Abstract
This study examined the expression of a neuron-specific cell adhesion molecule, OBCAM (opioid-binding cell adhesion molecule), at both the mRNA and protein levels in the cat primary visual cortex at various postnatal ages, using cDNA array analysis and immunocytochemistry. Results obtained using both methods showed that the expression level of OBCAM was high in young and low in older and adult visual cortex. OBCAM-immunoreactivities were associated predominantly with perikarya and dendrites of pyramidal neurons, and OBCAM-immunopositive neurons were present in all cortical layers. Immunostaining of OBCAM in adult visual cortex showed a reduced number of immunopositive neurons and neurites and relatively lower staining intensities as compared with younger animals. In addition, the number of OBCAM-immunopositive neurons was significantly higher in the visual cortex of 4-month-old animals dark-reared from birth than those in age-matched normally reared animals. These results suggest that OBCAM may play an important role in visual cortex development and plasticity.
Keywords: cDNA microarray, dark-rearing, opioid-binding cell adhesion molecule, striate cortex
Introduction
Since the pioneering work of Wiesel and Hubel (1963), extensive investigations have improved our understanding of visual cortex development and plasticity (Hubel and Wiesel, 1970; Movshon and van Sluyters, 1981; Sherman and Spear, 1982; Fregnac and Imbert, 1984; Daw, 1995). Much is now known about the functional characteristics of visual cortex plasticity, such as the conditions and timing of interventions that can affect visual cortex development. The time-course of maturation of visual response parameters such as the receptive field properties of cortical neurons, as well as the critical period during which neuronal connectivity in the visual cortex is sensitive to experience-dependent modifications, have been well characterized. At the cellular and molecular levels, a number of neurotransmitters, neurotrophins and hormones have been shown to play important roles in visual cortex plasticity (Rauschecker, 1991; Cellerino and Maffei, 1996; Katz and Shatz, 1996; Gu, 2002; Berardi et al., 2003; Daw, 2004). Recently, it has also been shown that blockade of protein synthesis in the visual cortex but not in the lateral geniculate nucleus (LGN) prevents ocular dominance plasticity (Taha and Stryker, 2002), suggesting that gene expression in the visual cortex is crucial for its developmental plasticity. Despite the progress in our understanding of visual cortex plasticity, many issues at the molecular level remain to be elucidated. With emerging techniques such as cDNA array analysis, it is now possible to examine the expression pattern of a large number of genes at once, and questions concerning visual cortex development and plasticity at the molecular level can be investigated in greater detail. We have been using high-density cDNA array technology to analyze gene expression patterns in the visual cortex during postnatal development (Prasad et al., 2002). Our hypothesis is that differential gene expression may contribute to neuronal plasticity in the developing visual cortex. This hypothesis requires continuing expression of crucial molecules when visual cortex is adjusting and fine-tuning its neural circuitry within a critical period during postnatal development. The expression levels of plasticity candidate genes have to be either high at the peak of visual cortex plasticity during early postnatal development and low in mature visual cortex, or else the opposite, depending upon their particular role. Thus, for example, elevated expression levels may be expected early in life for genes that code for proteins necessary for activity-dependent sprouting and retraction of axons, as well as consolidation and elimination of synaptic connectivity.
In the present study, we focused on a neuron-specific cell adhesion molecule, OBCAM (opioid-binding cell adhesion molecule), which was first purified from rat brain as an opioid-binding protein with the molecular weight of 58 kDa (Cho et al., 1986). Subsequent sequence and structure analyses have revealed that it contains 345 amino acids, including three immunoglobulin-like domains anchored to the membrane through a glycosylphosphatidylinositol tail, and that it belongs to the immunoglobulin (Ig) superfamily of cell adhesion molecules (Schofield et al., 1989). In the nervous system, a variety of Ig-superfamily cell adhesion proteins have been discovered which are important regulators of axonal growth, fasciculation, synaptogenesis and synaptic plasticity (Brummendorf and Rathjen, 1996; Faivre-Sarrailh and Rougon, 1997; Walsh and Doherty, 1997; Boulanger et al., 2001). Although a specific physiological function of OBCAM has not been established, available evidence concerning the general properties of cell adhesion molecules suggests that they may be of particular relevance to neuronal plasticity since they intervene in most cell–cell and cell–matrix interactions and participate in the establishment and remodeling of neural circuits (Doherty et al., 1995; Hoffman, 1998; Ronn et al., 1998; Boulanger et al., 2001; Kiss and Muller, 2001).
We used a commercially obtained cDNA array (Prasad et al., 2002) to detect OBCAM expression at the mRNA level at various postnatal developmental stages, and employed a specific monoclonal antibody against amino acids 185–202 of OBCAM (Hachisuka et al., 1996; Nakajima et al., 1997) to examine the distribution and developmental changes of OBCAM in cat primary visual cortex. Our results revealed similar expression profiles of OBCAM at both mRNA and protein levels, namely high expression levels at ~30 days of age and lower expression levels in adult visual cortex. In addition, there was a higher expression level of OBCAM in the visual cortex of dark-reared 4-month-old animals than in the visual cortex of age-matched normal animals. These data indicate that OBCAM expression is positively correlated with the level of visual cortex plasticity, an observation that suggests that OBCAM may play a crucial role in the postnatal development of the visual cortex, specifically through involvement in activity-dependent synaptic modifications.
Materials and Methods
Animals
Domestic cats were obtained either from inbred colonies of the Animal Services Center at the University of British Columbia or at Dalhousie University (dark-reared animals) or from the following commercial suppliers: Laka (Montreal, Quebec, Canada), University of California (Davis, CA) and Harlan Sprague Dawley (Madison, WI). The experimental protocols involving animals were approved by the Animal Care Committees of the University of British Columbia and of Dalhousie University (dark-rearing), respectively.
cDNA Microarray
Procedures dealing with cDNA array experiments have been described in detail elsewhere (Prasad et al., 2002). In brief, animals were deeply anaesthetized with pentobarbital (Euthanyl, 150mg/kg body weight) and transcardially perfused with ice-cold phosphate buffer (0.1 M, pH 7.4) for a few minutes. The brains were quickly removed. A block of tissue from the primary visual cortex was cut and then frozen at −80°C. Total RNAs were isolated from tissue blocks by using Trizol reagent (Canadian Life Technologies, Burlington, Ontario, Canada), as per the manufacturer’s instructions, and mRNAs were extracted from the total visual cortex RNA using magnetic Dynal beads (Dynal, Lake Sciences, New York, NY) according to the manufacturer’s instructions. To ensure the absence of any genomic DNA contamination, the mRNA samples were incubated with DNase I followed by phenol/chloroform extraction and ethanol precipitation. The concentration of mRNA was determined by spectrophotometric m easurement at a wavelength of 260 nm. Single-stranded cDNA probes were derived from the poly(A)+ RNAs. A 2.5 μg quantity of poly(A)+ RNA was reverse-transcribed using Superscript II reverse transcriptase (Canadian Life Technologies), as described in the manufacturer’s protocol, using oligo dT 18-mer for priming. Radioactive labeling was achieved by incorporating 50 μCi of [α-33P]dATP (Amersham, Baie d’Urfe, Quebec, Canada; 3000 Ci/mmol) in the reverse transcription reaction. The unincorporated nucleotides were removed using G-50 Micro Columns (Pharmacia, Baie d’Urfe, Quebec, Canada) as described in the manufacturer’s protocols. Following the removal of unincorporated nucleotides, the radioactively labeled cDNA probes were boiled for 3 min and then chilled on ice. To degrade RNA, 1 N NaOH was added to a final concentration of 0.25 N and incubated at 37°C for 10 min. The reaction was then neutralized by adding 1 M Tris (pH 6.8) and 1 M HCl to final concentrations of 0.2 and 0.1 M respectively. The degraded RNA was then removed using the G-50 Micro Columns as above. Approximately 20% of the labeled nucleotides were incorporated in the final cDNA probes. The cDNA probes were hybridized to high-density human gene discovery array nylon membranes, which were spotted with 18 732 non-redundant human cDNA clones (Genome Systems Inc., Palo Alto, CA). The incubation was conducted within glass tubes in a hybridization oven in a volume of 10 ml with the DNA side facing the inside of the tubes. The array membranes were prehybridized for periods ranging from 2 h to overnight at 37°C in a buffer containing 0.75 M NaCl, 0.1 M sodium phosphate (pH 7.5), 0.15 M Tris (pH 7.5), 0.1% sodium pyrophosphate, 5× Denhardt’s solution, 0.2% sodium dodecyl sulfate (SDS), 50% formamide, 1 μg/ml poly(A) and 100 μg/ml denatured salmon sperm DNA. Hybridization was carried out in a fresh 10 ml buffer containing 2 × 107 c.p.m. of cDNA probe for 18 h at 37°C. The 33P-labeled cDNA probes were blocked for repeat elements using Cot1 DNA (Canadian Life Technologies) as recommended by the supplier and hybridized individually to each filter overnight. Following hybridization the array membranes were rinsed first in 500 ml of 2× saline sodium citrate (SSC) at room temperature for 5 min. Each array membrane was then washed twice in 500 ml of 2× SSC, 0.1% SDS at 65°C for 30 min followed by two high stringency washes in 500 ml of 0.6× SSC, 1% SDS each at 65°C for 30 min. The array membranes were then wrapped in plastic wrap and exposed to the phosphor imaging screens for 2–4 days. The images on the exposed screens were scanned on a STORM Phosphor Imaging System (Molecular Dynamics, Sunnyvale, CA) for quantitative analysis of hybridization intensities. After image acquisition, the scanned 16-bit images were imported to a PC Pentium III computer and image analysis was performed using purpose-built grid analysis extract software (Prasad et al., 2000, 2002).
Western Blot
A 4-week-old kitten was deeply anaesthetized with pentobarbital (Euthanyl, 150 mg/kg body weight) and transcardially perfused with ice-cold phosphate buffer (0.1 M, pH 7.4) for 5 min. A block of tissue from the primary visual cortex was cut and then frozen at −80°C. Ten volumes of ice-cold triple detergent containing 50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 0.02% sodium azide, 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate, 100 μg/ml phenylmethylsulfonyl fluoride and 1 μg/ml aprotinin were added to one unit per weight of frozen brain tissue (10 ml/g tissue). The brain tissue was teased apart using a pair of forceps and the solution was incubated on ice for 30 min. The mixture was pipetted several times to improve solublization of the tissue. High-molecular-weight chromosomal DNA was sheared by sonication for 1 min at maximum power. The solution was then centrifuged at 15 000 g for 5 min at 4°C. The proteins were denatured by boiling in buffer for 5 min. Following spin down of the protein solution for 30 s, protein samples (30 μl) were loaded onto 8% SDS–polyacrylamide gel and electrophoresed at 200 V for ~40 min until the dye front migrated to ~5 mm from the bottom of the gel. The gel was then washed with transfer buffer for 15 min and transferred onto a polyvinylidene difluoride membrane at 20 V and 135 mA for ~30–45 min. The membrane was then incubated with 5% bovine serum albumin (BSA) and 0.1% Tween-20 in 0.1 M phosphate buffer at 4°C overnight. After rinses, the membrane was incubated with the anti-OBCAM antibody (1:10 000) for 1 h at room temperature. After rinses, the membrane was incubated in HRP-conjugated anti-mouse antibody (1:1000) for 1 h at room temperature. After further rinses, the excess liquid from the washed membrane was drained onto a paper towel and the membrane was placed protein side up on a piece of Saran wrap. The membrane was covered with the detection solution (Amersham ECL™ Western blotting analysis system) for 1 min. Photographic film (Kodak BioMax MR film) was placed on the membrane for 1 min and the ECL signal detected by subsequent standard development of the film.
Immunocytochemistry
Experimental procedures concerning immunocytochemistry were similar to those described before (Gu et al., 1993, 1994). In brief, animals were deeply anesthetized with an i.p. injection of pentobarbital (150 mg/kg body weight) and perfused briefly with 0.9% saline in 0.1 M phosphate buffer, pH 7.4 (PBS), followed by a fixative solution containing 4% paraformaldehyde. The brains were removed and preserved in fixative at 4°C overnight. The visual cortices were sectioned (40 μm) in either the frontal or the parasagittal plane with a vibratome. The sections were washed in PBS for 15 min four times. To block endogenous peroxidase activity, the sections were immersed in PBS with 3% hydrogen peroxide and 10% methanol for 20 min. After four washes with PBS for 15 min each, the sections were incubated with normal goat serum to block nonspecific binding of the secondary antibody for 1 h at a concentration of 20% in PBS with 3% BSA at room temperature, and then incubated with the primary antibody (1:2500) at 4°C overnight. After four rinses in PBS for 15 min each, the sections were incubated for 1 h in biotinylated anti-mouse antibody diluted 1:200 in PBS containing 3% BSA, again rinsed four times in PBS, and finally incubated for 1 h in a Vectastain avidin-biotin-complex (ABC) kit. Following this incubation and four rinses, the sections were incubated for 5 min with diaminobenzidine (0.7 mg/ml PBS) and then 0.01% hydrogen peroxide was added. After 10 min incubation and a final wash in PBS, they were mounted on slides, dehydrated in increasing alcohol concentrations to xylene and coverslipped.
Sections adjacent to those used for immnocytochemical labeling were Nissl-stained. For classification of cortical laminae, we relied on the criteria of Mower and Chen (2003) for the neonatal age (1-week-old) and Otsuka and Hassler (1962) for all other ages. Considering that the laminar thickness varies within the primary visual cortex, and that the thickness of the same layer changes during development, we adopted a semi-quantitative measurement to compare relative density of immunopositive cells in a given cortical region. Two 100 μm wide strips from each animal’s primary visual cortex were randomly selected. Data were calculated to the relative cell numbers per 100 × 100 μm and averaged from these two strips for each animal. For statistical analyses of OBCAM-immunopositive cell densities in the primary visual cortex, we used a 5 × 5 (ages × laminae) analysis of variance (ANOVA) to calculate the level of significance of differences among different cortical layers and different ages during postnatal development, and a 4 × 5 (repeated measures × laminae) ANOVA to determine the level of significance of differences in different cortical layers between normal and dark-reared animals, respectively. Following ANOVA, differences between pairs of group means were tested using Fisher’s PLSD post hoc tests.
Results
Using cDNA arrays, mRNA levels of OBCAM in newborn, 30-day-old and adult visual cortex were examined. In addition, determinations of mRNA levels of OBCAM in the visual cortex of 4-month-old dark-reared animals (which should retain a high level of plasticity) as well as in retinal choroidal tissue of adult cats, which is a structure of the visual system that should exhibit little plasticity were carried out. The cDNA array results of OBCAM hybridization signals are shown in Figure 1. The expression level of OBCAM mRNAs was high in 30-day-old normal and in 4-month-old dark-reared visual cortex and low in newborn and adult visual cortex, as well as in choroidal tissue. These data indicate that at the mRNA level, OBCAM expression is high when the visual cortex is more plastic and low when there is little plasticity.
Figure 1.
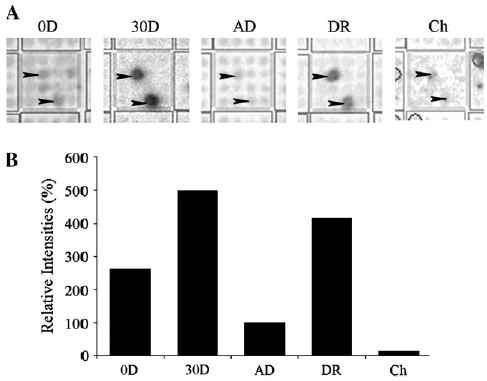
(A) cDNA-array images of OBCAM mRNA signals representing newborn (0D), 30-day-old (30D), adult (AD) and 4-month-old dark-reared (DR) visual cortex, as well as choroidal tissue (Ch). Each grid of the cDNA array (large square) has 16 spots with double spotted identical clones for eight different genes. Arrowheads indicate the expected locations of OBCAM signals. (B) Relative intensities of OBCAM mRNA signals are illustrated as percentages of adult values (100%).
As the level of mRNAs in biological systems may not truly reflect the amount of proteins present (Anderson and Seilhamer, 1997; Abbott, 1999; Gygi et al., 1999), we also performed immunocytochemistry to examine OBCAM-positive neurons in cat visual cortex. The anti-OBCAM antibody used in this study (OBC53) was directed against amino acids 185–202 of OBCAM and was previously shown to be specific against rat, mouse, guinea pig, rabbit and bovine tissue (Hachisuka et al., 1996), indicating that OBCAM is a relatively conserved molecule during evolution. To determine whether OBC53 was also specific against cat tissue, Western blots were performed using proteins derived from a 4-week-old kitten visual cortex. Our results showed two bands, at 58 and 51 kDa respectively (Fig. 2), which represent the two major glycosylated forms of OBCAM (Hachisuka et al., 1996). This labeling pattern is similar to that obtained using proteins from other species (Hachisuka et al., 1996), thereby indicating that the monoclonal antibody OBC53 remains specific in cat visual cortex.
Figure 2.
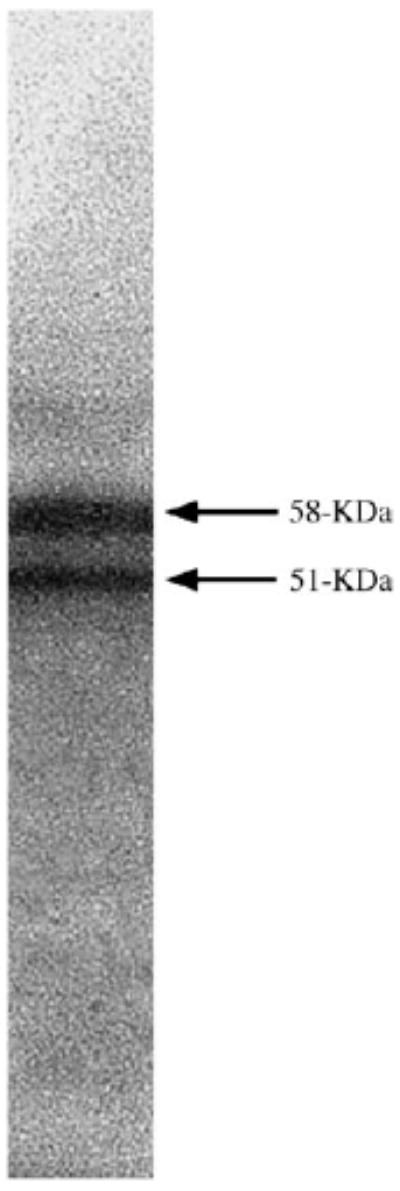
Immunoblot of the monoclonal anti-OBCAM antibody (OBC53) to proteins purified from a 4-week-old kitten visual cortex. The two bands represent the two glycosylated forms of OBCAM.
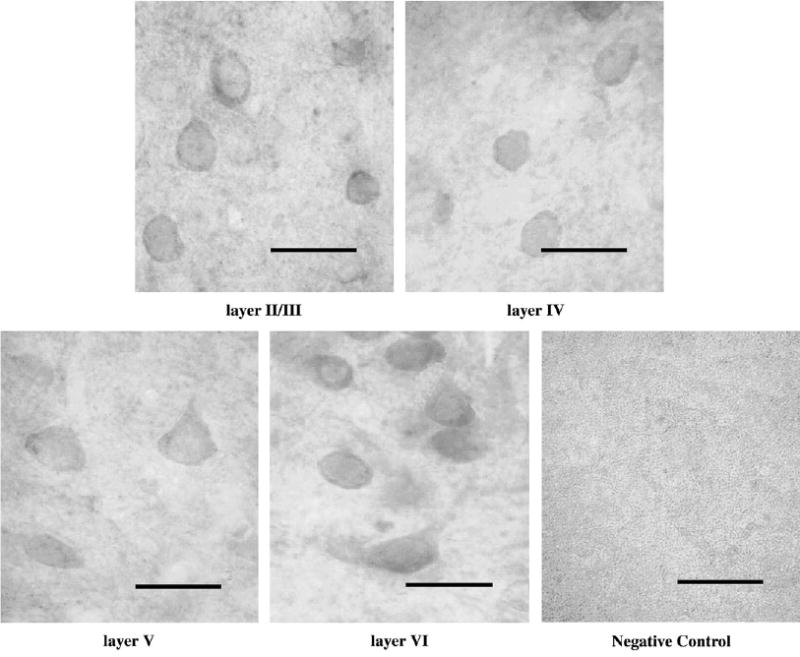
To determine the profile of postnatal development of OBCAM in the primary visual cortex at the protein level, immunocytochemical staining was performed using tissue sections from 1-week-old, 2-week-old, 4-week-old, 6-week-old, 3-month-old and adult cat visual cortex. In 1-week-old animals, immunopositive neurons were abundant in the visual cortex (Fig. 3). OBCAM-immunoreactivity was predominantly associated with perikarya including both pyramidal and non-pyramidal neurons. Immuno-labeling of the dendrites of pyramidal neurons was also evident. The morphological characteristics of labeled OBCAM-immunopositive neurons did not show much change through postnatal development. However, in adult visual cortex the overall intensity of immunostaining was somewhat lower and there was less neurite labeling as compared with younger animals (cf. Figs 3–5). The density of OBCAM-immunopostive neurons in visual cortex of 1-week-old animals was approximately uniform in all cortical layers except for layer I, where the density of OBCAM-positive cells was lower (Fig. 6). A similar pattern of OBCAM-immunoreactivity was maintained in 4-week-old visual cortex (Fig. 7), while in adult visual cortex the density of OBCAM-immunopositive neurons was much reduced (Fig. 8). Figure 9 shows the changes in the relative density of OBCAM-immunopositive neurons in the visual cortex at different postnatal ages, as well as the laminar-specific changes in the pattern of OBCAM-immunoreactivity at different postnatal ages. Through postnatal one to four weeks, the overall number of OBCAM-immunoreactive neurons was high but had declined by six weeks of age. The number of OBCAM-immunopositive cells was reduced even further in the adult visual cortex.
Figure 3.
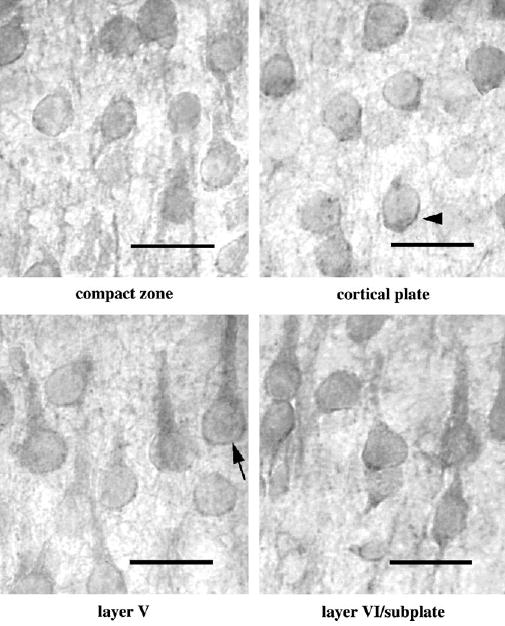
Immunolabeling of OBCAM in different cortical layers in postnatal 1-week-old visual cortex. Both pyramidal (arrow) and non-pyramidal (arrowhead) neurons were labeled. Scale bar = 30 μm.
Figure 5.
Immunolabeling of OBCAM in different cortical layers in adult visual cortex. Immunopositive neurons have a similar morphological appearance as those at other ages but with a reduced intensity of neurite labeling. Negative control shows the appearance of layer VI following application of the same immunohistochemical procedure but with the primary antibody omitted. Scale bar = 30 μm.
Figure 6.
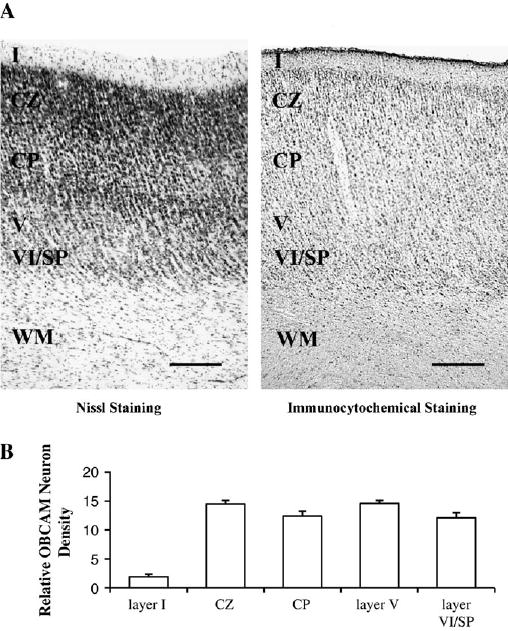
(A) Photomicrographs illustrating the laminar distribution of OBCAM protein in 1-week-old kitten primary visual cortex. The letters I, V, VI indicate cortical layers; CZ = compact zone; CP = cortical plate; SP = subplate; WM = white matter. Scale bar = 0.2 mm. (B) Histograms displaying the density of OBCAM neurons in the various cortical layers expressed as counts of the number of OBCAM-immunopositive neurons per 100 × 100 μm grid.
Figure 7.
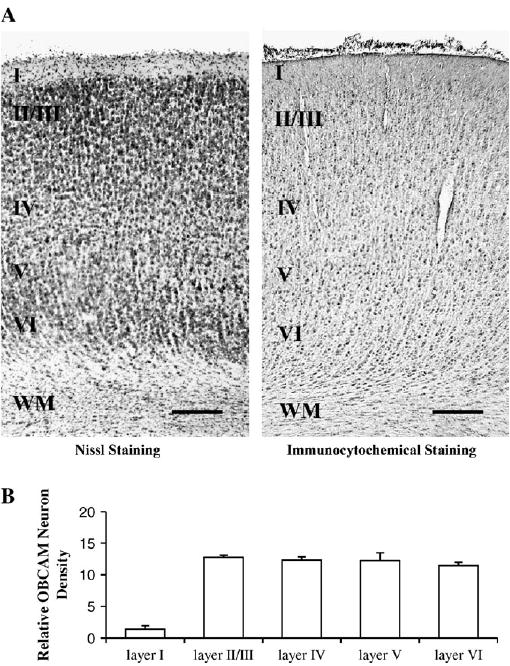
(A) Photomicrographs illustrating the laminar distribution of OBCAM protein in 4-week-old kitten primary visual cortex. The letters I–VI indicate cortical layers; WM = white matter. Scale bar = 0.2 mm. (B) Histograms displaying the density of OBCAM neurons in the various cortical layers expressed as counts of the number of OBCAM-immunopositive neurons per 100 × 100 μm grid.
Figure 8.
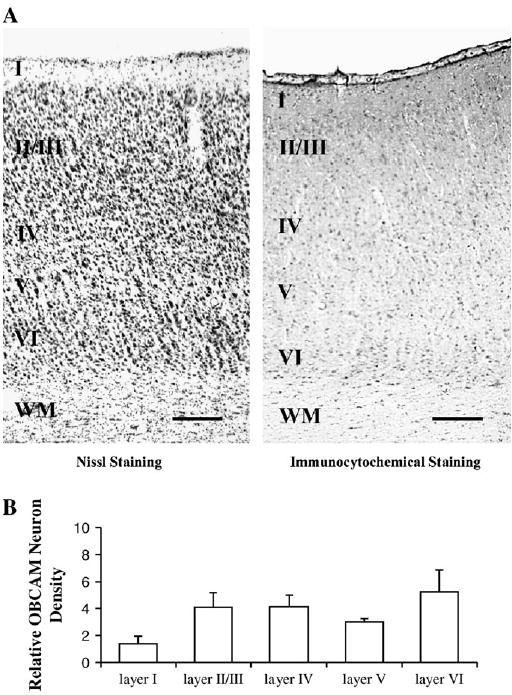
(A) Photomicrographs illustrating the laminar distribution of OBCAM protein in adult cat primary visual cortex. The letters I–VI indicate cortical layers; WM = white matter. Scale bar = 0.2 mm. (B) Histograms displaying the density of OBCAM neurons in the various cortical layers expressed as counts of the number of OBCAM-immunopositive neurons per 100 × 100 μm grid.
Figure 9.
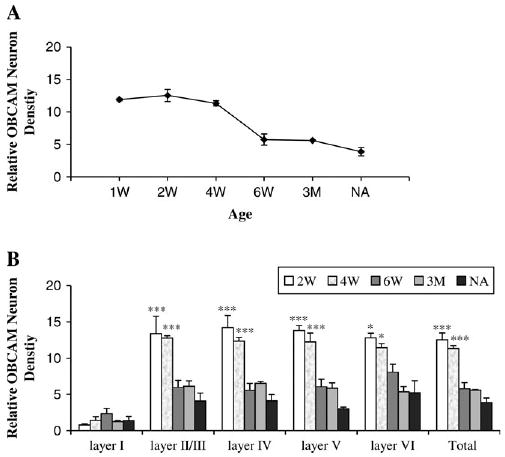
(A) Relative densities of OBCAM-immunopositive neurons in cat primary visual cortex at different ages: 1 week old (1W), 2 weeks old (2W), 4 weeks old (4W), 6 weeks old (6W), 3 months old (3M) and adult (NA). (B) Relative densities (expressed as the number of OBCAM-immunopositive neurons per 100 × 100 μm) of OBCAM-immunopositive neurons in different cortical layers at different ages. *P < 0.05, ***P < 0.001 comparing to normal adult (NA).
To further determine whether OBCAM expression was purely age-dependent or rather associated with other factors such as the level of cortical plasticity, we investigated OBCAM-immunoreactivity in the visual cortex of 4-month-old animals that had been dark-reared from birth with that observed in the visual cortex of age-matched normal animals. Dark-rearing extends the closure of the critical period so that the visual cortex of such animals still retains considerable plasticity at 4 months of age (Cynader and Mitchell, 1980; Mower, 1991). The results showed that the OBCAM-immunoreactivity in the visual cortex of dark-reared 4-month-old animals was higher than that of age-matched normal animals; more immunopositive neurons were evident in the former animals than the latter (Fig. 10a). In comparison to the visual cortex of normal 4-month-old animals, OBCAM-immunopositive neurons were significantly more numerous in cortical layers II/III, IV and VI of dark-reared visual cortex, while no significant difference was found in layers I and V (Fig. 10b). Additional experiments were carried out using frontal cortex of 4-month-old normal and dark-reared cats in order to confirm that dark-rearing did not affect OBCAM expression in non-visual cortical areas. OBCAM-immunoreactivity in the frontal cortex was not different between normal and dark-reared animals (Fig. 11).
Figure 10.
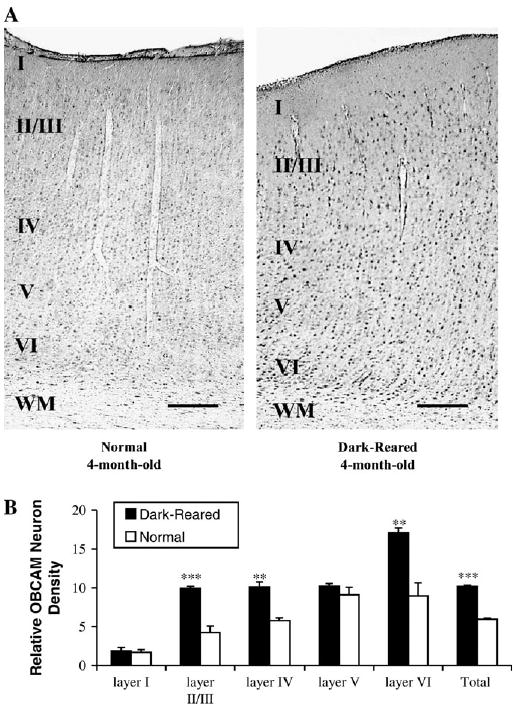
(A) Photomicrographs illustrating the effect of dark-rearing on OBCAM-immunoreactivity in the primary visual cortex of a normal and a dark-reared cat at 4 months of age. More OBCAM-immunopositive neurons were present in the primary visual cortex of the dark-reared animal. (B) Histograms that show the number of OBCAM-immunopositive neurons in visual cortex of 4-month-old normal and dark-reared animals. Black columns: dark-reared; white columns: normal. Other conventions are the same as in Figure 8. **P < 0.01, ***P < 0.001 (ANOVA analysis).
Figure 11.
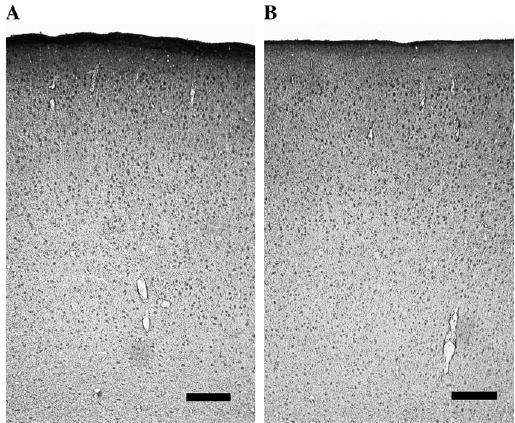
Photomicrographs that show OBCAM-immunoreactivity in the frontal cortex of a 4-month-old normal cat (A) and a 4-month-old dark-reared cat (B). Scale bars = 0.2 mm.
Discussion
It has been shown recently that blockade of protein synthesis in the primary visual cortex but not in the LGN disrupts ocular dominance plasticity in developing mouse visual cortex (Taha and Stryker, 2002), suggesting that gene expression in the visual cortex is crucial for visual cortex plasticity. However, the kind(s) of genes that are highly expressed during the critical period for visual cortex plasticity remain to be identified. Our results showing that OBCAM-expression in cat visual cortex was high during early postnatal development and low at older and adult ages, suggest that OBCAM may be one of the candidate genes involved in visual cortex development and plasticity. Evidence to further support this notion was the observation that OBCAM protein levels were higher in the visual cortex of 4-month-old dark-reared animals than that observed in age-matched normal animals, a finding that fits with the higher level of cortical plasticity observed in the former as compared with the latter (Cynader and Mitchell, 1980; Mower, 1991). The data from dark-reared and age-matched animals further suggest that the expression level of OBCAM in the visual cortex is not purely genetically preprogrammed or age-dependent, and could be affected by additional epigenetic factors such as the rearing condition. Because OBCAM-immunoreactivity did not appear to change between 1 and 2 weeks postnatally (before and after eye opening), it does not appear likely that the expression level of OBCAM is linked to the onset of visual input. Also, the fact that OBCAM-immunoreactivity in the visual cortex of 4-month-old dark-reared animals was greater than that observed in 4-month-old normal animals further suggests that OBCAM expression in the visual cortex is not positively correlated with the level of visually driven cortical activity. Previous experiments have shown that rearing animals in the dark to 5–6 weeks of age made visual cortex less plastic than normal, while rearing animals in the dark to 12–20 weeks made it more plastic. Animals dark-reared to 8–9 weeks of age exhibited a similar level of plasticity in the visual cortex plasticity to that observed in normal age-matched animals (Mower, 1991). Thus, future experiments to compare the OBCAM expression level in visual cortex of dark-reared animals at these ages would provide additional data linking the level of OBCAM and plasticity. While molecular and anatomical data are all suggestive, a definite confirmation of involvement of OBCAM in visual cortex development and plasticity will require functional assessments.
To date, little is known about the function of OBCAM in the nervous system. Molecular sequence analysis (Schofield et al., 1989) revealed OBCAM as a cell adhesion molecule and a member of the immunoglobulin superfamily which is defined as possessing one or more extracellular immunoglobulin-like domains. Since evidence is available to suggest that other members of the immunoglobulin superfamily of cell adhesion molecules (IgCAM) play important roles in nervous system development, including axonal guidance (Tang et al., 1994; Stoeckli and Landmesser, 1995; Cremer et al., 1997), contact-dependent neurite outgrowth and retraction (Burgoon et al., 1995; Norenberg et al., 1995), synaptogenesis (Hunkapillar and Hood, 1989) and neuronal plasticity (Faivre-Sarrailh and Rougon, 1997; Walsh and Doherty, 1997; Huh et al., 2000; Boulanger et al., 2001), our results support the idea that OBCAM may serve an important function in visual cortex development and plasticity.
Our results indicate a good match between mRNA and protein levels of OBCAM in the visual cortex. Comparison of both mRNA and protein level was necessary, as there is often a mismatch between the levels of mRNAs and proteins (Anderson and Seilhamer, 1997; Abbott, 1999; Gygi et al., 1999). High levels of mRNAs may not be translated into corresponding high levels of proteins and vice versa. For example, the mRNA level of brain-derived neurotrophic factor (BDNF) in the visual cortex was found to be lower in dark-reared animals than that in age-matched normal animals, while the protein level of BDNF was higher in dark-reared animals than that in normal age-matched animals (Pollock et al., 2001). In contrast, our results suggest that OBCAM belongs to the class of molecules whose expressions at mRNA and protein levels are positively correlated. Moreover, the positive correlation of OBCAM expression at mRNA and protein level also supports the validity of our cDNA microarray analysis, which is an emerging technique in neuroscience (Mirnics et al., 2001) and therefore requires independent data confirmation (Hess et al., 2001).
In the developing visual cortex a variety of molecules have been identified as important factors for visual cortex plasticity, such as members of neurotransmitter systems (Kasamatsu and Pettigrew, 1976; Bear and Singer, 1986; Ramoa et al., 1988; Reiter and Stryker, 1988; Bear et al., 1990; Gu and Singer, 1993, 1995; Wang et al., 1997; Hensch et al., 1998), neurotrophin family (Gu, 1995; Lo, 1995; Thoenen, 1995; Bonhoeffer, 1996; Cellerino and Maffei, 1996; Ghosh, 1996; Katz and Shatz, 1996; McAllister et al., 1999), hormones (Daw et al., 1991), proteases (Mataga et al., 1996, 2002; Müller and Griesinger, 1998) and protein kinases (Gordon et al., 1996; Beaver et al., 2001; Di Cristo et al., 2001; Taha et al., 2002). Our data showing that the level of OBCAM expression is higher in immature than in adult visual cortex and also higher in dark-reared older animals than in age-matched normal animals suggest that, in addition to the aforementioned molecules, cell adhesion molecules may also play an important role in visual cortex plasticity. Electrophysiological recording in hippocampus in vitro have already shown that members of IgCAM are critical for modulation of synaptic efficacy, such as the maintenance of long-term potentiation (Luthi et al., 1994, 1996; Cremer et al., 1998; Huh et al., 2000).
OBCAM shares stringent sequence homology with LAMP (limbic system-associated membrane protein) and neurotrimin, which are two other members of the IgCAM family. The initial letters of these three IgCAMs make up the highly homologous IgLON family. Neurotrimin and LAMP have activities that induce the outgrowth of neurites, and LAMP also has a function in the guidance of developing axons and remodeling of mature circuitry in the limbic system (Keller et al., 1989; Pimenta et al., 1995). These findings, together with our observation that OBCAM expression in the visual cortex appeared to be better associated with the level of cortical plasticity, suggest that OBCAM may play an important role in visual cortex plasticity. Additional investigations are required to provide further insight into the functional role of OBCAM in visual cortex development and plasticity.
Figure 4.
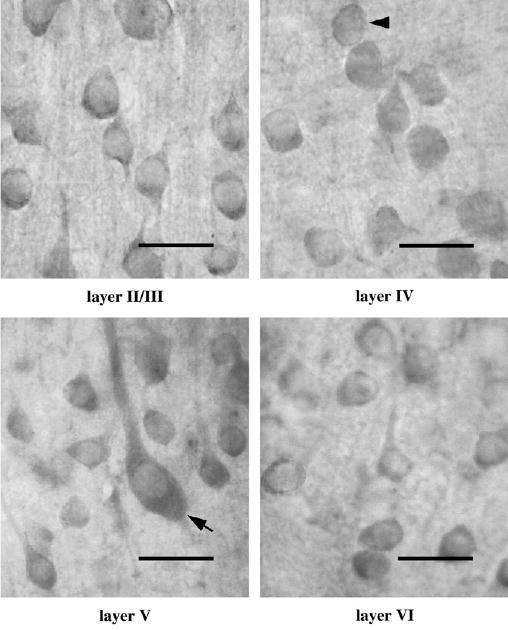
Immunolabeling of OBCAM in different cortical layers in postnatal 4-week-old visual cortex. Immunopositive neurons have a similar morphological appearance as those in neonatal visual cortex. Scale bar = 30 μm.
Footnotes
Notes This work was supported by British Columbia Health Research Foundation and NIH (EY14892) to QG and a Natural Science & Engineering Research Council grant to DEM. Q.G. was a joint scholar of British Columbia Health Research Foundation and Vancouver Hospital Health Sciences Center.
References
- Abbott A. A post-genomic challenge: learning to read patterns of protein synthesis. Nature. 1999;402:715–720. doi: 10.1038/45350. [DOI] [PubMed] [Google Scholar]
- Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18:533–537. doi: 10.1002/elps.1150180333. [DOI] [PubMed] [Google Scholar]
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Bear MF, Kleinschmidt A, Gu Q, Singer W. Disruption of experience-dependent synaptic modifications in striate cortex by infusion of an NMDA receptor antagonist. J Neurosci. 1990;10:909–925. doi: 10.1523/JNEUROSCI.10-03-00909.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver CJ, Ji Q, Fischer QS, Daw NW. Cyclic AMP-dependent protein kinase mediates ocular dominance shifts in cat visual cortex. Nat Neurosci. 2001;4:159–163. doi: 10.1038/83985. [DOI] [PubMed] [Google Scholar]
- Berardi N, Maffei L. From visual experience to visual function: roles of neurotrophins. J Neurobiol. 1999;41:119–126. [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- Boulanger LM, Huh GS, Shatz CJ. Neuronal plasticity and cellular immunity: shared molecular mechanisms. Curr Opin Neurobiol. 2001;11:568–578. doi: 10.1016/s0959-4388(00)00251-8. [DOI] [PubMed] [Google Scholar]
- Brummendorf T, Rathjen FG. Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr Opin Neurobiol. 1996;6:584–593. doi: 10.1016/s0959-4388(96)80089-4. [DOI] [PubMed] [Google Scholar]
- Burgoon MP, Hazan RB, Phillips GR, Crossin KL, Edelman GM, Cunningham BA. Functional analysis of posttranslational cleavage products of the neuron–glia cell adhesion molecule, Ng-CAM. J Cell Biol. 1995;130:733–744. doi: 10.1083/jcb.130.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellerino A, Maffei L. The action of neurotrophins in the development and plasticity of the visual cortex. Prog Neurobiol. 1996;49:53–71. doi: 10.1016/0301-0082(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Cho TM, Hasegawa J, Ge BL, Loh HH. Purification to apparent homogeneity of a mu-type opioid receptor from rat brain. Proc Natl Acad Sci USA. 1986;83:4138–4142. doi: 10.1073/pnas.83.12.4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer H, Chazal G, Goridis C, Represa A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol Cell Neurosci. 1997;8:323–335. doi: 10.1006/mcne.1996.0588. [DOI] [PubMed] [Google Scholar]
- Cremer H, Chazal G, Carleton A, Goridis C, Vincent JD, Lledo PM. Long-term but not short-term plasticity at mossy fiber synapses is impaired in neural cell adhesion molecule-deficient mice. Proc Natl Acad Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M, Mitchell DE. Prolonged sensitivity to monocular deprivation in dark-reared cats. J Neurophysiol. 1980;43:1026–1040. doi: 10.1152/jn.1980.43.4.1026. [DOI] [PubMed] [Google Scholar]
- Daw NW, Sato H, Fox K, Carmichael T, Gingerich R. Cortisol reduces plasticity in the kitten visual cortex. J Neurobiol. 1991;22:158–168. doi: 10.1002/neu.480220206. [DOI] [PubMed] [Google Scholar]
- Daw NW (1995) Visual development. New York: Plenum Publishing.
- Daw NW (2004) Mechanisms of plasticity in the visual cortex. In: The visual neurosciences (Chalupa LM, Werner JS, eds), pp. 126–145. New York: MIT Press.
- Di Cristo G, Berardi N, Cancedda L, Pizzorusso T, Putignano E, Ratto GM, Maffei L. Requirement of ERK activation for visual cortical plasticity. Science. 2001;292:2337–2340. doi: 10.1126/science.1059075. [DOI] [PubMed] [Google Scholar]
- Doherty P, Fazeli MS, Walsh FS. The neural cell adhesion molecule and synaptic plasticity. J Neurobiol. 1995;26:437–446. doi: 10.1002/neu.480260315. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Rougon G. Axonal molecules of the immunoglobulin superfamily bearing a GPI anchor: their role in controlling neurite outgrowth. Mol Cell Neurosci. 1997;9:109–115. doi: 10.1006/mcne.1997.0609. [DOI] [PubMed] [Google Scholar]
- Fregnac Y, Imbert M. Development of neuronal selectivity in primary visual cortex of cat. Physiol Rev. 1984;64:325–434. doi: 10.1152/physrev.1984.64.1.325. [DOI] [PubMed] [Google Scholar]
- Ghosh A. Cortical development: with an eye on neurotrophins. Curr Biol. 1996;6:130–133. doi: 10.1016/s0960-9822(02)00442-6. [DOI] [PubMed] [Google Scholar]
- Gordon JA, Cioffi D, Silva AJ, Stryker MP. Deficient plasticity in the primary visual cortex of alpha-calcium/calmodulin-dependent protein kinase II mutant mice. Neuron. 1996;17:491–499. doi: 10.1016/s0896-6273(00)80181-6. [DOI] [PubMed] [Google Scholar]
- Gu Q. Involvement of nerve growth factor in visual cortex plasticity. Rev Neurosci. 1995;6:329–351. doi: 10.1515/revneuro.1995.6.4.329. [DOI] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Gu Q, Singer W. Effects of intracortical infusion of anticholinergic drugs on neuronal plasticity in kitten visual cortex. Eur J Neurosci. 1993;5:475–485. doi: 10.1111/j.1460-9568.1993.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Gu Q, Singer W. Involvement of serotonin in developmental plasticity of kitten visual cortex. Eur J Neurosci. 1995;7:1146–1153. doi: 10.1111/j.1460-9568.1995.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Gu Q, Perez-Velazquez JL, Angelides KJ, Cynader MS. Immunohistochemical study of GABAA receptors in the cat visual cortex. J Comp Neurol. 1993;333:94–108. doi: 10.1002/cne.903330108. [DOI] [PubMed] [Google Scholar]
- Gu Q, Liu YL, Cynader MS. Immunocytochemical study of tachykinins in the cat visual cortex. Brain Res. 1994;640:336–340. doi: 10.1016/0006-8993(94)91890-2. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachisuka A, Yamazaki T, Sawada J, Terao T. Characterization and tissue distribution of opioid-binding cell adhesion molecule (OBCAM) using monoclonal antibodies. Neurochem Int. 1996;28:373–379. doi: 10.1016/0197-0186(95)00108-5. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess KR, Zhang W, Baggerly KA, Stivers DN, Coombes KR. Microarrays: handling the deluge of data and extracting reliable information. Trends Biotechnol. 2001;19:463–468. doi: 10.1016/s0167-7799(01)01792-9. [DOI] [PubMed] [Google Scholar]
- Hoffman KB. The relationship between adhesion molecules and neuronal plasticity. Cell Mol Neurobiol. 1998;18:461–475. doi: 10.1023/A:1026371124366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol (Lond) 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, Shatz CJ. Functional requirement for class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapillar T, Hood L. Diversity of the immunoglobulin gene superfamily. Adv Immunol. 1989;44:1–63. doi: 10.1016/s0065-2776(08)60639-2. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T, Pettigrew JD. Depletion of brain catecholamines: failure of ocular dominance shift after monocular occlusion in kittens. Science. 1976;194:206–209. doi: 10.1126/science.959850. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Keller F, Rimvall K, Barbe MF, Levitt P. A membrane glycoprotein associated with the limbic system mediates the formation of the septo-hippocampal pathway in vitro. Neuron. 1989;3:551–561. doi: 10.1016/0896-6273(89)90265-1. [DOI] [PubMed] [Google Scholar]
- Kiss JZ, Muller D. Contribution of the neural cell adhesion molecule to neuronal and synaptic plasticity. Rev Neurosci. 2001;12:297–310. doi: 10.1515/revneuro.2001.12.4.297. [DOI] [PubMed] [Google Scholar]
- Lo DC. Neurotrophic factors and synaptic plasticity. Neuron. 1995;15:979–981. doi: 10.1016/0896-6273(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Luthi A, Laurent JP, Figurov A, Muller D, Schachner M. Hippocampal long-term potentiation and neural cell adhesion molecules L1 and NCAM. Nature. 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- Luthi A, Mohajeri H, Schachner M, Laurent JP. Reduction of hippocampal long-term potentiation in transgenic mice ectopically expressing the neural cell adhesion molecule L1 in astrocytes. J Neurosci Res. 1996;46:1–6. doi: 10.1002/(SICI)1097-4547(19961001)46:1<1::AID-JNR1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Mataga N, Imamura K, Shiomitsu T, Yoshimura Y, Fukamauchi K, Watanabe Y. Enhancement of mRNA expression of tissue-type plasminogen activator by L-threo-3,4-dihydroxyphenylserine in association with ocular dominance plasticity. Neurosci Lett. 1996;218:149–152. doi: 10.1016/s0304-3940(96)13139-6. [DOI] [PubMed] [Google Scholar]
- Mataga N, Nagai N, Hensch TK. Permissive proteolytic activity for visual cortical plasticity. Proc Natl Acad Sci USA. 2002;99:7717–7721. doi: 10.1073/pnas.102088899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001;24:479–486. doi: 10.1016/s0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- Movshon JA, van Sluyters RC. Visual neural development. Annu Rev Psychol. 1981;32:477–522. doi: 10.1146/annurev.ps.32.020181.002401. [DOI] [PubMed] [Google Scholar]
- Mower GD. The effect of dark rearing on the time course of the critical period in cat visual cortex. Dev Brain Res. 1991;58:151–158. doi: 10.1016/0165-3806(91)90001-y. [DOI] [PubMed] [Google Scholar]
- Mower GD, Chen L. Laminar distribution of NMDA receptor subunit (NR1, NR2A, NR2B) expression during the critical period in cat visual cortex. Mol Brain Res. 2003;119:19–27. doi: 10.1016/j.molbrainres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Müller CM, Griesinger CB. Tissue plasminogen activator mediates reverse occlusion plasticity in visual cortex. Nat Neurosci. 1998;1:47–53. doi: 10.1038/248. [DOI] [PubMed] [Google Scholar]
- Nakajima O, Hachisuka A, Takagi K, Yamazaki T, Ikebuchi H, Sawada J. Expression of opioid-binding cell adhesion molecule (OBCAM) and neurotrimin (NTM) in E. coli and their reactivity with monoclonal anti-OBCAM antibody. Neuroreport. 1997;8:3005–3008. doi: 10.1097/00001756-199709290-00003. [DOI] [PubMed] [Google Scholar]
- Norenberg U, Hubert M, Brummendorf T, Tarnok A, Rathjen FG. Characterization of functional domains of the tenascin-R (restrictin) polypeptide: cell attachment site, binding with F11, and enhancement of F11-mediated neurite outgrowth by tenascin-R. J Cell Biol. 1995;130:473–484. doi: 10.1083/jcb.130.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka R, Hassler R. Über Aufbau und Gliederung der corticalen Sehsphäre bei der Katze. Arch Psychiat Zeitschr Ges Neurol. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Pimenta AF, Zhukareva V, Barbe MF, Reinoso BS, Grimley C, Henzel W, Fischer I, Levitt P. The limbic system-associated membrane protein is an Ig superfamily member that mediates selective neuronal growth and axon targeting. Neuron. 1995;15:287–297. doi: 10.1016/0896-6273(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Pollock GS, Vernon E, Forbes ME, Yan Q, Ma YT, Hsieh T, Robichon R, Frost DO, Johnson JE. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. J Neurosci. 2001;21:3923–3931. doi: 10.1523/JNEUROSCI.21-11-03923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad SS, Kojic LZ, Lee SS, Hetherington PA, Cynader MS. Identification of differentially expressed genes in the visual structures of brain using high-density cDNA grids. Mol Brain Res. 2000;82:11–24. doi: 10.1016/s0169-328x(00)00172-8. [DOI] [PubMed] [Google Scholar]
- Prasad SS, Kojic LZ, Li P, Mitchell DE, Hachisuka A, Sawada J, Gu Q, Cynader MS. Gene expression patterns during enhanced periods of visual cortex plasticity. Neuroscience. 2002;111:35–45. doi: 10.1016/s0306-4522(01)00570-x. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, Paradiso MA, Freeman RD. Blockade of intracortical inhibition in kitten striate cortex: effects on receptive field properties and associated loss of ocular dominance plasticity. Exp Brain Res. 1988;73:285–296. doi: 10.1007/BF00248220. [DOI] [PubMed] [Google Scholar]
- Reiter HO, Stryker MP. Neural plasticity without postsynaptic action potentials: less-active inputs become dominant when kitten visual cortical cells are pharmacologically inhibited. Proc Natl Acad Sci USA. 1988;85:3623–3627. doi: 10.1073/pnas.85.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronn LC, Hartz BP, Bock E. The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp Gerontol. 1998;33:853–864. doi: 10.1016/s0531-5565(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Mechanisms of visual plasticity: Hebb synapses, NMDA receptors, and beyond. Physiol Rev. 1991;71:587–615. doi: 10.1152/physrev.1991.71.2.587. [DOI] [PubMed] [Google Scholar]
- Schofield PR, McFarland KC, Hayflick JS, Wilcox JN, Cho TM, Roy S, Lee NM, Loh HH, Seeburg PH. Molecular characterization of a new immunoglobulin superfamily protein with potential roles in opioid binding and cell contact. EMBO J. 1989;8:489–495. doi: 10.1002/j.1460-2075.1989.tb03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Spear PD. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982;62:738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995;14:1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- Taha S, Stryker MP. Rapid ocular dominance plasticity requires cortical but not geniculate protein synthesis. Neuron. 2002;34:425–436. doi: 10.1016/s0896-6273(02)00673-6. [DOI] [PubMed] [Google Scholar]
- Taha S, Hanover JL, Silva AJ, Stryker MP. Phosphirylation of alpha CAMKII is required for ocular dominance plasticity. Neuron. 2002;36:483–491. doi: 10.1016/s0896-6273(02)00966-2. [DOI] [PubMed] [Google Scholar]
- Tang J, Rutishauser U, Landmesser L. Polysialic acid regulates growth cone behavior during sorting of motor axons in the plexus region. Neuron. 1994;13:405–414. doi: 10.1016/0896-6273(94)90356-5. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Walsh FS, Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu Rev Cell Dev Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- Wang Y-C, Gu Q, Cynader M. Blockade of serotonin-2C receptors by mesulergine reduces ocular dominance plasticity in kitten visual cortex. Exp Brain Res. 1997;114:321–328. doi: 10.1007/pl00005640. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single cell responses in striate cotex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]


