polarity opposite to that normally produced by a central spot. However, the responses of bipolar cells did not appear to be due to a simple summation of opposite polarity signals contributed from central and peripheral parts of their receptive fields.
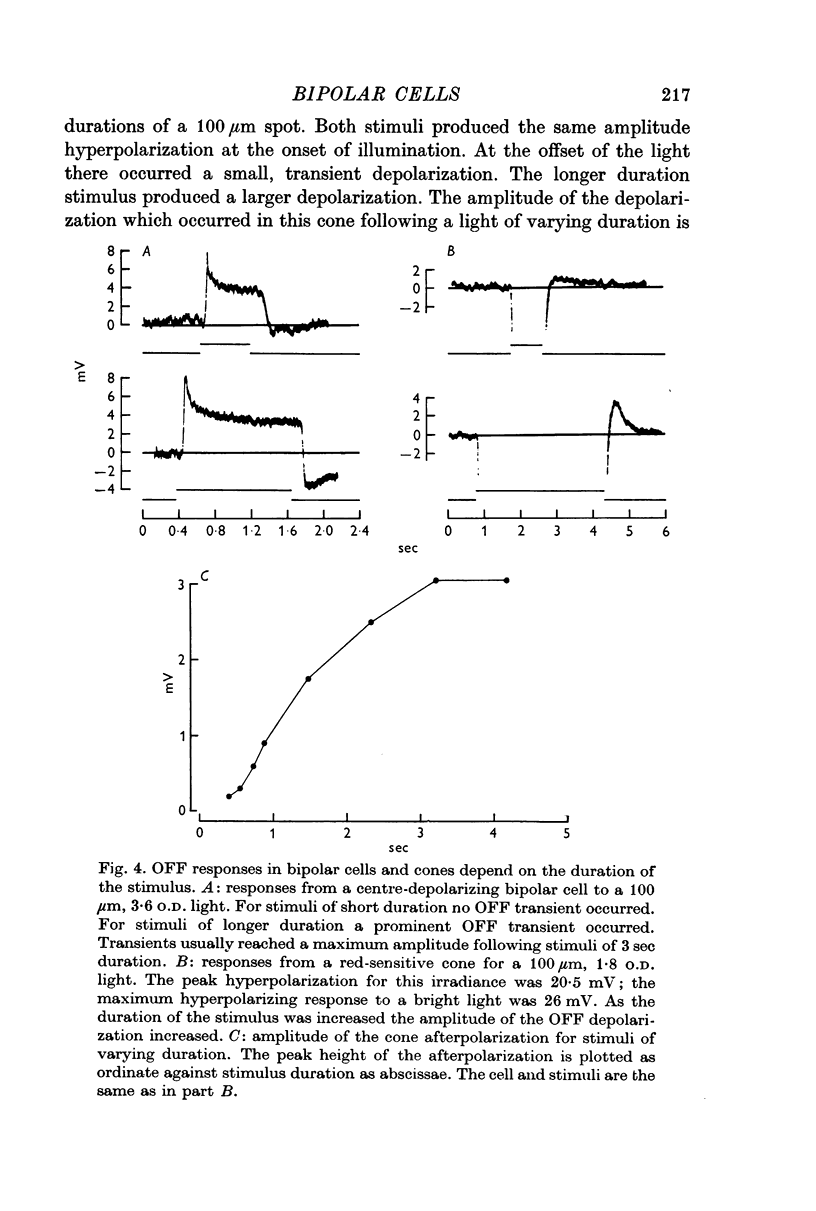
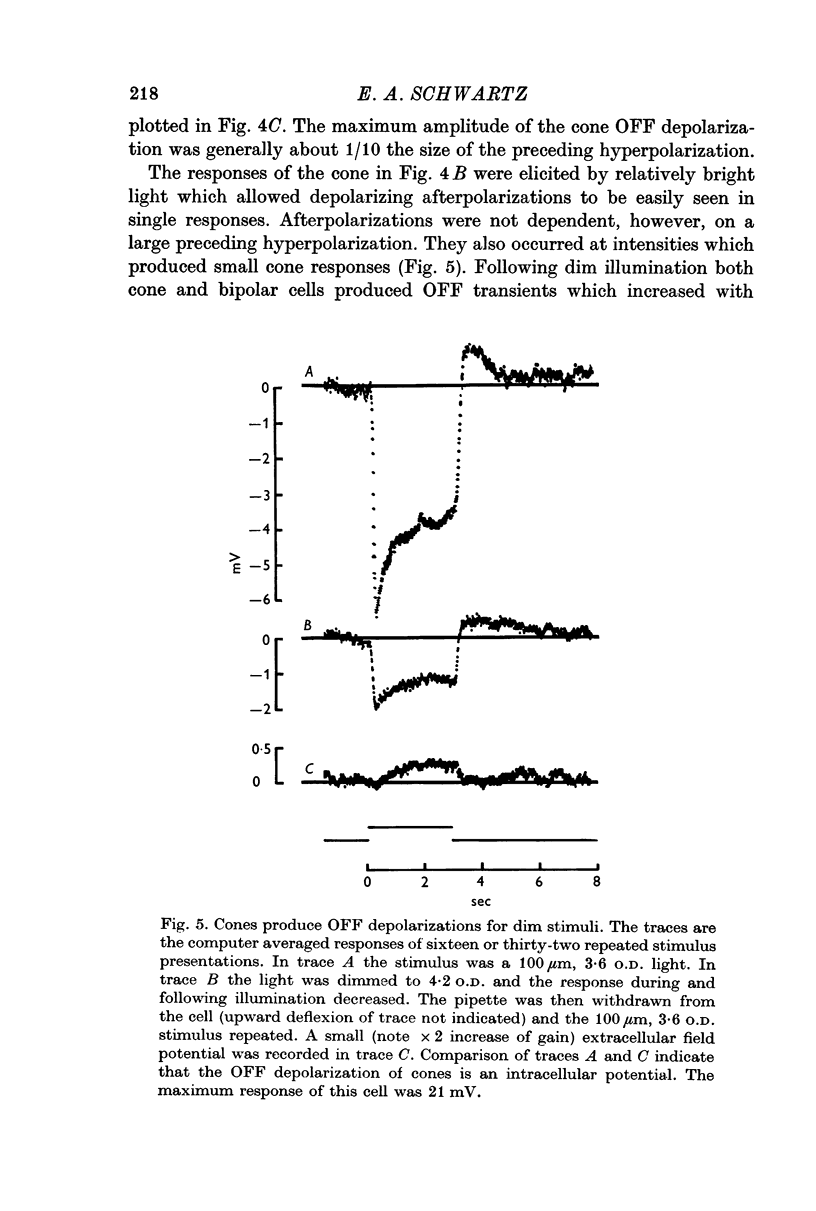
3. When small spots or annuli of light were turned off there frequently occurred an overshooting OFF transient. The occurrence of OFF transients depended on the duration of the stimulus. Cones recorded under similar conditions produced an OFF depolarization. The size of cone OFF depolarizations increased with increasing duration of the preceding light; following approximately 3 sec of illumination their maximum amplitude was roughly 1/10 the amplitude of the preceding hyperpolarization. The size of OFF responses in both cone and bipolar cells was increased when horizontal cells were hyperpolarized by light.
It is concluded that bipolar cells produce large responses for very small cone responses, and, as a consequence, a small depolarization in cones following illumination produces large OFF transients in bipolar cells. Furthermore, the responses of bipolar cells do not appear to represent a simple summation of opposite polarity input from receptor and horizontal cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENOLKEN R. M. Reversal of photoreceptor polarity recorded during the graded receptor potential response to light in the eye of Limulus. Biophys J. 1961 Sep;1:551–564. doi: 10.1016/s0006-3495(61)86908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Lisman J. E. An electrogenic sodium pump in Limulus ventral photoreceptor cells. J Gen Physiol. 1972 Jun;59(6):720–733. doi: 10.1085/jgp.59.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuortes M. G., Schwartz E. A., Simon E. J. Colour-dependence of cone responses in the turtle retina. J Physiol. 1973 Oct;234(1):199–216. doi: 10.1113/jphysiol.1973.sp010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIKUCHI R., NAITO K., TANAKA I. Effect of sodium and potassium ions on the electrical activity of single cells in the lateral eye of the horseshoe crab. J Physiol. 1962 May;161:319–343. doi: 10.1113/jphysiol.1962.sp006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H., Brown H. M., Hagiwara S. Hyperpolarization of a barnacle photoreceptor membrane following illumination. J Gen Physiol. 1971 Jun;57(6):723–737. doi: 10.1085/jgp.57.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Cell junctions at the outer synaptic layer of the retina. Invest Ophthalmol. 1972 May;11(5):265–275. [PubMed] [Google Scholar]
- Matsumoto N., Naka K. I. Identification of intracellular responses in the frog retina. Brain Res. 1972 Jul 13;42(1):59–71. doi: 10.1016/0006-8993(72)90042-x. [DOI] [PubMed] [Google Scholar]
- NAKA K. I. Recording of retinal action potentials from single cells in the insect compound eye. J Gen Physiol. 1961 Jan;44:571–584. doi: 10.1085/jgp.44.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Nye P. W. Role of horizontal cells in organization of the catfish retinal receptive field. J Neurophysiol. 1971 Sep;34(5):785–801. doi: 10.1152/jn.1971.34.5.785. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Organization of on-off cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):1–14. doi: 10.1113/jphysiol.1973.sp010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J. Two types of luminosity horizontal cells in the retina of the turtle. J Physiol. 1973 Apr;230(1):199–211. doi: 10.1113/jphysiol.1973.sp010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J. Membrane resistance changes underlying the bipolar cell response in the carp retina. Vision Res. 1973 Feb;13(2):283–294. doi: 10.1016/0042-6989(73)90107-7. [DOI] [PubMed] [Google Scholar]
- WATANABE K., TOSAKA T. Functional organization of the cyprinid fish retina as revealed by discriminative responses to spectral illumination. Jpn J Physiol. 1959 Mar 25;9(1):84–93. doi: 10.2170/jjphysiol.9.84. [DOI] [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]


