Abstract
The PucR protein of Bacillus subtilis has previously been suggested to regulate the expression of 15 genes, pucABCDE, pucFG, pucH, pucI, pucJKLM, pucR, and gde, all of which encode proteins involved in purine catabolism. When cells are grown under nitrogen-limiting conditions, the expression of these genes is induced and intermediary compounds of the purine catabolic pathway affect this expression. By using pucR deletion mutants, we have found that PucR induces the expression of pucFG, pucH, pucI, pucJKLM, and gde while it represses the expression of pucR and pucABCDE. Deletions in the promoters of the five induced operons and genes combined with bioinformatic analysis suggested a conserved upstream activating sequence, 5′-WWWCNTTGGTTAA-3′, now named the PucR box. Potential PucR boxes overlapping the −35 and −10 regions of the pucABCDE promoter and located downstream of the pucR transcription start point were also found. The positions of these PucR boxes are consistent with PucR acting as a negative regulator of pucABCDE and pucR expression. Site-directed mutations in the PucR box upstream of pucH and pucI identified positions that are essential for the induction of pucH and pucI expression, respectively. Mutants with decreased pucH or increased pucR expression obtained from a library of clones containing random mutations in the pucH-to-pucR intercistronic region all contained mutations in or near the PucR box. The induction of pucR expression under nitrogen-limiting conditions was found to be mediated by the global nitrogen-regulatory protein TnrA. In other gram-positive bacteria, we have found open reading frames that encode proteins similar to PucR located next to other open reading frames encoding proteins with similarity to purine catabolic enzymes. Hence, the PucR homologues are likely to exert the same function in other gram-positive bacteria as PucR does in B. subtilis.
The soil bacilli can utilize different nitrogen-containing compounds as their sole source of nitrogen. Bacillus subtilis prefers ammonia or glutamine as its nitrogen source, and it assimilates all of its nitrogen by the glutamine synthetase (GS)-catalyzed reaction NH3 + glutamate + ATP → glutamine + ADP + Pi. When B. subtilis is grown in media with a less preferred nitrogen source, a number of enzymes and permeases involved in the assimilation of nitrogen from alternative nitrogen-containing compounds are induced. Among them are asparaginase (1), gabP-encoded γ-aminobutyric acid permease (6), ureABC-encoded urease (4, 20), amtB-glnK-encoded ammonium transport proteins (19), and nasABCDEF-encoded nitrate assimilatory enzymes (9). The induction of these genes is dependent on the global nitrogen-regulatory protein TnrA. A second global nitrogen-regulatory protein, GlnR, is active during nitrogen excess conditions and negatively regulates the expression of the glnRA operon encoding GlnR and GS. TnrA and GlnR are homologous and bind to the same DNA sequence, known as the TnrA/GlnR box (5′-TGTNAN7TNACA-3′). During growth with excess nitrogen (glutamine or ammonia plus glutamate), feedback-inhibited GS stimulates GlnR binding activity but inhibits the action of TnrA through a direct protein-protein interaction (24), thereby preventing the expression of alternative nitrogen-assimilatory pathways. When cells are grown with limited nitrogen, the binding of GS to TnrA disappears and TnrA becomes active while GlnR binding activity is inhibited. This leads to the induction of alternative nitrogen-assimilatory pathways (7, 21, 23).
B. subtilis is capable of taking up purine bases and using them for nucleotide synthesis and as a source of nitrogen. To enter the catabolic pathway, guanine, adenine, and hypoxanthine must be converted to xanthine (10). The genes encoding the enzymes and permeases necessary for a complete degradation of purine bases to ammonia and for the transport of uric acid and allantoin have recently been identified (15). They are pucABCDE (xanthine dehydrogenase), pucF (allantoate amidohydrolase), pucH (allantoinase), pucI (allantoin permease), pucJK (uric acid transport), and pucLM (uricase). Inactivation of the pucR gene prevents the expression of gde (guanine deaminase) and all of the puc genes (except pucA). The PucR protein (purine catabolism regulator) shows limited sequence homology to hypothetical proteins from various organisms and was suggested to be a transcriptional regulator responsible for the regulation of expression of the genes of the purine catabolic pathway (15).
This work presents genetic evidence that the PucR protein controls the expression of the genes involved in the purine catabolic pathway and that induction requires a cis-acting regulatory element. Furthermore, we have shown that TnrA induces transcription of pucR under nitrogen-limiting conditions and that PucR is not fully active unless purine degradation products are also present.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains used in this work are listed in Tables 1 and 2 and Fig. 3 through 6. All strains are isogenic derivatives of B. subtilis strain 168. Under nitrogen excess conditions, B. subtilis was grown in Spizizen minimal salt medium containing ammonia and glutamate (11). Under nitrogen-limiting conditions, cells were grown in Spizizen minimal salt medium in which disodium sulfate (final concentration, 0.2%) was substituted for ammonium sulfate. The minimal media were supplemented with 0.4% glucose as a carbon source and 40 mg of l-tryptophan/liter. Allantoin (250 mg/liter) was added where indicated. Cells were cultured at 37°C. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added in a 0.1 mM concentration. Antibiotics were used for selection in the following final concentrations: ampicillin (Ap), 100 mg/liter; erythromycin (Er), 1 mg/liter; lincomycin (Ln), 25 mg/liter; neomycin (Neo), 5 mg/liter; chloramphenicol (Cm), 6 mg/liter.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| B. subtilis | ||
| 168 | trpC2 | C. Anagnostopoulosa |
| HØR18 | trpC2 pucR::pBOER | 15 |
| HØR26 | trpC2 pucR::Pspac-pucR | Transformation of 168 by pIMut4 selecting for Err |
| LCC26 | trpC2 tnrA62::Tn917 | Transformation of 168 with DNA from SF416R selecting for Err (2) |
| E. coli | ||
| MC1061 | F−araD139 Δ(ara-leu)7697 galE15 galK16 Δ(lac)74 rpsL (Strr) hsdR2 (r− m+) mcrA mcrB1 | Laboratory stock |
| Plasmids | ||
| pDG268neo | Apr (E. coli) Neor (B. subtilis); vector used for construction of transcriptional lacZ fusion designed to integrate into the amyE gene of B. subtilis | 11 |
| pIMut4 | Lnr, Err, Pspac-pucR, LacZ−; pMUTIN4 digested with EcoRI-SacI, ligated with PCR-generated fragment containing RBS and 211 bp of the 5′-end of pucR; integration into the pucR locus results in a 3′ truncated PucR copy expressed from the pucR promoter and an intact PucR copy expressed from Pspac | This work |
| pMUTIN4 | Integrational plasmid used for gene inactivation by recombination and for generation of transcriptional lacZ fusions | 17 |
| pBOE335 | Apr (E. coli) Cmr (B. subtilis); integration vector, pUC19 containing cat gene cloned into KasI site | 11 |
| pBOER | Cmr; pBOE335 containing an internal part of the pucR transcriptional unit; integration of pBOER results in pucR inactivation | 15 |
Centre National de la Recherche Scientifique, Jouy-en-Josas, France.
TABLE 2.
β-Galactosidase produced from transcriptional fusionsa
| Strain | Relevant genotype | β-Galactosidase activity (U/mg of protein)
|
||
|---|---|---|---|---|
| Glutamate plus NH4+ | Glutamate | Glutamate plus allantoin | ||
| HH422 | amyE::pucA′-lacZ | 1 | 335 | 12 |
| CJ13 | amyE::pucA′-lacZ pucR::pBOER | 1 | 145 | 117 |
| LB032 | amyE::pucF′-lacZ | 1 | 37 | 1,818 |
| CJ12 | amyE::pucF′-lacZ pucR::pBOER | 1 | 2 | 19 |
| CO7 | amyE::pucH′-lacZ | 2 | 17 | 354 |
| CJ10 | amyE::pucH′-lacZ pucR::pBOER | 2 | 2 | 3 |
| HØR19 | amyE::pucI′-lacZ | 1 | 23 | 400 |
| HØR29 | amyE::pucI′-lacZ pucR::pBOER | 1 | 10 | 60 |
| LB035 | amyE::pucJ′-lacZ | 2 | 1,002 | 2,336 |
| CJ14 | amyE::pucJ′-lacZ pucR::pBOER | 1 | 3 | 8 |
| CO3 | amyE::pucR′-lacZ | 1 | 27 | 6 |
| LB175 | amyE::pucR′-lacZ pucR::pBOER | 1 | 508 | 420 |
| LB031 | amyE::gde′-lacZ | 4 | 12 | 160 |
| ED441 | amyE::gde′-lacZ pucR::pBOER | 5 | 5 | 3 |
Fusions were between lacZ and the regulatory region upstream of pucA, pucF, pucH, pucI, pucJ, pucR, or gde integrated at the amyE locus of the wild-type B. subtilis strain and a pucR derivative grown under different nitrogen conditions. Cells were grown in glucose minimal medium supplemented with the indicated nitrogen source.
FIG. 3.
Deletion analysis of PucR-induced promoters. The sequences upstream of the −35 sequence of the indicated gene-lacZ fusions, including the PucR box, are shown. The downstream sequence fusion points are +97 for pucH′-lacZ, +74 for pucI′-lacZ, +137 for pucJ′-lacZ, +94 for pucF′-lacZ, and +78 for gde′-lacZ. In the case of pucJ, the upstream TnrA/GlnR box (Fig. 2B) is also indicated. Cells containing the various lacZ fusions were grown under nitrogen-limiting conditions (glutamate) either with or without allantoin. nt, nucleotides. Boldface indicates a match to the consensus sequence.
DNA manipulations and genetic techniques.
Isolation of DNA and RNA and basic molecular techniques, including primer extension analysis, were performed as described previously (12, 26).
Construction of transcriptional lacZ fusions.
DNA fragments containing native promoters, promoters with deletions, or site-directed mutagenized sequences generated by PCR amplification were used. Random mutagenized sequences were generated by a modified PCR procedure (26). Specific DNA primers fitted with appropriate 5′-positioned restriction sites were used in a PCR to amplify the DNA fragments in question. PCR products were purified and digested with the restriction enzymes specific to the restriction sites incorporated at the 5′ ends of the PCR primers. The digested DNA fragments were ligated to plasmid pDG268neo, which had been digested with the same enzymes. The ligation mixtures were transformed into Escherichia coli MC1061 by selection for Apr. Clones containing the promoter DNA fragments fused in front of lacZ in pDG268neo were identified, and the plasmid construct was isolated. The purified plasmids were linearized by digestion with KpnI and transformed into B. subtilis 168 by selection for Neor. Linearized pDG268neo derivatives were integrated by double homologous recombination into the amyE gene, resulting in Neor and amylase-negative transformants. The promoter containing fragments cloned in front of lacZ was amplified by flanking neo- and lacZ-specific primers, and the nucleotide sequence was determined to identify clones with the correct DNA sequence.
Strains carrying both a lacZ fusion and either the pucR, Pspac-pucR, or tnrA genotype were obtained by transforming the lacZ fusion strains with DNA isolated from HØR18, HØR26, or LCC26, respectively.
Enzyme assay.
Cells were harvested in the exponential growth phase and homogenized by sonication in Z buffer (0.06 M Na2HPO4, 0.04 M NaH2PO4, 0.01 M KCl, 0.001 M MgSO4; pH 7). β-Galactosidase activity was measured as previously described (2). One unit of activity is defined as 1 nmol of substrate converted per min per mg of protein. The total protein content was determined by the Lowry method. All enzyme levels reported are the means of results of at least three experiments. The variation was less than 25%.
Homology searches.
Preliminary sequence data were obtained from The Institute for Genomic Research website (http://www.tigr.org).
RESULTS
Mapping of the transcription start site of the pucABCDE, pucFG, pucH, pucI, pucJKLM, and pucR transcriptional units.
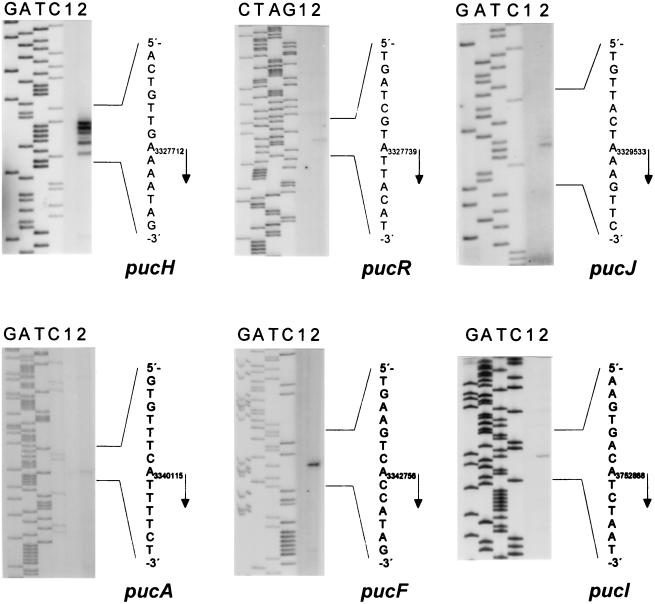
Based on inspection of the nucleotide sequence and on the pattern of gene expression described by Schultz et al. (15), six potential transcriptional units, pucABCDE, pucFG, pucH, pucI, pucJKLM, and pucR, were suggested. For all six potential promoter-containing regions, the transcription start site (+1 position) was determined by primer extension analysis (Fig. 1). No cDNA was produced in the reactions with RNA extracted from cells grown with excess nitrogen, whereas the presence of RNA from cells grown under induced conditions (glutamate plus allantoin) resulted in detectable amounts of specific cDNA products. The pattern of five specific cDNA products generated in the reaction containing the pucH-specific DNA primer was repeatedly obtained in three independent experiments and may be due to transcriptional slippage (25). However, the position corresponding to the 5′ end of the longest cDNA product was chosen as the +1 position for pucH transcription.
FIG. 1.
Mapping of the transcription start sites for pucA, pucF, pucH, pucI, pucJ, and pucR by primer extension. Total RNA was isolated from strain 168 grown with ammonia and glutamate (lanes 1) or glutamate and allantoin (lanes 2) as the nitrogen source. The most likely transcription start point for each gene is indicated by an arrow.
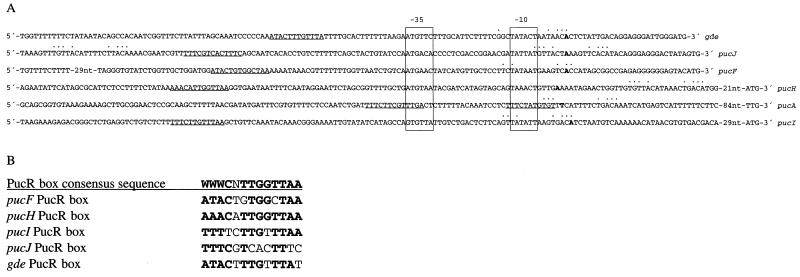
The nucleotide sequences of five of the promoter regions together with that of gde (10) were aligned with respect to the deduced −10 and −35 promoter elements (Fig. 2A). A sequence resembling the consensus TnrA/GlnR box (5′-TGTNAN7TNACA-3′) was detected overlapping the +1 position of all of the genes except for pucH, for which it was located downstream of the +1 position (Fig. 2A). In the case of pucJ, a second TnrA/GlnR box was located upstream of the −35.
FIG. 2.
(A) Alignment of the PucR-regulated promoters of pucA, pucF, pucH, pucI, pucJ, and gde with respect to the −10 and −35 sequences. Boldface letters indicate transcription start sites (as determined from results shown in Fig. 1), −10 and −35 sequences are boxed, PucR boxes (5′-WWWCNTTGGTTAA-3′) are underlined, and matches to TnrA/GlnR boxes (5′-TGTNAN7TNACA-3′) are indicated by dots above the nucleotides. nt, nucleotides. (B) Alignment of the potential PucR boxes in the PucR-induced genes. Boldface indicates a match to the consensus sequence.
Transcriptional control of pucA, pucF, pucH, pucI, pucJ, pucR, and gde expression requires PucR.
DNA fragments containing the regulatory regions upstream of pucA, pucF, pucH, pucI, pucJ, pucR, and gde were fused to the lacZ reporter gene and integrated into the amyE locus. The β-galactosidase activity in cultures of the various fusion strains grown under different nitrogen conditions was determined. All lacZ fusions were induced during growth with limited nitrogen (glutamate). The addition of allantoin to glutamate cultures resulted in a further 2- to 49-fold increase of expression of all lacZ fusions, except for pucA′-lacZ and pucR′-lacZ expression, which were repressed 28- and 4-fold, respectively (Table 2). These results are in agreement with the results described by Schultz et al. (15).
To demonstrate the role of PucR in the expression of the puc gene lacZ fusions, a pucR disruption mutation (pucR::pBOER) was introduced into the lacZ fusion strains. Compared to the expression in wild-type fusion strains grown under nitrogen-limiting conditions (glutamate), the expression of the lacZ fusions—except for that of pucR—was decreased in the respective pucR derivatives grown under the same conditions (Table 2). The decrease in expression varied between 2-fold for pucI and 334-fold for pucJ. The β-galactosidase level in the pucR derivatives grown with allantoin was in general reduced to approximately less than 1% of the levels in the wild-type strains, except for pucI, in which it was significantly higher. This may suggest that additional regulatory factors have an influence on pucI expression. Again, pucA′-lacZ and pucR′-lacZ expression were derepressed in the pucR strain compared to those in the wild type (Table 2). We therefore conclude that pucR acts as a positive factor in relation to the pathway-specific induction of pucH, pucJ, pucF, pucI, and gde expression and as a negative factor in relation to pucR and pucA expression.
We introduced the lacZ fusions into a pucD disruption strain to test whether the PucR-dependent induction also would occur under nitrogen-limiting conditions (glutamate) in a strain unable to produce purine degradation products, as a pucD disruption strain cannot degrade guanine or hypoxanthine to uric acid. The lacZ fusion strains carrying the pucD disruption displayed the same expression pattern (data not shown) as the wild-type strains. Thus, we can conclude that PucR is able to induce transcription without purine degradation products but that the induction is much more effective when purine degradation products are available.
Deletion analysis of puc gene promoter regions.
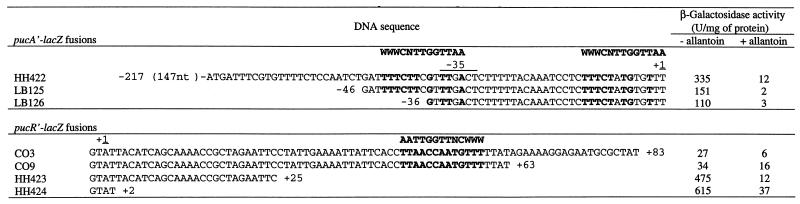
Since PucR induces transcription from the promoters of pucF, pucH, pucI, pucJ, and gde and the PucR C-terminal sequence shows some similarity to a LysR-like DNA binding domain (15), we looked for the presence of a conserved sequence upstream of the promoters that might function as a recognition site for PucR. As we could not identify any conserved sequence by visual inspection, we made a systematic deletion analysis of all five promoter regions. In all five analyses, transcription ceased when the deletions reached a certain point (Fig. 3), but no obviously common DNA sequence could be immediately identified around these points. The Ann-spec (18) bioinformatics computer program was used to search for conserved sequences within a region spanning 100 bp upstream of the −35 sequence of the promoters of pucF, pucH, pucI, pucJ, and gde. Since LysR-like regulators normally recognize the sequence T-N11-A (8, 13), the request was for a 13-bp sequence. The five 100-bp sequences were searched for a conserved sequence and compared to approximately 4,100 other upstream gene sequences from B. subtilis. Several 13-bp sequences were suggested. However, only one of the suggested sequences was supported by the experimental data presented in Fig. 3. This sequence was 5′-WWWCNTTGGTTAA-3′, tentatively called the PucR box. The potential PucR box sequences in front of the puc genes and gde are aligned in Fig. 2B. Partial or total deletion of the PucR box can explain the loss of promoter activity in deletion mutants of the pucF′-, pucH′-, pucI′-, pucJ′-, and gde′-lacZ promoter fusions. It appears that the activation of transcription from these five genes requires a cis-acting sequence containing the PucR box motif and that this sequence could be part of a recognition site for the putative activator protein encoded by pucR.
The effect of the TnrA/GlnR box located upstream of pucJ was analyzed in strains LB035 and LB056, in which the TnrA/GlnR box was deleted (Fig. 3). A deletion of the TnrA/GlnR box did not affect expression under nitrogen-limiting conditions (glutamate), but with limited nitrogen plus allantoin the expression was elevated by a factor of 2.5. Hence, the TnrA/GlnR box upstream of the −35 sequence in the pucJ promoter exerts a negative regulatory function on pucJ expression under induced-growth conditions.
When the potential PucR box in front of pucR was deleted, the activity from the pucR′-lacZ promoter fusion increased almost 20-fold when cells were grown under nitrogen-limiting conditions (Fig. 4). Surprisingly, the activity was only slightly increased for limited nitrogen supplied with allantoin. This indicates that there might be another sequence important for the repression of the pucR promoter when allantoin is present (see below). Two potential PucR boxes overlapping the −35 and −10 sequences in the pucA promoter were also found (Fig. 2A and 4). This finding agrees with the data showing that PucR inhibited transcription from the pucA promoter under nitrogen-limiting conditions with allantoin present (Table 2).
FIG. 4.
Deletion analysis of PucR-repressed promoters. The upstream sequences and the positions of the upstream fusion points of the pucA′-lacZ fusions are shown (the downstream sequence fusion point is +180), as are the positions of the downstream sequences and the downstream fusion points of the pucR′-lacZ fusions (the upstream sequence fusion point is −123). The PucR box is indicated (boldface indicates a match with the consensus sequence), as is the −35 sequence. A complementary PucR box in the pucR′-lacZ fusions is located in the noncoding strand. Cells containing the various lacZ fusions were grown under nitrogen-limiting conditions (glutamate) either with or without allantoin. nt, nucleotides.
Site-directed and random mutagenesis analysis of the pucH and pucI regulatory regions.
In order to verify the PucR box motif, site-directed mutagenesis was performed in all of the positions of the PucR box in the pucH promoter and β-galactosidase activity was measured in the respective fusion strains (Fig. 5). The three substitutions in LB079, LB094, and LB067 caused an almost complete loss of activity, as observed in some of the promoter deletions (see Fig. 3, strain LB086). Other substitutions had a less dramatic effect. The substitutions in LB076, LB078, and LB081 decreased the activity two- to fourfold compared to that of the wild type. To test whether some of these substitutions had the same effect on the expression from other puc promoters, site-directed substitutions were made in the PucR box of the pucI regulatory region at the same three positions. Promoter activity in LB129 and LB131 was greatly reduced, as it was in LB079 and LB067, and the promoter activity was impaired in strain LB130, as it was in LB078. Thus, changes in the same positions in the PucR boxes upstream of pucI and pucH resulted in similar decreases in transcription in the mutants. We therefore conclude that the PucR box suggested by the use of bioinformatics covers some very important bases for PucR activation. Interestingly, the G-to-C mutation in the pucH′-lacZ fusion in strain LB094 almost abolished activity; however, in the pucI PucR box of the wild type, there is a T instead of a G at this position. This indicates that the PucR box might be more degenerate than suggested, as other deviations from the proposed PucR box consensus sequence were observed in the other candidate PucR boxes.
FIG. 5.
Site-directed mutational analysis of the proposed PucR box in the pucH and pucI regulatory regions. The sequences upstream of the −35 region of the pucH′-lacZ and pucI′-lacZ fusions are listed. The PucR box is indicated (boldface indicates a match to the consensus sequence), and the positions of the upstream fusion points are shown. The downstream sequence fusion points are +97 for pucH′-lacZ and +74 for pucI′-lacZ. Cells containing the various lacZ fusions were grown under nitrogen-limiting conditions (glutamate) either with or without allantoin. wt, wild type.
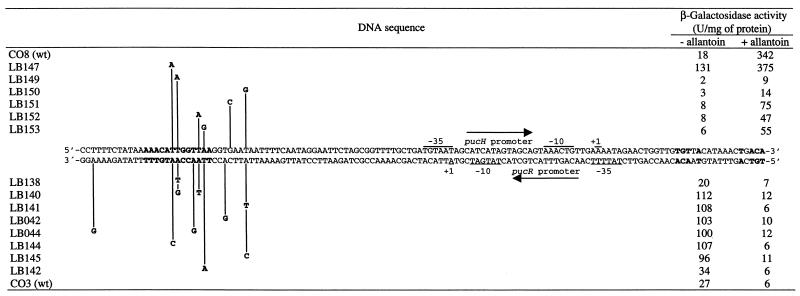
The pucH and pucR reading frames are divergently oriented and separated by a 174-bp intercistronic sequence that contains a PucR box. To investigate whether there were important positions other than the putative PucR box, random mutagenesis was performed on the upstream promoter sequences of the pucH and pucR genes fused to lacZ. Clones from the pucH′-lacZ library in which β-galactosidase activity was decreased under nitrogen-limiting conditions with allantoin were isolated and sequenced. All of the mutations were found in or downstream of the deduced PucR box (Fig. 6). From a similar pucR′-lacZ library, clones that showed increased β-galactosidase activity when grown under nitrogen-limiting conditions without allantoin were selected and analyzed. Here the mutations were found in or near the PucR box, corresponding to the same area found in the pucH′-lacZ library. The fivefold increase in pucR′-lacZ expression in the randomly mutagenized pucR′-lacZ mutants was observed only in glutamate-grown cultures (Fig. 6). The same tendency, but more pronounced, was observed in a mutant strain with a deletion of the entire PucR box (475 U/mg of protein; strain HH423, Fig. 4). In both cases, the expression level in glutamate-plus-allantoin-grown cultures was similar to the wild-type level (approximately 6 to 12 U/mg of protein). Only in a pucR-defective strain was pucR′-lacZ expression constitutively high under both nitrogen conditions (strain LB175, Table 2). Deletion of or mutations in the same PucR box in a pucH′-lacZ fusion strain (Fig. 3, 5, and 6) resulted in a complete loss of pucH expression. This leads us to suggest that the PucR box in the pucH-to-pucR intercistronic region is strictly required for the induction of pucH expression while it is only partially required for the repression of pucR expression and that a second cis-acting element located upstream of the PucR box is required for complete pucR repression. The second cis-acting element may be an additional PucR box located upstream of the pucR +1 position overlapping the −35 pucR sequence. This potential PucR box has less homology to the consensus sequence, and due to the lower homology, it is possible that PucR may bind to it only when an inducer molecule, i.e., allantoin, is present. Hence, PucR binds to this additional PucR box only under nitrogen-limiting conditions with purine degradation products available, and this binding is responsible for the repression of pucR expression under these conditions.
FIG. 6.
Random mutational analysis of the proposed PucR box in the pucH and pucR regulatory region. Part of the pucH-to-pucR intercistronic region is presented as a double-stranded DNA sequence. The pucH′-lacZ fusion strains (the upstream and downstream sequence fusion points are −110 and +97) are listed above the double-stranded DNA sequence, and the pucR′-lacZ fusion strains (the upstream and downstream sequence fusion points are −123 and +84) are listed below. The PucR box (5′-WWWCNTTGGTTAA-3′) and TnrA/GlnR box (5′-TGTNAN7TNACA-3′) are listed (boldface indicates a match to the consensus sequence), and the −10 and −35 sequences and the +1 positions are indicated. Cells containing the various lacZ fusions were grown under nitrogen-limiting conditions (glutamate) either with or without allantoin. wt, wild type.
The global nitrogen regulator TnrA controls pucR expression.
A single copy of the palindromic TnrA/GlnR box was identified in the pucH-to-pucR intercistronic region (Fig. 6). The TnrA/GlnR box is located 10 bp upstream of the deduced −35 region of the pucR promoter. TnrA/GlnR boxes were found at a similar distance upstream of other genes that have been shown to be activated by the TnrA activator protein (22). To test whether the induction of pucR expression is mediated by TnrA, a pucR′-lacZ fusion strain (CO6) with a deletion of the TnrA/GlnR box was constructed and its pucR′-lacZ expression was compared to that of strain CO3 (Table 2), which contains a pucR′-lacZ fusion including the TnrA/GlnR box. The β-galactosidase level in CO6 grown under nitrogen-limiting conditions (glutamate) was 4 U/mg of protein, compared to 27 U/mg of protein in CO3. A strain (CJ015) was constructed that contained the pucR′-lacZ fusion of strain CO3 in a tnrA genetic background, and as found in strain CO6, pucR′-lacZ expression could no longer be induced (4 U/mg of protein during growth with glutamate). We therefore conclude that TnrA activates the expression of pucR during nitrogen-limiting conditions.
To test whether TnrA also plays a direct role in the PucR-dependent activation of puc genes and gde, the pucR gene expression was put under the control of the IPTG-inducible Pspac promoter. This construction (pIMut4, Table 1) was introduced into the pucA′-, pucF′-, pucH′-, pucI′-, pucJ′-, and gde′-lacZ fusion strains. The strains were grown under nitrogen excess conditions (ammonia plus glutamate), in which the TnrA protein is not active. The basal level of pucR expression under nitrogen excess conditions for the pucH′-lacZ fusion strain under the Pspac promoter was 4 U/mg of protein. In the presence of IPTG, which induces pucR expression, the level was increased to 11 U/mg of protein, while the addition of both IPTG and allantoin led to a strong induction of expression of the pucH′-lacZ fusion (371 U/mg of protein). These levels were similar to the β-galactosidase level of strain CO7, which carries the same lacZ fusion in a PucR+ background. The same pattern of expression was observed for pucF′-, pucI′-, pucJ′-, and gde′-lacZ fusion strains (data not shown). However, in the case of a pucA′-lacZ fusion strain, the β-galactosidase level was low (2 to 3 U/mg of protein) under all three conditions. This experiment demonstrates that synthesis of PucR, even under growth conditions where pucR normally is not expressed (excess nitrogen conditions), stimulates transcription from the promoters of pucF, pucH, pucI, pucJ, and gde. Expression was further stimulated 15- to 42-fold by allantoin, and the PucR protein did not require active TnrA protein to induce expression. PucR induction and the addition of allantoin in the Pspac-pucR strain did not induce pucA transcription. This is consistent with the observation that in the presence of allantoin, PucR most likely has a negative effect on pucA expression. The induction of pucA is PucR independent, since induction is observed in a pucR genetic background (Table 2). TnrA may be a candidate for a transcription factor responsible for the induction of pucA under nitrogen-limiting conditions. Evidence for this view comes from the finding that the expression of pucABCDE is high in a glnA background (10). However, this was not tested experimentally.
DISCUSSION
Similarity searches in the recently fully sequenced genomes and in partially finished sequenced genomes revealed that at least five other gram-positive bacteria, Enterococcus faecalis V583, Bacillus halodurans C-125, Mycobacterium smegmatis, Listeria monocytogenes 4-b, and Clostridium acetobutylicum, possess proteins with significant amino acid similarity to PucR. In both E. faecalis and B. halodurans, the pucR-like genes are located together with other genes encoding putative purine catabolic functions. The gene homologous to pucR in E. faecalis (open reading frame [ORF] EF2995) and ORF EF2994, which encodes a putative transaminase similar to B. subtilis PucG, are divergently oriented, which suggests that the two genes are divergently transcribed. ORFs encoding a PucF-like allantoate amidohydrolase (EF2997), a PucH-like allantoinase (EF2999), and a PucI-like allantoin permease (EF3000) are located downstream of the putative pucR in E. faecalis. In B. halodurans, the pucR (ORF BH0757) is also located at a position that suggests that it is divergently transcribed from three ORFs (BH0758, BH0759, and BH0760) which encode a putative uricase similar to the uricase encoded by the B. subtilis pucLM operon. In C. acetobutylicum, a PucR homologue (ORF CAC1426) was not found to be linked to other genes recognized as being involved in purine catabolism. In fact, only very limited similarity was found to genes from the purine catabolic pathway in B. subtilis in this bacterium. However, ORFs with amino acid sequence similarity to PucR, PucH, and PucJ have been found in the partially sequenced genomes of Clostridium perfringens, L. monocytogenes, and M. smegmatis. Thus, PucR is found not only in B. subtilis but also in other gram-positive bacteria and is often linked to other ORFs with similarity to purine catabolic enzymes. Therefore, the PucR homologues in these bacteria are likely to exert the same function as PucR in B. subtilis.
Two proteins, SrmR and SdaR, with known functions as transcriptional activators were recorded among the proteins showing similarity to PucR (15). An alignment (15) indicated that PucR, SrmR, and SdaR might contain a LysR-like DNA-binding domain in their C termini. However, the general sequence similarity to the LysR family and other known families of regulatory proteins is not significant, indicating that PucR is not related to any of the other known families. We therefore suggest that PucR, SrmR, SdaR, and the PucR homologues in E. faecalis, B. halodurans, M. smegmatis, C. perfringens, and C. acetobutylicum may constitute a novel family of transcriptional regulators.
Based on the results presented in this paper and on the work of Schultz et al. (15), we suggest a model for the global nitrogen catabolite repression and pathway-specific regulation of the PucR regulon genes in B. subtilis. Purine catabolic genes, together with other genes encoding alternative nitrogen-assimilatory pathways, are not expressed during nitrogen excess conditions due to the inhibition of TnrA activity by GS encoded by glnA. The glnRA operon repressor GlnR controls the level of glnA expression during excess nitrogen conditions (7, 14). Under nitrogen-limiting conditions (glutamate as nitrogen source), TnrA becomes active while GlnR becomes inactive. TnrA activates pucR transcription most likely by binding to the TnrA/GlnR box located upstream of the pucR promoter (Fig. 6). As shown in Table 2, expression of the pucABCDE, pucFG, pucI, pucH, pucJKLM, pucR, and gde operons is induced during growth with glutamate as the nitrogen source and, except in the cases of pucABCDE and pucR, PucR appears to be required for the expression of all puc genes, including gde (Table 2). The expression of pucABCDE, which encodes the subunits of xanthine dehydrogenase (XDH), which oxidizes hypoxanthine and xanthine to uric acid, is induced to a relatively high level during growth with glutamate as the nitrogen source. Uric acid acts together with allantoin and allantoic acid as the effector molecules for PucR-activated transcription (15).
The combination of nitrogen-limiting conditions and excess purine or purine degradation intermediates (e.g., glutamate plus allantoin as the nitrogen source) results in PucR-dependent repression of pucABCDE and pucR expression and strong induction of pucFG, pucH, pucI, pucJKLM, and gde expression (Table 2). These observations are in agreement with previous findings (15). Based on the position of the putative PucR recognition site (PucR box) as revealed by genetic and bioinformatic analysis, we are able to explain both the positive and negative roles of PucR in puc and gde gene expression. The PucR-induced promoters contain PucR boxes located 17 bp (gde) to 39 bp (pucH and pucI) upstream of the −35 elements (Fig. 2 and 3). These upstream promoter positions are consistent with the suggested transcription activator function of PucR. The PucR-repressed promoters in front of pucR and pucABCDE (Fig. 2 and 4) have PucR boxes overlapping the promoter −35 element (pucABCDE) and/or located downstream of the transcription start point (pucABCDE and pucR). Binding of PucR to these sites in the presence of an inducer represses transcription. An alternative explanation for the repression of pucR is that because (i) the pucR and pucH promoters overlap and (ii) PucR activates pucH transcription, the activation of pucH transcription (by PucR) reduces pucR transcription due to the competition of RNA polymerase for the two overlapping promoters. Since the pucH promoter is expressed at low levels in strains containing either a pucR mutation or an inactive PucR-binding site, this competition would not occur and the pucR promoter would be transcribed at higher levels. By subjecting pucABCDE (XDH) expression to uric acid-, allantoin-, or allantoic acid-induced PucR repression, B. subtilis is able to modulate the cellular contents of purine catabolic enzymes in response to the available nitrogen source. During nitrogen-limiting conditions in the absence of purine degradation intermediates, XDH (pucABCDE) is expressed at higher levels than the other puc gene-encoded catabolic enzymes. The presence of uric acid, allantoin, or allantoic acid, which are the inducers of PucR-activated transcription, results in a shift in the relative gene expression towards the expression of pucLM (uricase), pucH (allantoinase), and pucF (allantoic acid amidohydrolase) instead of pucABCDE (XDH), which is not required for purine degradation under these growth conditions.
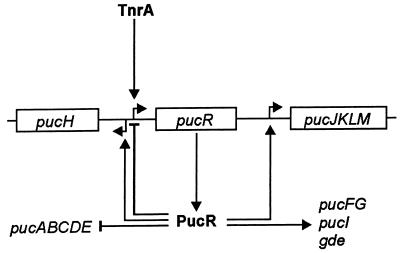
A model for the role of PucR in the regulation of the purine catabolic genes under nitrogen-limiting conditions with purine degradation products available based on the experimental results is presented in Fig. 7. Under nitrogen-limiting conditions, TnrA is activated and induces the expression of PucR. When purine degradation products also are present, PucR induces transcription of gde and the puc genes, except for pucABCDE and pucR, which it represses.
FIG. 7.
Model for the role of PucR in the regulation of purine degradation in B. subtilis under nitrogen-limiting conditions with purine degradation products (e.g., allantoin) available. TnrA induces transcription from pucR, and PucR induces transcription from the purine catabolic genes, as purine degradation products are available. PucR also inhibits its own transcription and transcription from the pucABCDE operon. The sizes of and distances between genes and operons are not drawn to scale. Short bent arrows indicate promoters; long arrows indicate induction; lines ending in a bar indicate repression; boxes denote genes or operons.
The control of purine degradation in the two bacterial species B. subtilis and E. coli has now been revealed and turns out to be quite different between the species. While purine degradation in B. subtilis is subjected to a two-level positive control mechanism involving TnrA (global nitrogen state) and PucR (pathway-specific regulation), the degradation of allantoin in E. coli is controlled by a two-level negative control mechanism involving aerobic-anaerobic control (global state) and the all operon repressor AllR (pathway-specific induction) (5). The two-level positive control mechanism reported here for B. subtilis is similar to those reported for the control of allantoin degradation in Saccharomyces cerevisiae (3) and purine degradation in Aspergillus nidulans (16). In these two lower eukaryotes, allantoin and purine degradation are also subjected to global nitrogen catabolite control and pathway-specific regulation. The GLN3 protein of S. cerevisiae and the AREA protein of A. nidulans sense the nitrogen state of the cell and activate transcription under nitrogen-limiting conditions. In concert with the pathway-specific regulatory proteins DAL81 and DAL82, GLN3 activates allantoin-degradative genes in S. cerevisiae in the presence of the pathway-specific inducer allophanate. The AREA protein in A. nidulans together with the regulatory protein UaY activates purine-degradative genes when uric acid is present. In both S. cerevisiae and A. nidulans, the global and the pathway-specific regulators act in concert on the relevant promoters, while B. subtilis has evolved a hierarchical mechanism by which the global regulator TnrA controls the level of the pathway-specific activator PucR.
The TnrA/GlnR box located upstream of the pucR promoter was found to be required for the induction of pucR expression. Except in the case of pucR, TnrA/GlnR boxes were located around the +1 position of all puc genes and gde. The presence of TnrA/GlnR boxes in front of puc genes and gde may indicate that TnrA is required for gene expression. However, this is not likely the case, since the induction of PucR synthesis in strains growing under excess nitrogen conditions—conditions during which TnrA is inactive—resulted in the activation of puc and gde gene expression. However, in the case of pucJKLM, TnrA may play a role in gene expression. The pucJKLM operon has two potential TnrA/GlnR boxes, and deletion of the upstream promoter box resulted in derepression of the PucR-dependent pucJ expression (Fig. 3). The TnrA/GlnR box and the PucR box are separated by 12 nucleotides (Fig. 2), and binding of TnrA to the TnrA/GlnR box under nitrogen-limiting conditions may reduce the binding efficiency of PucR to the downstream-located PucR box. TnrA may therefore be directly involved in the modulation of functions connected to the uptake and oxidation of uric acid; however, this remains to be shown experimentally.
Acknowledgments
We thank Kirsten Hansen for invaluable technical assistance.
This work was supported by EU contract BIO2-CT95-0278 and by Danish National Science Research Council grant 9901855. This project also received financial support from the Novo Nordisk Foundation and from the Saxild Family Foundation.
REFERENCES
- 1.Atkinson, M. R., and S. H. Fisher. 1991. Identification of genes and gene products whose expression is activated during nitrogen-limited growth in Bacillus subtilis. J. Bacteriol. 173:23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christiansen, L. C., S. Schou, P. Nygaard, and H. H. Saxild. 1997. Xanthine metabolism in Bacillus subtilis: characterization of the xpt-pbuX operon and evidence for purine- and nitrogen-controlled expression of genes involved in xanthine salvage and catabolism. J. Bacteriol. 179:2540-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper, T. G. 1996. Regulation of allantoin catabolism in Saccharomyces cerevisiae, p. 139-169. In R. Brambl and G. A. Marzluf (ed.), The mycota III: biochemistry and molecular biology. Springer-Verlag KG, Berlin, Germany.
- 4.Cruz-Ramos, H., P. Glaser, L. V. Wray, Jr., and S. H. Fisher. 1997. The Bacillus subtilis ureABC operon. J. Bacteriol. 179:3371-3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cusa, E., N. Obradors, L. Baldoma, J. Badia, and J. Aguilar. 1999. Genetic analysis of a chromosomal region containing genes required for assimilation of allantoin nitrogen and linked glyoxylate metabolism in Escherichia coli. J. Bacteriol. 181:7479-7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferson, A. E., L. V. Wray, Jr., and S. H. Fisher. 1996. Expression of the Bacillus subtilis gabP gene is regulated independently in response to nitrogen and amino acid availability. Mol. Microbiol. 22:693-701. [DOI] [PubMed] [Google Scholar]
- 7.Fisher, S. H. 1999. Regulation of nitrogen metabolism in Bacillus subtilis: vive la différence! Mol. Microbiol. 32:223-232. [DOI] [PubMed] [Google Scholar]
- 8.Goethals, K., M. Van Montagu, and M. Holsters. 1992. Conserved motifs in a divergent nod box of Azorhizobium caulinodans ORS571 reveal a common structure in promoters regulated by LysR-type proteins. Proc. Natl. Acad. Sci. USA 89:1646-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakano, M. M., F. Yang, P. Hardin, and P. Zuber. 1995. Nitrogen regulation of nasA and the nasB operon, which encode genes required for nitrate assimilation in Bacillus subtilis. J. Bacteriol. 177:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nygaard, P., S. M. Bested, K. A. Andersen, and H. H. Saxild. 2000. Bacillus subtilis guanine deaminase is encoded by the yknA gene and is induced during growth with purines as the nitrogen source. Microbiology 146:3061-3069. [DOI] [PubMed] [Google Scholar]
- 11.Saxild, H. H., L. N. Andersen, and K. Hammer. 1996. dra-nupC-pdp operon of Bacillus subtilis: nucleotide sequence, induction by deoxyribonucleosides, and transcriptional regulation by the deoR-encoded DeoR repressor protein. J. Bacteriol. 178:424-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saxild, H. H., J. H. Jacobsen, and P. Nygaard. 1994. Genetic and physiological characterization of a formate-dependent 5′-phosphoribosyl-1-glycinamide transformylase activity in Bacillus subtilis. Mol. Gen. Genet. 242:415-420. [DOI] [PubMed] [Google Scholar]
- 13.Schell, M. A. 1993. Molecular biology of the LysR transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 14.Schreier, H. J., S. W. Brown, K. D. Hirschi, J. F. Nomellini, and A. L. Sonenshein. 1989. Regulation of Bacillus subtilis glutamine synthetase gene expression by the product of the glnR gene. J. Mol. Biol. 210:51-63. [DOI] [PubMed] [Google Scholar]
- 15.Schultz, A. C., P. Nygaard, and H. H. Saxild. 2001. Functional analysis of 14 genes that constitute the purine catabolic pathway in Bacillus subtilis and evidence for a novel regulon controlled by the PucR transcription activator. J. Bacteriol. 183:3293-3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suárez, T., M. V. de Queiroz, N. Oestreicher, and C. Scazzocchio. 1995. The sequence and binding specificity of UaY, the specific regulator of the purine utilization pathway in Aspergillus nidulans, suggest an evolutionary relationship with the PPR1 protein of Saccharomyces cerevisiae. EMBO J. 14:1453-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 18.Workman, C. T., and G. D. Stormo. 2000. Ann-spec: a method for discovering transcription factor binding sites with improved specificity, p. 467-478. In Proceedings from the Pacific Symposium on Biocomputing 2000. [DOI] [PubMed]
- 19.Wray, L. V., Jr., M. R. Atkinson, and S. H. Fisher. 1994. The nitrogen-regulated Bacillus subtilis nrgAB operon encodes a membrane protein and a protein highly similar to the Escherichia coli glnB-encoded PII protein. J. Bacteriol. 176:108-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wray, L. V., Jr., A. E. Ferson, and S. H. Fisher. 1997. Expression of the Bacillus subtilis ureABC operon is controlled by multiple regulatory factors including CodY, GlnR, TnrA, and Spo0H. J. Bacteriol. 179:5494-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wray, L. V., Jr., A. E. Ferson, K. Rohrer, and S. H. Fisher. 1996. TnrA, a transcription factor required for global nitrogen regulation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 93:8841-8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wray, L. V., Jr., J. M. Zalieckas, A. E. Ferson, and S. H. Fisher. 1998. Mutational analysis of the TnrA-binding sites in the Bacillus subtilis nrgAB and gabP promoter regions. J. Bacteriol. 180:2943-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wray, L. V., Jr., J. M. Zalieckas, and S. H. Fisher. 2000. Purification and in vitro activities of the Bacillus subtilis TnrA transcription factor. J. Mol. Biol. 300:29-40. [DOI] [PubMed] [Google Scholar]
- 24.Wray, L. V., Jr., J. M. Zalieckas, and S. H. Fisher. 2001. Bacillus subtilis glutamine synthetase controls gene expression through a protein-protein interaction with transcription factor TnrA. Cell 107:427-435. [DOI] [PubMed] [Google Scholar]
- 25.Xiong, X. F., and W. S. Reznikoff. 1993. Transcriptional slippage during the transcription initiation process at a mutant lac promoter in vivo. J. Mol. Biol. 231:569-580. [DOI] [PubMed] [Google Scholar]
- 26.Zeng, X., and H. H. Saxild. 1999. Identification and characterization of a DeoR-specific operator sequence essential for induction of dra-nupC-pdp operon expression in Bacillus subtilis. J. Bacteriol. 181:1719-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]