Abstract
1. The abdominal frog skin was mounted between two chambers containing Ringer with 1 mM-Na on the outside and 115 mM-Na on the inside. When the Na concentration of the outer solution ([Na]o) is instantaneously raised from 1 to 50 mM, the short circuit current (I) increases to a new value in less than a second, and becomes essentially time-independent. Only in a few experiments was it observed to increase further, although at a much slower rate.
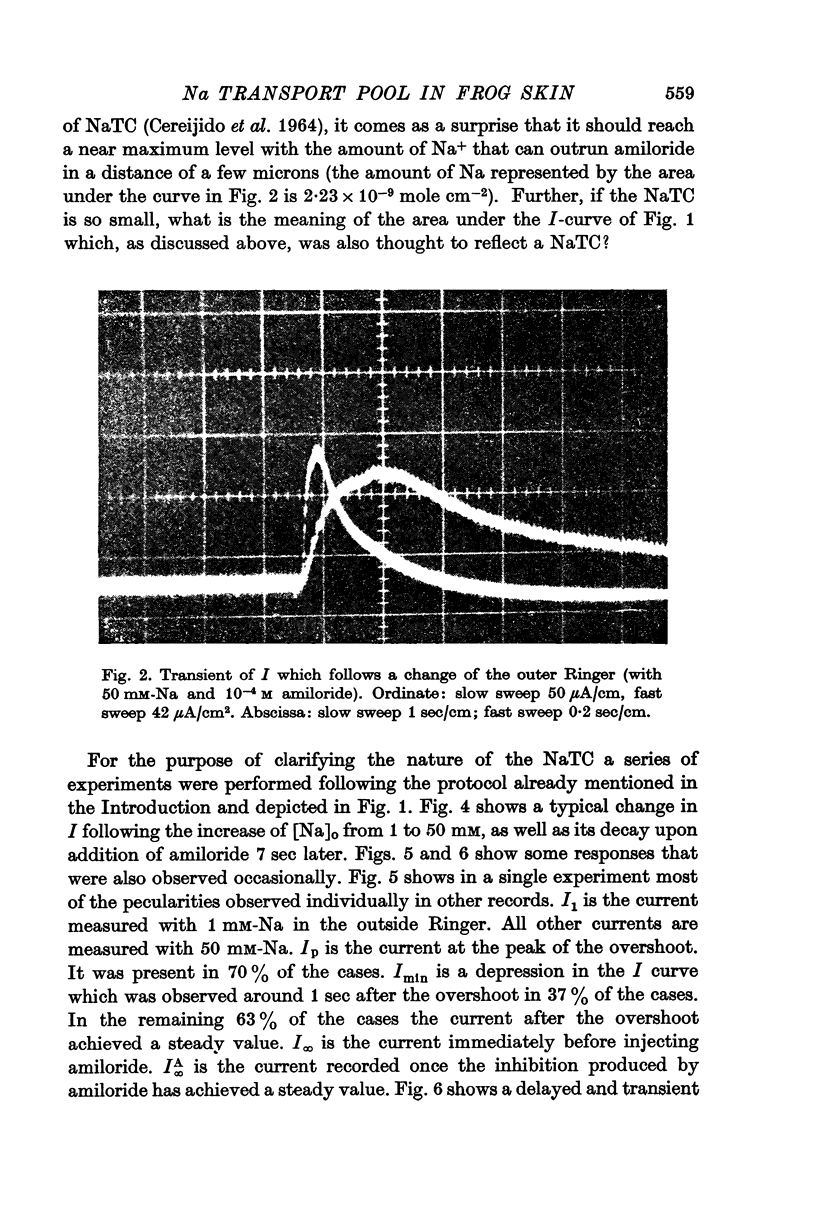
2. At a time t after this increase, the addition of 10-4 M amiloride to the outer solution produces an exponential decrease of I. The area under this exponential curve is generally taken to reflect the existence of a Na- transporting compartment (NaTC).
3. The amount of Na represented by NaTC is a function of t: it increases from 1·7 × 10-9 mole. cm-2, at t = 10 sec, to 22·8 × 10-9 mole. cm-2 at t = 10 min.
4. In view of the fact that (a) I is not a function of the size of the `NaTC' and (b) that whereas I reaches a steady value in a fraction of a second the size of NaTC keeps increasing for minutes, it is proposed that the `NaTC' represents an amount of Na which is not located along the main route of transepithelial transport.
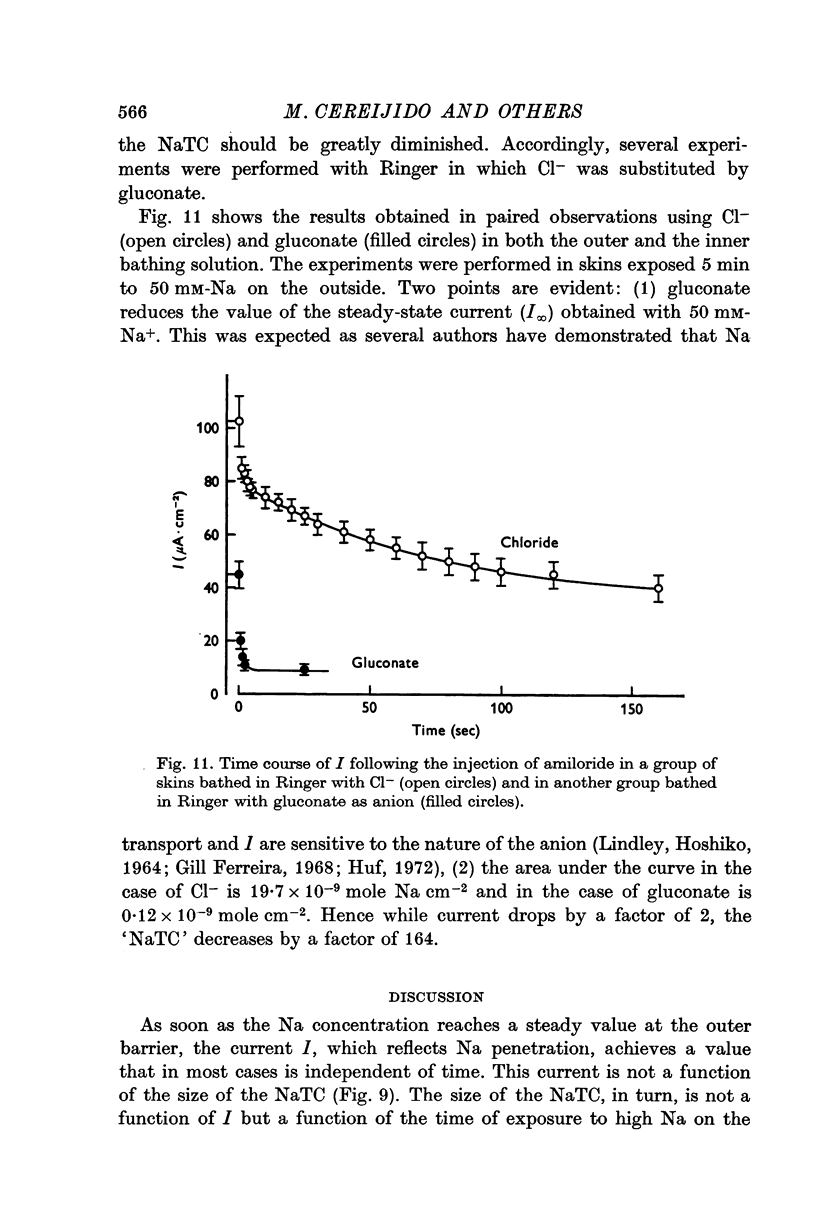
5. On the assumption that the NaTC is located in a cellular compartment and that, in order to accumulate in this compartment Na should be accompanied by a permeable anion, a series of experiments were performed with Ringer in which Cl- was replaced by gluconate. It was observed as expected, that NaTC in gluconate is 164 times smaller than in Cl-, but I only decreases to one half its value in Cl- Ringer.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN B., ZERAHN K. METHOD FOR NON-DESTRUCTIVE DETERMINATION OF THE SODIUM TRANSPORT POOL IN FROG SKIN WITH RADIOSODIUM. Acta Physiol Scand. 1963 Dec;59:319–329. doi: 10.1111/j.1748-1716.1963.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Aceves J., Cereijido M. The effect of amiloride on sodium and potassium fluxes in red cells. J Physiol. 1973 Mar;229(3):709–718. doi: 10.1113/jphysiol.1973.sp010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aceves J., Erlij D. Sodium transport across the isolated epithelium of the frog skin. J Physiol. 1971 Jan;212(1):195–210. doi: 10.1113/jphysiol.1971.sp009317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber T. U., Chez R. A., Curran P. F. Na transport across frog skin at low external Na concentrations. J Gen Physiol. 1966 Jul;49(6):1161–1176. doi: 10.1085/jgp.0491161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber T. U., Curran P. F. Direct measurement of uptake of sodium at the outer surface of the frog skin. J Gen Physiol. 1970 Jul;56(1):83–99. doi: 10.1085/jgp.56.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber T. U. Effect of changes in transepithelial transport on the uptake of sodium across the outer surface of the frog skin. J Gen Physiol. 1971 Aug;58(2):131–144. doi: 10.1085/jgp.58.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEREIJIDO M., CURRAN P. F. INTRACELLULAR ELECTRICAL POTENTIALS IN FROG SKIN. J Gen Physiol. 1965 Mar;48:543–557. doi: 10.1085/jgp.48.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEREIJIDO M., HERRERA F. C., FLANIGAN W. J., CURRAN P. F. THE INFLUENCE OF NA CONCENTRATION ON NA TRANSPORT ACROSS FROG SKIN. J Gen Physiol. 1964 May;47:879–893. doi: 10.1085/jgp.47.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., HERRERA F. C., FLANIGAN W. J. The effect of Ca and antidiuretic hormone on Na transport across frog skin. II. Sites and mechanisms of action. J Gen Physiol. 1963 May;46:1011–1027. doi: 10.1085/jgp.46.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Rotunno C. A. Fluxes and distribution of sodium in frog skin. A new model. J Gen Physiol. 1968 May;51(5 Suppl):280S+–280S+. [PubMed] [Google Scholar]
- Cuthbert A. W. A double (series) pump model for transporting epithelia. J Theor Biol. 1972 Sep;36(3):555–568. doi: 10.1016/0022-5193(72)90008-2. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W. Neurohypophyseal hormones and sodium transport. Philos Trans R Soc Lond B Biol Sci. 1971 Aug 20;262(842):103–109. doi: 10.1098/rstb.1971.0081. [DOI] [PubMed] [Google Scholar]
- Dainty J., House C. R. Unstirred layers in frog skin. J Physiol. 1966 Jan;182(1):66–78. doi: 10.1113/jphysiol.1966.sp007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörge A., Nagel W. Effect of amiloride on sodium transport in frog skin. II. Sodium transport pool and unidirectional fluxes. Pflugers Arch. 1970;321(2):91–101. doi: 10.1007/BF00586365. [DOI] [PubMed] [Google Scholar]
- Ferreira K. T. Anionic dependence of sodium transport in the frog skin. Biochim Biophys Acta. 1968 Jun 11;150(4):587–598. doi: 10.1016/0005-2736(68)90048-5. [DOI] [PubMed] [Google Scholar]
- Finn A. L., Rockoff M. L. The kinetics of sodium transport in the toad bladder. I. Determination of the transport pool. J Gen Physiol. 1971 Mar;57(3):326–348. doi: 10.1085/jgp.57.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Huf E. G. The role of Cl - and other anions in active Na + transport in isolated frog skin. Acta Physiol Scand. 1972 Mar;84(3):366–381. doi: 10.1111/j.1748-1716.1972.tb05188.x. [DOI] [PubMed] [Google Scholar]
- KIDDER G. W., 3rd, CEREIJIDO M., CURRAN P. F. TRANSIENT CHANGES IN ELECTRICAL POTENTIAL DIFFERENCES ACROSS FROG SKIN. Am J Physiol. 1964 Oct;207:935–940. doi: 10.1152/ajplegacy.1964.207.4.935. [DOI] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- LINDLEY B. D., HOSHIKO T. THE EFFECTS OF ALKALI METAL CATIONS AND COMMON ANIONS ON THE FROG SKIN POTENTIAL. J Gen Physiol. 1964 Mar;47:749–771. doi: 10.1085/jgp.47.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel L. J., Curran P. F. Response of the frog skin to steady-state voltage clamping. I. The shunt pathway. J Gen Physiol. 1972 May;59(5):503–518. doi: 10.1085/jgp.59.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel L. J., Curran P. F. Response of the frog skin to steady-state voltage clamping. II. The active pathway. J Gen Physiol. 1973 Jul;62(1):1–24. doi: 10.1085/jgp.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. H., Reisin I. L., Rodríguez Boulan E., Rotunno C. A., Cereijido M. Barriers to sodium movement across frog skin. J Membr Biol. 1973;11(2):99–115. doi: 10.1007/BF01869815. [DOI] [PubMed] [Google Scholar]
- Nagel W., Dörge A. Effect of Amiloride on sodium transport of frog skin. I. Action on intracellular sodium content. Pflugers Arch. 1970;317(1):84–92. doi: 10.1007/BF00586701. [DOI] [PubMed] [Google Scholar]
- Rotunno C. A., Zylber E. A., Cereijido M. Ion and water balance in the epithelium of the abdominal skin of the frog Leptodactylus ocellatus. J Membr Biol. 1973 Oct 10;13(3):217–232. doi: 10.1007/BF01868229. [DOI] [PubMed] [Google Scholar]
- Routunno C. A., Pouchan M. I., Cereijido M. Location of the mechanism of active transport of sodium across the frog skin. Nature. 1966 May 7;210(5036):597–599. doi: 10.1038/210597a0. [DOI] [PubMed] [Google Scholar]
- Schwartz T. L., Snell F. M. Nonsteady-state three compartment tracer kinetics. II. Sodium flux transients in the toad urinary bladder in response to short circuit. Biophys J. 1968 Jul;8(7):818–841. doi: 10.1016/S0006-3495(68)86523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H., WINDHAGER E. E. NATURE OF SHUNT PATH AND ACTIVE SODIUM TRANSPORT PATH THROUGH FROG SKIN EPITHELIUM. Acta Physiol Scand. 1964 Aug;61:484–504. [PubMed] [Google Scholar]
- Vanatta J. C., Bryant L. A. Compartmentation of the sodium transport pool of the toad bladder. Proc Soc Exp Biol Med. 1970 Feb;133(2):385–393. doi: 10.3181/00379727-133-34479. [DOI] [PubMed] [Google Scholar]
- Voute C. L., Ussing H. H. Quantitative relation between hydrostatic pressure gradient, extracellular volume and active sodium transport in the epithelium of the frog skin (R. temporaria). Exp Cell Res. 1970 Oct;62(2):375–383. doi: 10.1016/0014-4827(70)90568-9. [DOI] [PubMed] [Google Scholar]
- ZADUNAISKY J. A., CANDIA O. A., CHIARANDINI D. J. THE ORIGIN OF THE SHORT-CIRCUIT CURRENT IN THE ISOLATED SKIN OF THE SOUTH AMERICAN FROG LEPTODACTYLUS OCELLATUS. J Gen Physiol. 1963 Nov;47:393–402. doi: 10.1085/jgp.47.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E. A., Rotunno C. A., Cereijido M. Ion and water balance in isolated epithelial cells of the abdominal skin of the frog Leptodactylus ocellatus. J Membr Biol. 1973 Oct 10;13(3):199–216. doi: 10.1007/BF01868228. [DOI] [PubMed] [Google Scholar]