Abstract
The guanylate cyclase-activating proteins (GCAPs) are Ca2+ -binding proteins of the calmodulin (CaM) gene superfamily that function in the regulation of photoreceptor guanylate cyclases (GCs). In the mammalian retina, two GCAPs (GCAP 1-2) and two transmembrane GCs have been identified as part of a complex regulatory system responsive to fluctuating levels of free Ca2+. A third GCAP, GCAP3, is expressed in human and zebrafish (Danio rerio) retinas, and a guanylate cyclase-inhibitory protein (GCIP) has been shown to be present in frog cones. To explore the diversity of GCAPs in more detail, we searched the pufferfish (Fugu rubripes) and zebrafish (Danio rerio) genomes for GCAP-related gene sequences (fuGCAPs and zGCAPs, respectively) and found that at least five additional GCAPs (GCAP4-8) are predicted to be present in these species. We identified genomic contigs encoding fuGCAPl-8, fuGCIP, zGCAPl-5, zGCAP7 and zGCIP. We describe cloning, expression and localization of three novel GCAPs present in the zebrafish retina (zGCAP4, zGCAP5, and zGCAP7). The results show that recombinant zGCAP4 stimulated bovine rod outer segment GC in aCa2+-dependent manner. RT-PCR with zGCAP specific primers showed specific expression of zGCAPs and zGCIP in the retina, while zGCAPl mRNA is also present in the brain. In situ hybridization with anti-sense zGCAP4, zGCAP5 and zGCAP7 RNA showed exclusive expression in zebrafish cone photoreceptors. The presence of at least eight GCAP genes suggests an unexpected diversity within this subfamily of Ca2+-binding proteins in the teleost retina, and suggests additional functions for GCAPs apart from stimulation of GC. Based on genome searches and EST analyses, the mouse and human genomes do not harbor GCAP4-8 or GCIP genes.
Keywords: Guanylate cyclase, Guanylate cyclase-activating proteins, Phototransduction, Ca2+-binding proteins, Rod and cone photoreceptors
Introduction
Photoreceptor guanylate cyclases (GCs) convert GTP to cGMP, a cyclic nucleotide that serves as internal transmitter of phototransduction in vertebrates (McBee et al. 2001; Polans et al. 1996). An important feature of photoreceptor GCs is their Ca2+ sensitivity, which is mediated by Ca2+-binding proteins termed GCAPs (Palczewski et al. 1994, 2000; Sokal et al. 2000). In mammalian systems, GCAPs have been well characterized biochemically and were shown to stimulate GCs in low [Ca2+]free but inhibit GCs at high Ca2+. Stimulation of GC at low Ca2+ constitutes a negative feedback mechanism responsible for return of photoreceptors to the dark state. GCs are integral membrane proteins with one transmembrane domain; GCAPs are soluble CaM-like Ca2+-binding proteins (Polans et al. 1996).
In the mammalian retina, two related membrane proteins, GC1 and GC2 (Garbers and Lowe 1994; Lowe et al. 1995; Shyjan et al. 1992), have been identified; the teleost retina (Oryzias latipes) harbors additional closely related GCs termed olGC3, olGC4, olGC5, and olGC-R2 (Harumi et al. 2003; Hisatomi et al. 1999; Seimiya et al. 1997). Three GCAPs (GCAP 1-3) have been cloned from vertebrate retinas (Dizhoor et al. 1994, 1995; Gorczyca et al. 1994, 1995; Haeseleer et al. 1999; Imanishi et al. 2002). GCAP mRNAs are abundant transcripts present in vertebrate rod and cone photoreceptors (Imanishi et al. 2002; Otto-Bruc et al. 1997b; Palczewski et al. 1994; Subbaraya et al. 1994). As judged by immunoreactivity, GCAP1 is most abundant in cone outer segments of all mammalian species tested (Cuenca et al. 1998; Kachi et al. 1999). GCAP2 was localized primarily in rod photoreceptors (Dizhoor et al. 1995) but was also found in cones (Kachi et al. 1999; Otto-Bruc et al. 1997b). GCAP3 was only identified in human and zebrafish (Danio rerio) retinas. In zebrafish retina, zGCAP1-3 were localized to rod cells, short single cones (zGCAP1-2), and all subtypes of cones (zGCAP3) (Imanishi et al. 2002). A protein related to GCAPs, guanylate cyclase-inhibitory protein (GCIP), has been shown to be present in frog cones (Li et al. 1998).
The GCAP 1/2 genes are arranged in a tail-to-tail array in mammals (Howes et al. 1998; Surguchov et al. 1997) and chicken (Semple-Rowland et al. 1999), separated by short intergenic sequences (<5 kb). The tail-to-tail gene arrangement facilitates the construction of GCAP 1/2 double knockout mice with a single construct (Mendez et al. 2001). GCAPs-/-mice show increased amplitudes of single photon responses and a delay in dark adaptation consistent with a lack of GC stimulation. Mice expressing transgenic GCAP1 on a GCAP double null background show normal response kinetics under dim light conditions in rods expressing normal levels of GCAP1 (Howes et al. 2002), as well as in cones under bright flash conditions (Pennesi et al. 2003). Defects in the GCAP1 gene have been linked to cone dystrophies in several human families (Newbold et al. 2001; Payne et al. 1998; Sokal et al. 1998; Wilkie et al. 2001). The role of GCAP2 in regulation of mammalian phototransduction is less well understood. No defects in the human GCAP2 gene have been linked to retina disease to date (Payne et al. 1999).
In this study, we explore the presence of additional GCAP genes in zebrafish and pufferfish (Fugu rubripes). Zebrafish and pufferfish have secured their place in studies of vertebrate development (Malicki 2000; Raz 2003) and also as models of retina function (Kainz et al. 2003). The zebrafish genome sequencing project (currently ∼50% finished in draft form; http://www2.ebi.ac.uk/genornes/mot/) and the Fugu rubripes genome (sequenced to over 95% coverage ([Aparicio et al. 2002]) added new and powerful tools to unravel novel genes expressed in the retina. We show that the exon/intron arrangements of the zGCAP1-5/GCAP7 and fuGCAPl-8 genes are identical to those of known vertebrate GCAP genes, suggesting that the GCAP genes arose by gene duplications from a common ancestor. Three of the four introns of the GCIP genes were positioned identically to those in GCAP genes, indicating a close evolutionary relationship between GCAPs and GCIP. We found no evidence that the teleost GCAP1 and GCAP2 genes are arranged in a tail-to-tail array, while the fuGCAP7/fuGCAP8 genes are in a head-to-tail array separated by a less than 2-kb intergenic region. GCAP7 and GCAP8 form new subtypes in the large GCAP subfamily and the discovery of GCIP genes in pufferfish and zebrafish establishes GCIPs as a novel subfamily.
Materials and Methods
Identification of Zebrafish GCAP1-5 (zGCAP1-5) and zGCAP7 Genomic and cDNA Sequences
Zebrafish gene fragments were identified by searching the zebrafish Genome Data Base at http://www.ncbi.nlm.nih.gov/genome/seq/DrBlast.html with GCAP1-3 amino acid sequences using the BLAST algorithm (Altschul et al. 1990). Complete or partial contigs were obtained for zGCAP1-5 and zGCAP7 genes. The exon/intron boundaries of zGCAP1-3 genes were derived using the zGCAP1-3 amino acid sequences (Imanishi et al. 2002) as a template. The zGCAPl gene was contained in a 222,736-bp genomic contig (BX537162). ATG was at position 137,812, and the stop codon was at position 143,953. The contig contained uninterrupted 4-kb 5′-UTR and 1-kb 3′-UTR. Exon 1 of the zGCAP2 gene was contained in a 710-bp genomic fragment (GI 25817282) deposited in the zebrafish genome database, exon 2 in an 853-bp genomic fragment (GI 226245091), and exons 3 and 4 in a 996-bp genomic fragment (GI 233521263). The gene identifiers (GIs) for corresponding zGCAP3 genomic fragments are GI 131324543 (GCAP3_xl), GI 100379500 (GCAP3_x2), GI 25521086 (GCAP3_x3), and GI 110877272 (GCAP3_x4). The zGCAP4 gene was contained in a 164,721-bp contig (AL627325) deposited by the Wellcome Trust Sanger Institute. ATG was at position 914, and the stop codon at position 4782. The stop codon is followed by 40 kb of uninterrupted 3′ region. The zGCAP4 cDNA and amino acid sequences were derived in silico by taking advantage of the sequence similarity to the GCAP1-3 genes, and the contiguous mRNA sequence was verified by RT/PCR using zebrafish retina RNA (see below). The zGCAP5 gene was contained in the following short contigs: GI 226210856 (GCAP5_x3x4), GI 83199735 (GCAP5_xl), and GI 98848481 (GCAP5_x2). The cDNA sequence was assembled from these contigs using two ESTs (AL922711, BI876167) covering most of the zGCAP5 coding sequence. Exon 4 of the zGCAP7 gene (orthologue of the fuGCAP7 gene) was contained in a large, 219,383-bp contig (BX323069). The remainder of the zGCAP7 gene was found in the following contigs: GI 111452178 (zGCAP7_x3), GI 158654090 (zGCAP7_xl), and GI 30535992 (zGCAP7_x2). The zGCAP7 mRNA was derived from the gene fragments using zGCAPl-5 as templates. The mRNA sequence was confirmed by RT/PCR with N- and C-terminal primers. The complete cDNA sequences of zGCAP4, zGCAP5, and zGCAP7 have been deposited in GenBank.
Cloning and Expression of Zebrafish GCAPs
The coding regions (ORF in Table 1) of zGCAPs were amplified (Qiagen one-step RT-PCR kit) by forward and reverse primers which introduced ribosome binding sites and expression enhancer sequences upstream of the translation start (ATG) and His6 tags preceding the translational stop codons, respectively (Table 1). The amplified cDNAs were first cloned into pENTR/D-TOPO vector, then recloned into pDEST 14 vector, and expressed in BL21-(DE3)pLysS bacteria (Invitrogen). The recombinant zGCAP-His6 isoforms were purified using the Histrap kit (Amersham Pharmacia). For generation of zGCAP-specific antisense probes, the 3′-UTRs of zGCAP4 and zGCAP7 were amplified with sense and antisense primers (Table 1). The 3′-UTR of zGCAP5 was cloned using the 3′-RACE (rapid amplification of CDNA ends) system (Invitrogen). The forward primer (Table 1) was paired with a universal antisense primer to amplify the whole 3′-UTR fragment. Then a reverse primer (Table 1) based on DNA sequencing was used to amplify the 800-bp 3′-UTR region. The 3′-UTR fragments were cloned into the TOPO-PCRII vector for production of anti-sense and sense RNA for in situ hybridization.
Table 1.
List of oligonucleotide sequences used for amplification of zGCAP coding sequences (ORFs) and 3'-UTRs
| RT/PCR sense primers | RT/PCR antisense primers | |
|---|---|---|
| zGCAP4 ORF | 5′-CACCTTGTTTAACTTTAAGAAGGAGTCCCCCACCATGGGTAACAACCATGCCAG | 5′-TTAATGATGATGATGATGATGTTTCTGTCGCCCTTCGACAATTATTAATAGG |
| 3′-UTR | 5′-GCTTCGAA CTTCATAGTA AGGA | 5′-GCTCACAAGCTTGCTGCATTG |
| zGCAP5 ORF | 5′-CACCTTGTTTAACTTTAAGAAGGAGTCCC CCACCATGGGGGACTCCTCCAGCATG | 5′-TTAATGATGATGATGATGATGTGCTTGATCCTCGATGATCTCGG |
| 3′-UTR | 5′-GACTCCTACATCGAGCAGGAGG | 5′-GAGCTCAACAGCGCTCCTAAG |
| zGCAP7 ORF | 5′-CACCTTGTTTAACTTTAAGAAGGAGTCCCCCACCATGGGCCA GAATCAAAGC GATG | 5′-TTAATGATGATGATGATGATGTGTTTTCT TCCCCAAGTTGTCCCTG |
| 3′-UTR | 5′-CTCA GGCTACGGTC TAGACTTG | 5′-CAACATACGCAGAAGACATTTGCAC |
Note. In ORF sense primers, the sequences upstream of ATG contain ribosome binding sites and expression enhancer sequences. In ORF antisense primers, the oligonucleotides contain a His-tag sequence (cursive) preceding the translational stop codon. Underlined sequences are zGCAP coding sequences.
Identification of Pufferfish GCAP Genomic and Amino Acid Sequences
The Blastp and/or Tblastn programs were used to identify fuGCAPl-8 sequences in the NCBI BLAST homepage with zGCAPs as probes (http://www.ncbi.nlm.nih.gov/PMGifs/Genomes/fugu.html). The following genomic contigs were identified: CAAB01000049 (fuGCAPl; 277,098 bp), CAAB01000358 (fuGCAP2, 137,344 bp), CAAB0100041 (fuGCAP3, 279,872 bp), CAAB01001815 (fuGCAP4; 43,707 bp), CAAB0100594 (fuGCAP5; 112,939 bp), CAAB0100322 (fuGCAP6; 143,735 bp), and CAAB0101451 (fuGCAP7 and GCAP8; 63,209 bp). The fuGCAP1-8 amino acid sequences were found in the “predicted proteins” database and verified by conceptual translation of the genomic contigs above. The fuGCAPl-8 cDNA sequences were derived from the gene contigs using exon/intron and intron/exon junction consensus sequences and the fuGCAP amino acid sequences as template. The software used was Omiga 2.0 and DS-Gene 1.5 (Accelrys).
Identification of GCIP Genes and cDNAs
The zebrafish GCIP genomic sequences were identified in five short unlinked contigs: WGS_137550740 (GCIP exon 4; 701 bp), WGS_174713351 (GCIP exon 1; 1175 bp), WGS_25810388 (GCIP exon 3; 724 bp), WGS_36001292 (GCIP exon 5; 613 bp), and WGS_42995461 (GCIP exon 2; 616 bp). The pufferfish GCIP gene was contained in a 161,141-bp contig (accession No. 22418311), where ATG is at position 142,977 and the stop codon at position 144,173. The salmon (Salmo salar) GCIP coding sequence was assembled from the following five ESTs: CA056338, CA062777, CB511886, CB516382, and CB517780.
Identification of GCs in Pufferfish and Zebrafish
Using medaka (Oryzias latipes) photoreceptor cyclases (olGC3, BAA19205; olGC4, BAA19206; olGC5, BAA19207; and olGCR2, BAA76301) (Hisatomi et al. 1999; Seimiya et al. 1997) as seeds, the corresponding pufferfish orthologues were identified. The pufferfish cyclase sequences were found in the Predicted Protein database under accession numbers SINFRUP00000059727 (GC3) (Fugu9727 in Fig. 6B), SINFRUP00000057919 (GC4) (Fugu 7919 in Fig. 6B), SINFRUP00000075651 (GC5) (Fugu5651 in Fig. 6B), and SINFRUP00000063925 (GCR2) (Fugu3925 in Fig. 6B). The corresponding gene sequences were found in the following contigs: 22421540_GC3, 22420508_GC4, 22419305_GC5, and 22422053_GCR2. In the zebrafish genome, the information contained in GenBankis limited. Three short mRNA sequences (AY050503, GC4 homolog; AY050504, GCR2 homolog; and AY050505, GC3 homolog) have been identified. There are only two partial genomic contigs encoding GCs in zebrafish (BX294181, GC3; BX537138, GC4).
Fig. 6.
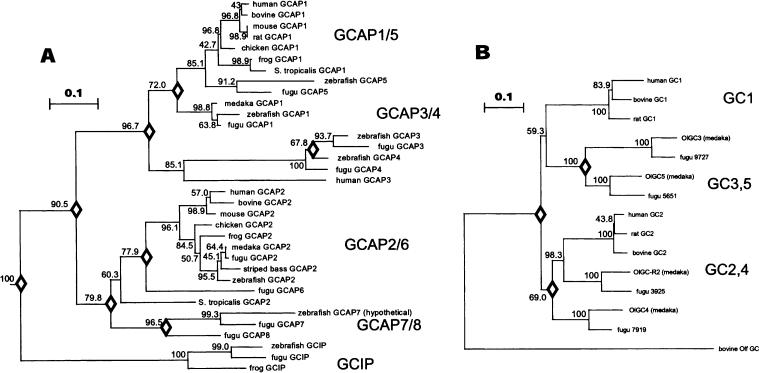
Phylogenetic analysis of GCAPs and GCs. A A phylogenetic tree calculated from the amino acid sequences of GCAPs. Numbers at the nodes indicate the clustering percentage obtained from 1000 bootstrap resamplings. Bar indicates 10% replacement of an amino acid per site (k = 0.1; see Materials and Methods). The diamonds located at the nodes indicate the estimated gene duplication events. Sequence data used in the present analyses were taken from the GenBank, EMBL, SWISS-PROT, and NCBI databases, except for mouse GCAP2. The accession numbers for the amino acid sequences are as follows: bGCAP1 (Bos taurus), AAB31698; hGCAP1 (Homo sapiens), P_00040; mGCAP1 (Mus musculus), NP_03221; accession rGCAP1, composite sequence of two overlapping ESTs (BF543297 and AI579371), 27681847; cGCAP1 (Gallus gallus), P79880; fuGCAP1 (Rana pipiens) O73761; zGCAP1, AAK95947; fuGCAP1 (Takifugu rubripes), CAD12779; siluGCAP1 (Silurana tropicalis), translated from EST AL874865 (unpublished); oryGCAP1 (Oryzias latipes), BAB83093; bGCAP2, translated from L43001; hGCAP2, 8928106; mGCAP2 (taken from Howes et al. 1998); sbGCAP2 (striped bass; Morone saxatilis), K. Zhang and W. Baehr, unpublished; cGCAP2 (Gallus gallus), P79881; fuGCAP2 (Rang pipiens), O73762; siluGCAP2 (Silurana tropicalis), translated from EST AL797721; oryGCAP2 (Oryzias latipes), BAB83094; fuGCAP2, CAD12780; zGCAP2 (Danio rerio), AAK95948; hGCAP3, (Homo sapiens), AAD19944; zGCAP3 (Danio rerio), AAK95949; zGCAP4, 5, 7, (to be submitted to GenBank); fuGCIP (Rana pipiens), O73763. B A phylogenetic tree calculated from the amino acid sequences of photoreceptor GCs. Conserved amino acid sequences including transmembrane domain and intracellular domains are used for calculation. Bar indicates 10% replacement of an amino acid per site (k = 0.1; see Materials and Methods). The accession numbers of the amino acid sequences are as follows: bovine GC1, AAB86385; human GC1, Q02846; rat GC1, P51840; bovine GC2, O02740: human GC2, P51841; rat GC2, P51842; bovine GColf, AAC31208. For teleost olGC accession numbers, see Materials and Methods.
GC Assay
The GC assay was performed using washed bovine ROS membranes prepared from fresh slaughtered cattle (Schenk Packing Company, Stanwood, WA) (Papermaster 1982) Membranes were reconstituted with recombinant GCAPs and assayed as described previously (Otto-Bruc et al. 1997a). Ca2+ was calculated using the Chelator 1.00 (Schoenmakers et al. 1992).
Immunoblotting
Immunoblot analysis was performed as described previously (Maeda et al. 2001). The purified GCAPs (30 μg) were analyzed by SDS-PAGE, using a 12.5% polyacrylamide gel. Separated proteins in a gel were electrotransferred to PVDF membranes in 10 mM BTP buffer, pH 8.4, containing 10% methanol. After blocking with 5% skim milk in PBS, the expression of proteins was probed with an appropriate primary antibody, followed by a horseradish peroxidase (HRP)-labeled secondary antibody (Amersham, NJ).
Zebrafish Multiple Tissue RT-PCR
RNA derived from zebrafish tissues was isolated using the MicroAqueous RNA Isolation Kit (Ambion). Each tissue (10 mg) was processed according to the manufacturer's recommendations. The brain tissue included the pineal organ. Then the mRNAs were reverse transcribed using Superscript II RT (Invitrogen) according to the manufacturer's protocol. Diagnostic zGCAP cDNA fragments from each tissue were amplified using the primers listed in Table 2. To avoid false positives from trace genomic DNA, each primer pair was designed such that they flanked at least one intron.
Table 2.
List of oligonucleotide primers used for amplification of zGCAPs, zGCIP, and zβ-actin from various zebrafish tissues
| Sense primer | Antisense primer | |
|---|---|---|
| zGCAP1 | 5′-AAGAAGTTCATGACAGAGTGTCCATCC | 5′-GCGTAATTTGTGCTCCATTTTTCCT |
| zGCAP2 | 5′-CATGCACGATTTCAAGAGCTTTTTC | 5′-CTCCTTGAGCTCTGTTTTGTCGATG |
| zGCAP3 | 5′-TGGTACAACAAGTTCATGAGGGAATCT | 5′-TATGGTCTCCATTTCATCTCTGTCGAT |
| zGCAP4 | 5′-AGGACATGCACCACTGGTATAACAAAT | 5′-TCCATCCTGGTCAAAAAGTTTGAAGTA |
| zGCAP5 | 5′-CCAGCTCACCTTCTACGAGTTCAAGA | 5′-GTCCATATCGAAGAGCTTGAAATACCA |
| zGCAP6 | 5′-TAACAGAAATCCAGCCTCTTTACACCA | 5′-CTTGTCTGTCCAACTTTCCATTCTCAT |
| zGCIP | 5′-GTACGTCACCGAGCTTTATGAATGG | 5′-GATGGCGGTCACATATTCTCTGAAG |
| zβ-actin | 5′-CCCCTTGTTCACAATAACCT | 5′-TCTGTTGGCTTTGGGATTCA |
In Situ Hybridization
Anterior segments of zebrafish eyes were removed and then eyecups were fixed for 4 h in 4% paraformaldehyde in 0.1 M PB (100 mM sodium phosphate, pH 7.4). Retinal tissues were infiltrated with 20% sucrose in PB and then embedded in 33% OCT compound (Miles) diluted with 20% sucrose in PB. Eye tissues were sectioned at 5 μm. The 3′-UTRs of zGCAP4, GCAP5, and GCAP7 were cloned into PCRII-TOPO vector and linearized with appropriate endonucleases. Antisense and sense RNA probes (0.7-2013;1.0 kb) were synthesized by runoff transcription from the SP6 or T7 promoter with digoxigenin-UTP, as recommended in the manufacturer's protocol (Roche Molecular Biochemicals). In situ hybridization techniques for retinal sections are as described previously (Imanishi et al. 2002).
Calculation of the Phylogenetic Tree
A phylogenetic tree was constructed from the aligned sequences using the ClustalW program. Evolutionary distances of the sequences (k) were estimated using the proportion of different amino acids between the two sequences (p), with correction for multiple substitutions of k = -ln(1- p-0.2p2) (Kimura 1983) by Protdist in the PHYLIP package (version 3.6a). The phylogenetic tree was constructed by the neighbor-joining (NJ) method using the Neighbor program in PHYLIP package (version 3.6a). Bootstrap resamplings were performed by the Seqboot program in the PHYLIP package (version 3.6a).
Homology Model of GCAP1
A homology model of GCAPl was created on the basis of the NMR structure of unmyristoylated GCAP2 (Ames et al. 1999; see also Sokal et al., 1999, 2001), taking advantage of the sequence alignments. The model was generated with the HOMOLOGY module of the INSIGHTII software (Accelrys Inc., San Diego, CA). Superposition of the structures from this group of Ca2+-binding proteins showed that the main chain atoms of unmyristoylated, Ca2+-bound GCAP2, recoverin, and neurocalcin fold into a similar structure. The root mean square deviation of the main chain atoms (in the EF-hand motifs) is 2.2 ° comparing GCAP2 to recoverin and 2.0 ° comparing GCAP2 to neurocalcin.
Results and Discussion
Identification of Novel Zebrafish and Pufferfish GCAPs
We previously identified three GCAPs (zGCAPl-3) in zebrafish (Imanishi et al. 2002). These GCAPs were shown to be present in rods, short single cones (zGCAP1, 2), and all subtypes of cones (zGCAP3). Continuing these efforts, we used zGCAP amino acid and nucleotide sequences as seeds to identify additional GCAP sequences in the zebrafish and pufferfish genomic databases. The zebrafish genome yielded several large genomic contigs containing entire novel GCAP genes or multiple WGS (whole genome shotgun) fragments harboring single or pairs of exons. The zGCAP4 gene was found in a large contig of 163 kb (see Materials and Methods). Using exon/intron junction consensus sequences and zGCAP amino acid sequences as template, the GCAP4 cDNA and amino acid sequences were derived. Similarly, WGS fragments containing zGCAP5 and zGCAP7 exons were assembled to establish the corresponding gene sequences. Correct exon assembly was verified by amplification of the entire coding sequences by RT/PCR with N-terminal and C-terminal primers and zebrafish retina RNA as template. In addition to zebrafish, we identified six novel GCAPs (fuGCAP3-8) in the Fugu rubripes genome database. Previously, just two fugu GCAPs (fuGCAPl and fuGCAP2) had been identified (Wilkie et al. 2002). The novel fuGCAP3-8 genes were contained in large contigs, 43-280 kb in size (for accession numbers, see Materials and Methods). The cDNA and amino acid sequences were derived by conceptual translation after exon/intron junctions had been located.
Gene Arrangements and Gene Structures of Teleost GCAPs/GCIPs
The GCAP genes consist of four exons; the GCIP genes, of five exons (Figs. 1A and B). The zGCAPl structural gene (ATG to translation stop including all introns) spans 6140 bp and is thus nearly as large as its human counterpart (6786 bp; Table 3). The exact lengths of other zebrafish GCAP genes (except zGCAP4; 3868 bp) is unknown since the intron sequences are incomplete. Consistent with the small size of the pufferfish genome (∼1/10 the size of the human genome), the fuGCAP genes are more compact, with short intervening sequences (Table 3). The largest pufferfish structural GCAP gene, the fuGCAP4 gene, is 2759 bp in length; the shortest gene, fuGCAP8, consists of only 876 bp. The human GCAP3 gene (46 kb), in contrast, is nearly 40 times larger than the fuGCAP3 gene (1.2 kb). Importantly, the positions of the three introns in all sequenced GCAP genes (mammalian and teleost) are identical with respect to the GCAP coding sequences (Fig. 1C). This is a very remarkable conservation over several hundred million of years of evolution (the teleost and mammalian lineages diverged about 450 million years ago (Venkatesh et al. 2000). The GCIP genes, in contrast, have acquired an additional intron in the N-terminal region while preserving the positions of the three C-terminal introns (Fig. 1C). GCIPs have been identified in amphibian (Li et al. 1998) and teleost (this communication) and are closely related to GCAPs in sequence and structure (see below). GCIP genes/ESTs could not be retrieved from human, mouse, and rat genomic databases, suggesting that the GCIP genes were lost in mammals or arose in teleost/amphibian after mammalian and teleost divergence.
Fig. 1.
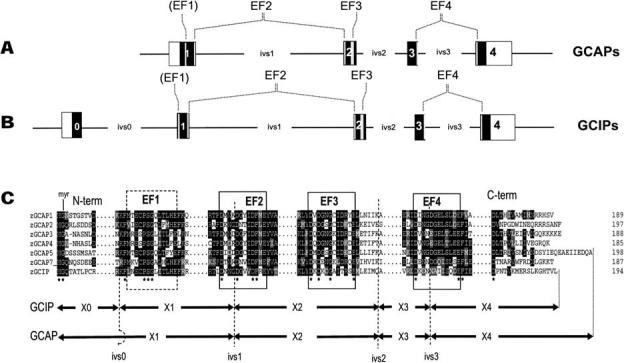
GCAP and GCIP genes. A, B Graphical depictions of GCAP and GCIP gene structures. Black boxes represent coding exons; white boxes, untranslated regions; and lines, introns (ivs0-3). Positions of introns (ivs1-3) are identical in GCAPs and GCIPs. The GCIP gene has acquired an additional intron (ivs0) in the N-terminal region, upstream of EF1. The locations of EF hands are indicated by gray lines. C Partial amino acid sequences and positions of exon/intron junctions of zGCAP and zGCIP genes. Intron positions in the amino acid sequences are indicated by dashed lines. The EF2 and EF4 Ca2+-binding loops are interrupted by introns, while EF1 and EF3 are contiguous. The EF loop sequences containing 12 amino acids, as well as flanking hydrophobic residues, are highly conserved among all GCAPs. The C-terminal regions are among the most divergent.
Table 3.
GCAP|GCIP gene and protein data of Fugu rubripes and Danio rerio
| Gene | Ivs0 | Ivs1 | Ivs2 | Ivs3 | Polypetide | Calc. pI | Mol. mass | Gene length | Gene length (human) |
|---|---|---|---|---|---|---|---|---|---|
| fuGCAP1 | — | 558 | 76 | 245 | 189 | 4.8 | 21,788 | 1448 | 6,786 |
| zGCAP1 | 5292 | 97 | 194 | 189 | 4.66 | 21,908 | 6140 | (NT_007592.13) | |
| fuGCAP2 | — | 158 | 81 | 219 | 197 | 4.25 | 22,929 | 1049 | 10,002 |
| zGCAP2 | 257 | >700 | 166 | 197 | 4.34 | 22,956 | >1800 | (NT_007592.13) | |
| fuGCAP3 | — | 184 | 418 | 103 | 189 | 4.17 | 21,888 | 1268 | 45,732 |
| zGCAP3 | >400 | >860 | >600 | 188 | 4.08 | 21,854 | >2400 | (NT_005612.14) | |
| fuGCAP4 | — | 1701 | 394 | 112 | 185 | 4.03 | 21,232 | 2759 | |
| zGCAP4 | 1260 | 1960 | 94 | 185 | 4.09 | 21,341 | 3868 | ||
| fuGCAP5 | — | 536 | 481 | 72 | 190 | 4.07 | 21,780 | 1657 | |
| zGCAP5 | 282 | >1000 | 133 | 198 | 3.97 | 22,423 | >2000 | ||
| fuGCAP6 | — | 273 | 205 | 116 | 189 | 4.91 | 21,895 | 958 | |
| fuGCAP7 | — | 78 | 541 | 207 | 187 | 4.76 | 21,768 | 1392 | |
| zGCAP7 | >250 | >400 | >650 | 187 | 4.61 | 22,037 | >2000 | ||
| fuGCAP8 | — | 93 | 76 | 141 | 190 | 4.96 | 22,205 | 876 | |
| fuGCIP | 308 | 79 | 117 | 105 | 198 | 4.64 | 22,724 | 1195 | |
| zGCIP | >650 | >800 | >150 | >950 | 194 | 4.80 | 22,239 | >2500 |
Note. Ivs, intervening sequences (nucleotides [nt]); polypeptide (number of amino acid residues); molecular masses (Da); gene length (nt) (translational start to stop). The numbers were calculated using Omiga 2.0 (Accelrys, Inc.). Rightmost column: human GCAP1-3 gene lengths, as a comparison, and GenBank accession numbers.
As has been described for human (Surguchov et al. 1997), mouse (Howes et al. 1998), and chicken (Semple-Rowland et al. 1999), the fuGCAP1 and fuGCAP2 genes were recently suggested to be arranged in a tail-to-tail array (genes on opposite strands in close proximity), separated by an intergenic region of ∼19 kb (Wilkie et al. 2002). We found the fuGCAP1 gene in a 277-kb contig (accession No. 22418112), which has 18 kb of uninterrupted 3′-UTR, and the fuGCAP2 gene in a 137-kb contig (accession No. 22418421) with a 20-kb 3′-UTR. The fuGCAP1 and fuGCAP2 3′-UTRs are nonoverlapping, thus the two genes must be at least 38 kb apart. The zebrafish contig containing the zGCAP1 gene has an uninterrupted 3′-UTR of ∼82 kb (complete zebrafish DNA sequence (from clone DKEY-9A20 in linkage group 4; accession No. BX537162), a region that has no sequence similarity to zGCAP2. This finding rules out that the GCAP1 and GCAP2 genes are arranged in close proximity in a tail-to-tail array in teleost species. However, the fuGCAP7 and fuGCAP8 genes are arranged in a head-to-tail gene array (both genes on the same strand in the same direction), separated by only 2 kb of genomic DNA (stop codon of fuGCAP7 to ATG of fuGCAP8). It is unknown whether a similar inferred gene duplication has occurred in zebrafish, since the zGCAP7 sequence was assembled from WGS fragments and the zGCAP8 gene is unidentified.
GCAP/GCIP Amino Acid Sequence Comparisons
GCAPs are typically 175 to 205 amino acids in length, are acidic, and contain four EF-hand motifs for Ca2+-binding, three of which are functional (EF2-4). The novel zGCAPs and fuGCAPs (zGCAP4, zGCAP5, zGCAP7, fuGCAP3-8) conform to the general structure of vertebrate GCAPs (Fig. 2A) and are 185-198 amino acids long, with calculated masses of ∼22 kDa (Table 3). All GCAPs also carry a consensus sequence for N-terminal myristoylation. Based upon earlier studies, post-translational processing is hypothesized to involve removal of Met-1 and N-myristoylation of Gly-2. The strongest homology among zGCAPs was observed around the EF Ca2+-binding motifs (Figs.1 and 2A). Sixteen of 22 amino acid residues invariant in all GCAPs sequenced to date are located in these areas. The strongest sequence conservation is in the central area of the molecule, around the EF2/EF3 motifs. The most divergent regions in the amino acid sequences are located between EF3 and EF4 and in the N- and C-terminal regions.
Fig. 2.
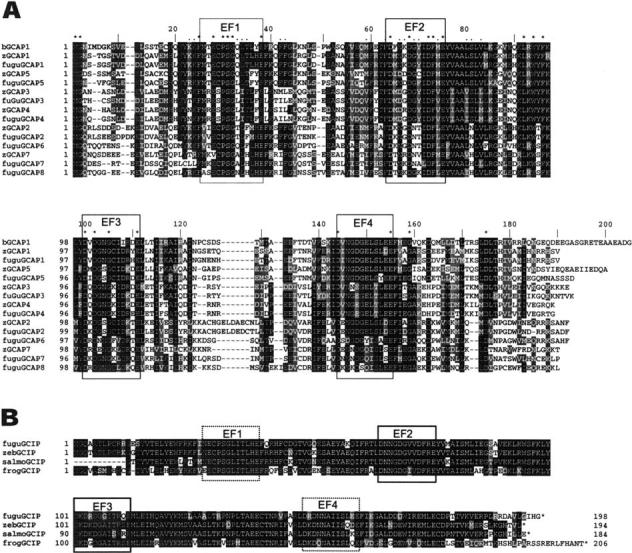
Sequence alignments. A Amino acid sequence alignment of Danio rerio and Fugu rubipes GCAPs, compared with bovine GCAP1 (bGCAP1; top). The three functional EF hand motifs (EF2-4) representing high-affinity Ca2+-binding sites are boxed. The nonfunctional EF1 in the N-terminal region is boxed by dashed line. Residues invariant in all GCAPs sequenced to date are marked by asterisks above the sequence; residues identified as subclass-specific are marked by dots. B Alignment of GCIPs (Fugu rubripes, Danio rerio, and Salmo salar) compared with frog (Rana pipiens) GCIP. The salmon GCIP sequence was assembled from several ESTs deposited in GenBank (see Materials and Methods). Note the much higher conservation of residues throughout the polypeptide. Residues conserved in more than 50% of the sequences shown are printed white on black. Conservative substitutions are on a gray background. The alignments were generated by Clustal W (version 1.82) at http://www.ebi.ac.uk/clustalw/ and shaded with boxshade at http://www.ch.embnet.org/software/BOX_form.html.
GCIPs (GC-inhibitory proteins), in contrast, are highly conserved polypeptides with little sequence variation among teleost and frog (Rana pipiens) (Fig. 2B), apart from the C-terminal ends. GCIP was first identified in Xenopus laevis cones by immuno-cytochemistry (Li et al. 1998). Recombinant GCIP can interact with GC but is unable to stimulate in high or low Ca2+. It competitively inhibits cyclase activity when the enzyme is constitutively activated by a Ca2+-insensitive mutant, consistent with GCIP binding sites on GC. In all GCIPs identified to date, EF1 and EF4 are not functional for Ca2+-binding (Li et al. 2001).
GCAP Structure and Biological Activity
The structure of Ca2+-bound vertebrate GCAPs (Fig. 3A) shows four EF-hand motifs arranged in a compact array like that seen in recoverin (Ames et al. 1999; Palczewski et al. 2000). Three Ca2+ ions are bound to EF2, EF3, and EF4, but Ca2+ is not bound to EF1 because the EF loop is distorted from a favorable Ca2+-binding geometry by a Pro residue at the fifth position of the 12-residue loop (Fig. 2A), a residue invariably present in all GCAPs. The GCIP structure is likely to be similar to that of GCAPs, since most of the hydrophobic residues in the hydrophobic core and in the exposed patch are highly conserved. The conservation of residues among GCAPs was calculated using the T-Coffee method (Notredame et al. 2000), which uses an algorithm for alignment and which scores by processing a data set of all pairwise alignments between the sequences. It not only calculates percentage identity for particular positions in the alignment, but also takes into consideration adjoining residues.
Fig. 3.
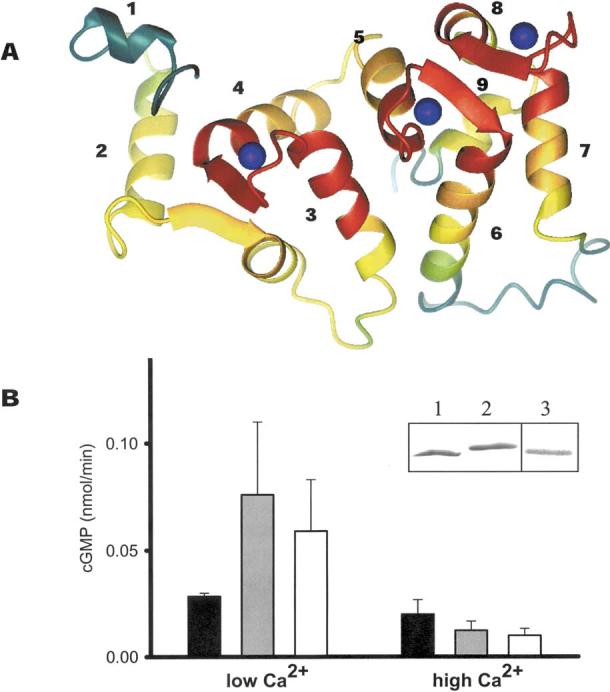
Structure and function of GCAPs. A Model of GCAPs based on the NMR structure of bovine GCAP2 (Ames et al. 1999). The conservation of residues between GCAPs was calculated using the T-Coffee method (Notredame et al. 2000). The polypeptide chain is colored as follows: red, 100-80%; orange, 80-60%; yellow, 60-40%; green, 40-20%; and green-blue, 20-0% similarity. Ca2+ions are shown as blue spheres. The most conserved regions are the Ca2+-binding sites (EF-hand loops). The GC-interacting site could involve helix 2 and the following β-sheet, helices 4, 5, and 7. B Reconstitution of ROS GC activity by recombinant bGCAP1 and zGCAP4. Blackbars correspond to ROS GC basal activity, gray bars correspond to ROS GC activity stimulated by bovine GCAP1, and white bars correspond to ROS GC activity stimulated by zGCAP4. Error bars represent standard deviations for GC activity stimulated by GCAPs. Assays were carried out at 50 nM and 1μM [Ca2+]free with the addition of 3 μM GCAPs and were repeated at least three times. Inset: SDS-PAGE (lanes 1 and lane 2) and immunoblotting (lane 3) of GCAP4. Lane 1 represents GCAP4 in the presence of 1 mM Ca2+; lane 2 represents GCAP4 in the presence of 1 mM EGTA.
Recently, we employed evolutionary trace analysis (ET) (Lichtarge et al. 1996) for the GCAP and NCBP families (Imanishi et al. 2002). ET uses the sequence identity tree for the gene family as a means of dividing the multiple sequence alignment into distinct subclasses. The subclasses are then examined for patterns of amino acid conservation and variation. ET analysis of the GCAP subfamily revealed a large surface cluster of both class-specific (italicized) and invariant (boldface) residues from EF1 and EF2 (residues based on bGCAP1 numbering: His19, Lys23, Lys24, Phe25, Glu28, Pro30, Ser31, Gly32, Gln33, Leu34, Thr35, Glu38, Phe42, Phe43, Tyr55, Met59, Phe63, Asn66, Lys67, Asp68, Gly69, Tyr70, Asp72, Phe73, Met74, Glu75, Ala78, Leu80, Ser81, Leu82, and Val83). The second identified cluster is composed predominantly of residues from EF3, with some contribution from EF4. The upstream helix of EF3 contains the invariant amino acids (Leu92, Trp94, and Phe96); the EF3 loop, another three (Asp100, Asp102, and Gly105). One face of the exiting helix of EF3 is composed entirely of class-specific residues (Leu112, Ile115, Ile116, Ile119, Arg120, Ile122, and Asn123). Residues from this region have previously been proposed to play a role in regulating GC (Ames et al. 1999; Li et al. 2001; Olshevskaya et al. 1999; Otto-Bruc et al. 1997a). As depicted in Fig. 3A, the nonfunctional EF-hand 1, the C-terminal region of helix 3, the loop and beginning of helix 4 (EF-hand 2), and the vicinity of Ca2+ loops constituting EF-hands 3 and 4 are the most conserved. Regions that are most likely not involved in the interaction with GC include the least conserved residues in the N- and C-terminal regions, and the loop connecting helices 6 and 7 (Fig. 2A).
We tested the biological activity of zGCAPs using bovine GC present in native ROS membranes as a target (Fig. 3B). Recombinant zGCAP3 was previously shown to stimulate bovine GC, suggesting conservation of residues critically important for interaction between partners of distantly related species. To measure zGCAP-mediated GC activation, His-tagged zGCAPs were expressed in bacteria and purified to apparent homogeneity. zGCAP4-His6 displayed a minor mobility change in the presence and absence of Ca2+ (Fig. 3B, inset), as observed for other GCAPs (Palczewski et al. 1994). When assayed in the presence of bovine rod outer segment GC, zGCAP4 modulated cyclase activity as expected in a dependent manner, stimulating at low and inhibiting at high [Ca2+] (Fig. 3B). These biochemical data demonstrate that zGCAP4 shares biochemical properties in stimulating mammalian GC, as observed previously for zGCAP3 (Imanishi et al. 2002). ZGCAP5 was not active under these conditions, and zGCAP7 could not be stably expressed.
Tissue Distribution of zGCAPs and zGCIP (RT-PCR)
To explore the expression of novel zebrafish GCAPs and GCIP in various tissues, we performed RT-PCR using zebrafish tissue-specific cDNAs as templates (Fig. 4). Monospecific primer pairs were produced that amplified a short (200- to 300-bp) diagnostic fragment for each gene. The results show that all GCAPs and GCIP are strongly expressed in the zebrafish retina, while other tissues (except brain) did not express detectable levels, similarly to observations in human (Subbaraya et al. 1994). GCAP1 mRNA and, to a lesser extent, zGCAP7 and zGCIP mRNA are also present in zebrafish brain, perhaps due to expression in the pineal gland, which is evolutionary related to photoreceptors. The GCAP1/GCAP2 gene array was previously shown to be expressed in chicken pineal (Semple-Rowland et al. 1999). Recently, a pineal expression promoting element (PIPE) was shown to be required for zebrafish pineal expression in addition to crx/otx binding sites (Asaoka et al. 2002). The zGCAPl gene upstream region, however, did not reveal the presence of such an element, thus it is possible that GCAP1 is expressed elsewhere in the brain.
Fig. 4.
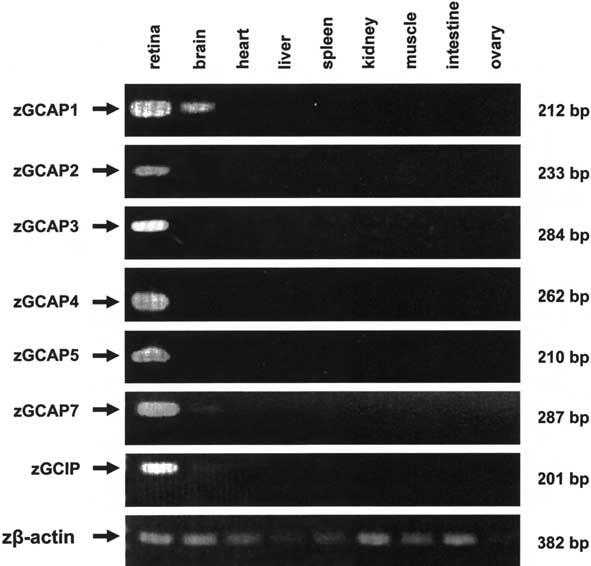
Tissue distribution of zGCAP1-7 and GCIP. Specific primers were used to amplify diagnostic cDNA fragments from different tissues by RT/PCR and the products were analyzed by agarose gel electrophoresis. Zβ-Actin primers were used to amplify control fragments from all tissues. Arrows indicate the positions of amplified PCR products; sizes in nucleotides are given at the right. Note amplification of GCAP1, and, to a lesser extent, of GCAP7 and GCIP, in brain.
In Situ Hybridization of zGCAP4, zGCAP5, and zGCAP7
The cyprinines have complex retinas with one rod type and up to seven cone classes with distinct morphologies (Marc and Cameron 2001). The zebrafish (belonging to cyprinines) retinal mosaic is patterned with four classes of cones, two individual double cones (long and short), long single cones, -and short single cones (Branchek and Bremiller 1984; Raymond et al. 1993; Tohya et al. 2003). Consistent with the morphological diversity of cones, zebrafish expresses at least nine types of opsins: Rh1 in rods, two Lws/Mws (red) in long double cones, four subtypes of Rh2 (green), Sws2 (blue) in long single cones, and Swsl in (UV) in short single cones (Chinen et al. 2003; Raymond et al. 1993; Vihtelic et al. 1999). It is unclear whether zebrafish has additional visual pigments and whether some cones express two or more pigments as has been shown for mouse cones (Applebury et al. 2000).
The subcellular distribution of zGCAP4, zGCAP5, and zGCAP7 mRNAs was investigated by in situ hybridization with antisense RNA produced from subcloned 3′-UTR regions. These regions have no sequence similarity and, thus, minimize cross-hybridization among various GCAPs under stringent conditions. Ellipsoids belonging to different cone types are separated vertically, as schematically shown in Fig. 5 (also see Raymond et al. 1993). The digoxigenin-labeled zGCAP4 antisense RNA probe hybridized specifically to the myoid region of double cones and long single cones protrude above the external limiting membrane. Only minimal signal was observed in short single cones. The sense probe gave no signal (Figs. 5A and B). Similarly, for the zGCAP5 antisense probe, signals were observed in inner segments of LS, SS, and both members of DC (Figs. 5C and D). zGCAP7 appeared to be weakly expressed in DC; the levels in single cones (long and short) were near-background (Figs. 5E and F). These results suggest that zGCAP4, zGCAP5, and zGCAP7 mRNAs are expressed in subtypes of cone photoreceptors but not in rods. No staining was observed in inner retinal neurons for zGCAP4, zGCAP5, and zGCAP7.
Fig. 5.
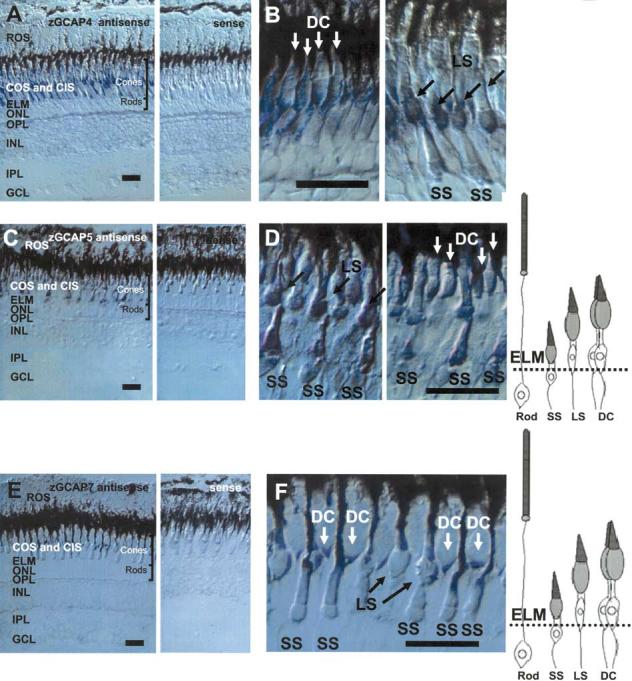
Expression of zGCAP4, zGCAP5, and zGCAP7 in the zebrafish retina by in situ hybridization. A In situ hybridization of GCAP4 transcripts using antisense (left) and sense (right) RNA. The strongest signals are in the cone inner segments. No signal is observed in rod myoid or cell bodies. B Localization of GCAP4 mRNA with a higher magnification. Signals are observed in short single cones (SS), long single cones (LS), and double cones (DC). C In situ hybridization of GCAP5 transcripts using antisense (left) and sense (right) RNA. The strong signal is in the cone inner segment. No signal is observed in rod myoid or cell bodies. D Localization of GCAP5 mRNA with a higher magnification. E In situ hybridization of GCAP7 transcripts using antisense (left) and sense (right) RNA. The signal is observed in a subpopulation of cone inner segments. No signal is observed in rod myoid or cell bodies. F Localization of GCAP7 mRNA with a higher magnification. Signals are strong in double cones (DC). Diagrams of zebrafish photoreceptors (modified from Imanishi et al. 2002) are shown at the right. ROS, rod photoreceptor outer segments; COS, cone photoreceptor outer segments; ELM, external limiting membrane; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Bar = 20 μm.
Diversity of GCAPs in Teleosts
A NJ tree was calculated from vertebrate GCAPs and GCIPs, using vertebrate recoverin and visinin as outgroups (Fig. 6A). This analysis suggests that a putative common ancestor diverged into GCIPs and GCAPs. The tree shows a group of GCIPs (frog, zebrafish, and fugu GCIPs) with a high clustering probability (100%). Thus, GCIPs form a new subfamily and the ancestor of GCIPs appeared before the divergence of teleosts and tetrapods. As we reported before (Imanishi et al. 2002), vertebrate GCAPs are categorized into three subtypes (GCAP1-3). From our analysis of additional GCAPs, teleost fish have at least five additional GCAP genes, among them GCAP4, GCAP5, and GCAP7, which form new subgroups within the GCAP1, GCAP3, and GCAP2 branches. Interestingly, teleost GCAP5s are closely related in sequence to mammalian, avian, and amphibian GCAP1s. The ancestor of zebrafish and pufferfish lived approximately 160-110 million years ago when euteleost fish emerged (Wittbrodt et al. 2002). Thus, the diversity of GCAP1, GCAP4, and GCAP7 is a common feature of euteleost fish that includes important model organisms of developmental biology, genetics, and evolution (Oryzias latipes, Xiphophorus maculates, Fugu rubripes, and Danio rerio).
Diversity of GCs in Teleosts
To investigate the diversity of photoreceptor GCs in teleosts, we searched the pufferfish genomic database with olGC3-5 sequences as seeds and retrieved guanylate cyclase sequences predicted from genomic contigs of four photoreceptor type GCs, fugu9727 (olGC3 ortholog), fugu5651 (olGC5), fugu3925 (olGC-R2), and fugu7919 (olGC4) (Fig. 6B). The lengths of the predicted proteins (1059-1153) correspond closely to those of medaka cyclases (1057-1151). A NJ tree was calculated from available vertebrate photoreceptor GCs (Fig. 6B). The ancestral gene appears to have duplicated at least three times (diamonds) to form a minimum of four photoreceptor cyclase subtypes (mammalian GC1, olGC3/GC5, mammalian GC2/olGC-R2, and olGC4). The tree shows the group of mammalian GC2, olGC-R2, and fugu3925 (GC-R2) with a high clustering probability (98.3%). Thus GC2 is conserved from lower (teleost) to higher (mammalian) vertebrates. The mammalian GC1 gene, expressed in rod and cone photoreceptors (Imanishi et al. 2002), functions in regulation of rod and cone phototransduction. The function of the mammalian GC2 gene, in contrast, presumed to be expressed in rods and cones at lower levels (Yang and Garbers 1997), is less well defined. The medaka cyclases olGC4 and olGC5 were shown to be expressed in the eye; olGC4 was also found in olfactory pits (Kusakabe and Suzuki 2001). OlGC3, in addition to the retina, was also found in multiple other tissues (Seimiya et al. 1997), while olGC-R2 appeared to be retina-specific (Hisatomi et al. 1999). These results suggest that guanylate cyclase gene duplications were relatively rare during vertebrate evolution. The relative paucity of GC genes expressed in the retina is in contrast to the multiple gene duplications of GCAP/ GCIP genes, which produced at least nine subfamilies (GCAP1-8, GCIPs).
Concluding Remarks
In summary, the diversity of GCAPs and GCIP was explored in the Fugu rubripes and Danio rerio genomes. We found that at least five additional GCAPs (GCAP4-8) are predicted to be present in these species. The exon/intron arrangements suggest that these genes arose by gene duplication from a common ancestor. Sequence analysis of the predicted polypeptides identified a myristoylation site at position 2 and three functional EF-hand Ca2+-binding motifs, EF2-4, characteristic of all GCAPs. We describe cloning, expression, and localization of three GCAPs present in the zebrafish retina (zGCAP4, zGCAP5, and zGCAP7). In situ hybridization with antisense zGCAP4, zGCAP5, and zGCAP7 RNA showed expression in zebrafish cone photoreceptors. The presence of at least eight GCAP genes suggests an unexpected complexity of regulation of photoreceptor GC in the teleost retina and contrasts with the much more limited number of genes encoding guanylate cyclases.
Acknowledgments
We would like to thank Darin Bronson for expert technical assistance with this project. This research was supported by grants from the NIH (EY08123, to W.B., and EY08061, to K.P.), the Ruth and Milton Steinbach Fund, the E.k. Bishop Foundation, the Alcon Research Institute, and Research to Prevent Blindness, Inc. (RPB), to the Departments of Ophthalmology at the University of Washington and the University of Utah, and a Center Grant from Foundation Fighting Blindness, Inc., to the University of Utah. W.B. and K.P. are recipients of an RPB Senior Investigator Award. W.B. acknowledges an endowment from Ralph and Mary Tuckto the Department of Ophthalmology at the University of Utah.
Abbreviations Used
- CaM
calmodulin
- DC
double cones
- EST
expressed sequence tag
- GC
guanylate cyclase
- GCAP
GC-activating protein
- fuGCAP
fugu GCAP
- zGCAP
zebrafish GCAP
- GCIP
GC-inhibitory protein
- LS
long single cones
- NCBP
neuronal Ca2+-binding protein
- PAGE
polyacrylamide gel electrophoresis
- PB
phosphate buffer
- PBS
phosphate-buffered saline
- PBST
phosphate-buffered saline with 0.1% Triton X-100
- TBST
Tris-buffered saline with 0.1% Tween 20
- PCR
polymerase chain reaction
- ROS
rod outer segments
- SS
short single cones
- SDS
sodium dodecyl sulfate
- PVDF
polyvinylidene digluoride
- RT
reverse transcriptase
- NJ
neighbor joining
- ORF
open reading frame
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ames JB, Dizhoor AM, Ikura M, Palczewski K, Stryer L. Three-dimensional structure of guanylyl cyclase activating protein-2, a calcium-sensitive modulator of photoreceptor guanylyl cyclases. J Biol Chem. 1999;274:19329–19337. doi: 10.1074/jbc.274.27.19329. [DOI] [PubMed] [Google Scholar]
- Aparicio S, Chapman J, Stupka E, Putnam N, Chia JM, Dehal P, Christoffels A, Rash S, Hoon S, Smit A, Gelpke MD, Roach J, Oh T, Ho IY, Wong M, Detter C, Verhoef F, Predki P, Tay A, Lucas S, Richardson P, Smith SF, Clark MS, Edwards YJ, Doggett N, Zharkikh A, Tavtigian SV, Pruss D, Barnstead M, Evans C, Baden H, Powell J, Glusman G, Rowen L, Hood L, Tan YH, Elgar G, Hawkins T, Venkatesh B, Rokhsar D, Brenner S. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science. 2002;297:1301–1310. doi: 10.1126/science.1072104. [DOI] [PubMed] [Google Scholar]
- Applebury ML, Antoch MP, Baxter LC, Chun LL, Falk JD, Farhangfar F, Kage K, Krzystolik MG, Lyass LA, Robbins JT. The murine cone photoreceptor: A single cone type expresses both S and M opsins with retinal spatial patterning. Neuron. 2000;27:513–523. doi: 10.1016/s0896-6273(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Asaoka Y, Mano H, Kojima D, Fukada Y. Pineal expression-promoting element (PIPE), a cis-acting element, directs pineal-specific gene expression in zebrafish. Proc Natl Acad Sci USA. 2002;99:15456–15461. doi: 10.1073/pnas.232444199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchek T, Bremiller R. The development of photoreceptors in the zebrafish, Brachydanio rerio I. Structure. J Comp Neurol. 1984;224:107–115. doi: 10.1002/cne.902240109. [DOI] [PubMed] [Google Scholar]
- Chinen A, Hamaoka T, Yamada Y, Kawamura S. Gene duplication and spectral diversification of cone visual pigments of zebrafish. Genetics. 2003;163:663–675. doi: 10.1093/genetics/163.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenca N, Lopez S, Howes K, Kolb H. The localization of guanylyl cyclase-activating proteins in the mammalian retina. Invest Ophthalmol Vis Sci. 1998;39:1243–1250. [PubMed] [Google Scholar]
- Dizhoor AM, Lowe DG, Olshevskaya EV, Laura RP, Hurley JB. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994;12:1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Olshevskaya EV, Henzel WJ, Wong SC, Stults JT, Ankoudinova I, Hurley JB. Cloning, sequencing, and expression of a 24-kDa Ca 2+-binding protein activating photoreceptor guanylyl cyclase. J Biol Chem. 1995;270:25200–25206. doi: 10.1074/jbc.270.42.25200. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. PHYLIP (phylogeny inference package), version 3.6. Department of Genetics, University of Washington; Seattle: 2002. [Google Scholar]
- Garbers DL, Lowe DG. Guanylyl cyclase receptors. J Biol Chem. 1994;269:30741–30744. [PubMed] [Google Scholar]
- Gorczyca WA, Gray-Keller MP, Detwiler PB, Palczewski K. Purification and physiological evaluation of a guanylate cyclase activating protein from retinal rods. Proc Natl Acad Sci USA. 1994;91:4014–4018. doi: 10.1073/pnas.91.9.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczyca WA, Polans AS, Surgucheva I, Subbaraya I, Baehr W, Palczewski K. Guanylyl cyclase activating protein: A calcium-sensitive regulator of phototransduction. J Biol Chem. 1995;270:22029–22036. doi: 10.1074/jbc.270.37.22029. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Sokal I, Li N, Pettenati M, Rao N, Bronson D, Wechter R, Baehr W, Palczewski K. Molecular characterization of a third member of the guanylyl cyclase-activating protein subfamily. J Biol Chem. 1999;274:6526–6535. doi: 10.1074/jbc.274.10.6526. [DOI] [PubMed] [Google Scholar]
- Harumi T, Watanabe T, Yamamoto T, Tanabe Y, Suzuki N. Expression of membrane-bound and soluble guanylyl cyclase mRNAs in embryonic and adult retina of the medaka fish Oryzias latipes. Zool Sci. 2003;20:133–140. doi: 10.2108/zsj.20.133. [DOI] [PubMed] [Google Scholar]
- Hisatomi O, Honkawa H, Imanishi Y, Satoh T, Tokunaga F. Three kinds of guanylate cyclase expressed in medaka photoreceptor cells in both retina and pineal organ. Biochem Biophys Res Commun. 1999;255:216–220. doi: 10.1006/bbrc.1999.0165. [DOI] [PubMed] [Google Scholar]
- Howes K, Bronson JD, Dang YL, Li N, Zhang K, Ruiz C, Helekar B, Lee M, Subbaraya I, Kolb H, Chen J, Baehr W. Gene array and expression of mouse retina guanylate cyclase activating proteins 1 and 2. Invest Ophthalmol Vis Sci. 1998;39:867–875. [PubMed] [Google Scholar]
- Howes KA, Pennesi ME, Sokal I, Church-Kopish J, Schmidt B, Margolis P, Frederick J, Rieke F, Palczewski K, Wu SM, Detwiler PB, Baehr W. GCAP1 rescues rod photoreceptor response in GCAP1/GCAP2 knockout mice. EMBO J. 2002;21:1545–1554. doi: 10.1093/emboj/21.7.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi Y, Li N, Sowa ME, Lichtarge O, Wensel TG, Saperstein DA, Baehr W, Palczewski K. Characterization of retinal guanylate cyclase-activating protein 3 (GCAP3) from zebrafish to man. Eur J Neurosci. 2002;15:63–78. doi: 10.1046/j.0953-816x.2001.01835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachi S, Nishizawa Y, Olshevskaya E, Yamazaki A, Miyake Y, Wakabayashi T, Dizhoor A, Usukura J. Detailed localization of photoreceptor guanylate cyclase activating protein-1 and) 2 in mammalian retinas using light and electron microscopy. Exp Eye Res. 1999;68:465–473. doi: 10.1006/exer.1998.0629. [DOI] [PubMed] [Google Scholar]
- Kainz PM, Adolph AR, Wong KY, Dowling JE. Lazy eyes zebrafish mutation affects Muller glial cells, compromising photoreceptor function and causing partial blindness. J Comp Neurol. 2003;463:265–280. doi: 10.1002/cne.10763. [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Suzuki N. A cis-regulatory element essential for photoreceptor cell-specific expression of a medaka retinal guanylyl cyclase gene. Dev Genes Evol. 2001;211:145–149. doi: 10.1007/s004270100136. [DOI] [PubMed] [Google Scholar]
- Li N, Fariss RN, Zhang K, Otto-Bruc A, Haeseleer F, Bronson JD, Qin N, Yamazaki A, Subbaraya I, Milam AH, Palczewski K, Baehr W. Guanylate cyclase inhibitory protein is a frog retinal Ca2+ binding protein related to mammalian guanylate cyclase activating proteins. Eur J Biochem. 1998;252:591–599. doi: 10.1046/j.1432-1327.1998.2520591.x. [DOI] [PubMed] [Google Scholar]
- Li N, Sokal I, Bronson JD, Palczewski K, Baehr W. Identification and functional regions of guanylate cyclase-activating protein 1 (GCAP1) using GCAP1/GCIP chimeras. Biol Chem. 2001;382:1179–1188. doi: 10.1515/BC.2001.148. [DOI] [PubMed] [Google Scholar]
- Lichtarge O, Bourne HR, Cohen FE. Evolutionary conserved G abc binding surfaces support a model of the G protein-receptor complex. Proc Natl Acad Sci USA. 1996;93:7507–7511. doi: 10.1073/pnas.93.15.7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DG, Dizhoor AM, Liu K, Gu Q, Spencer M, Laura R, Lu L, Hurley JB. Cloning and expression of a second photo-receptor-specific membrane retina guanylyl cyclase (RetGC), RetGC-2. Proc Natl Acad Sci USA. 1995;92:5535–5539. doi: 10.1073/pnas.92.12.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Maeda A, Maruyama I, Ogawa KI, Kuroki Y, Sahara H, Sato N, Ohguro H. Mechanisms of photoreceptor cell death in cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 2001;42:705–712. [PubMed] [Google Scholar]
- Malicki J. Harnessing the power of forward genetics—Analysis of neuronal diversity and patterning in the zebrafish retina. Trends Neurosci. 2000;23:531–541. doi: 10.1016/s0166-2236(00)01655-6. [DOI] [PubMed] [Google Scholar]
- Marc RE, Cameron D. A molecular phenotype atlas of the zebrafish retina. J Neurocytol. 2001;30:593–654. doi: 10.1023/a:1016516818393. [DOI] [PubMed] [Google Scholar]
- McBee JK, Palczewski K, Baehr W, Pepperberg DR. Confronting complexity: The interlinkof phototransduction and retinoid metabolism in the vertebrate retina. Prog Retin Eye Res. 2001;20:469–529. doi: 10.1016/s1350-9462(01)00002-7. [DOI] [PubMed] [Google Scholar]
- Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensititvity of rod photoreceptors. Proc Natl Acad Sci USA. 2001;98:9948–9953. doi: 10.1073/pnas.171308998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RJ, Deery EC, Walker CE, Wilkie SE, Srinivasan N, Hunt DM, Bhattacharya SS, Warren MJ. The destabilization of human GCAP1 by a proline to leucine mutation might cause cone-rod dystrophy. Hum Mol Genet. 2001;10:47–54. doi: 10.1093/hmg/10.1.47. [DOI] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- Olshevskaya EV, Boikov S, Ermilov A, Krylov D, Hurley JB, Dizhoor AM. Mapping functional domains of the guanylate cyclase regulator protein, GCAP-2. J Biol Chem. 1999;274:10823–10832. doi: 10.1074/jbc.274.16.10823. [DOI] [PubMed] [Google Scholar]
- Otto-Bruc A, Buczylko J, Surgucheva I, Subbaraya I, Rudnicka-Nawrot M, Crabb J, Arendt A, HArgrave PA, Baehr W, Palczewski K. Functional reconstitution of photoreceptor guanylate cyclase with native and mutant forms of guanylate cyclase activating protein 1. Biochemistry. 1997a;36:4295–4302. doi: 10.1021/bi963000d. [DOI] [PubMed] [Google Scholar]
- Otto-Bruc A, Fariss RN, Haeseleer F, Huang J, Buczylko J, Surgucheva I, Baehr W, Milam AH, Palczewski K. Localization of guanylate cyclase activating protein 2 in mammalian retinas. Proc Natl Acad Sci USA. 1997b;94:4727–4732. doi: 10.1073/pnas.94.9.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Subbaraya I, Gorczyca WA, Helekar BS, Ruiz CC, Ohguro H, Huang J, Zhao X, Crabb JW, Johnson RS, Walsh KA, Gray-Keller MP, Detwiler PB, Baehr W. Molecular cloning and characterization of retinal photoreceptor guanylyl cyclase activating protein (GCAP) Neuron. 1994;13:395–404. doi: 10.1016/0896-6273(94)90355-7. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Polans AS, Baehr W, Ames JB. Calcium binding proteins in the retina: Structure, function, and the etiology of human visual diseases. BioEssays. 2000;22:337–350. doi: 10.1002/(SICI)1521-1878(200004)22:4<337::AID-BIES4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Papermaster DS. Preparation of retinal rod outer segments. Methods Enzymol. 1982;81:48–52. doi: 10.1016/s0076-6879(82)81010-0. [DOI] [PubMed] [Google Scholar]
- Payne AM, Downes SM, Bessant DA, Taylor R, Holder GE, Warren MJ, Bird AC, Bhattacharya SS. A mutation in guanylate cyclase activator 1A (GUCA1A) in an autosomal dominant cone dystrophy pedigree mapping to a new locus on chromosome 6p21.1. Hum Mol Genet. 1998;7:273–277. doi: 10.1093/hmg/7.2.273. [DOI] [PubMed] [Google Scholar]
- Payne AM, Downes SM, Bessant DA, Plant C, Moore T, Bird AC, Bhattacharya SS. Genetic analysis of the guanylate cyclase activator IB (GUCA1B) gene in patients with autosomal dominant retinal dystrophies. J Med Genet. 1999;36:691–693. [PMC free article] [PubMed] [Google Scholar]
- Pennesi ME, Howes KA, Baehr W, Wu SM. GCAP1 rescues normal cone photoreceptor responses in GCAP1/GCAP2 knockout mice. Proc Natl Acad Sci USA. 2003;100:6783–6788. doi: 10.1073/pnas.1130102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polans A, Baehr W, Palczewski K. Turned on by Ca2+ The physiology and pathology of Ca2+ binding proteins in the retina. Trends Neurosci. 1996;19:547–554. doi: 10.1016/s0166-2236(96)10059-x. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Rounsifer ME, Sullivan SA, Knight JK. Expression of rod and cone visual pigments in goldfish and zebrafish: A rhodopsin-like gene is expressed in cones. Neuron. 1993;10:1161–1174. doi: 10.1016/0896-6273(93)90064-x. [DOI] [PubMed] [Google Scholar]
- Raz E. Primordial germ-cell development: The zebrafish perspective. Nat Rev Genet. 2003;4:690–700. doi: 10.1038/nrg1154. [DOI] [PubMed] [Google Scholar]
- Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHE LATOR: An improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- Seimiya M, Kusakabe T, Suzuki N. Primary structure and differential gene expression of three membrane forms of guanylyl cyclase found in the eye of the teleost Oryzias latipes. J Biol Chem. 1997;272:23407–23417. doi: 10.1074/jbc.272.37.23407. [DOI] [PubMed] [Google Scholar]
- Semple-Rowland SL, Larkin P, Bronson JD, Nykamp K, Streit WJ, Baehr W. Characterization of the chicken GCAP gene array and analyses of GCAP1, GCAP2, and GC1 gene expression in normal and rd chicken pineal. Mol Vis. 1999;5:14. [PubMed] [Google Scholar]
- Shyjan AW, deSauvage FJ, Gillett NA, Goeddel DV, Lowe DG. Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron. 1992;9:727–737. doi: 10.1016/0896-6273(92)90035-c. [DOI] [PubMed] [Google Scholar]
- Sokal I, Li N, Surgucheva I, Warren MJ, Payne AM, Bhattacharya SS, Baehr W, Palczewski K. GCAP1(Y99C) mutant is constitutively active in autosomal dominant cone dystrophy. Mol Cell. 1998;2:129–133. doi: 10.1016/s1097-2765(00)80121-5. [DOI] [PubMed] [Google Scholar]
- Sokal I, Otto-Bruc AE, Surgucheva I, Verlinde CL, Wang CK, Baehr W, Palczewski K. Conformational changes in guanylyl cyclase-activating protein 1 (GCAP1) and its tryptophan mutants as a function of calcium concentration. J Biol Chem. 1999;274:19829–19837. doi: 10.1074/jbc.274.28.19829. [DOI] [PubMed] [Google Scholar]
- Sokal I, Li N, Verlinde CL, Haeseleer F, Baehr W, Palczewski K. Ca2+-binding proteins in the retina: from discovery to etiology of human disease(1) Biochim Biophys Acta. 2000;1498:233–251. doi: 10.1016/s0167-4889(00)00099-9. [DOI] [PubMed] [Google Scholar]
- Sokal I, Li N, Klug CS, Filipek S, Hubbell WL, Baehr W, Palczewski K. Calcium-sensitive regions of GCAP1 as observed by chemical modifications, fluorescence and EPR spectroscopies. J Biol Chem. 2001;276:43361–43373. doi: 10.1074/jbc.M103614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaraya I, Ruiz CC, Helekar BS, Zhao X, Gorczyca WA, Pettenati MJ, Rao PN, Palczewski K, Baehr W. Molecular characterization of human and mouse photoreceptor guanylate cyclase activating protein (GCAP) and chromosomal localization of the human gene. J Biol Chem. 1994;269:31080–31089. [PubMed] [Google Scholar]
- Surguchov A, Bronson JD, Banerjee P, Knowles JA, Ruiz CC, Subbaraya I, Palczewski K, Baehr W. The human GCAP1 and GCAP2 genes are arranged in a tail-to-tail array on the short arm of chromosome 6 (p21.1) Genomics. 1997;39:312–322. doi: 10.1006/geno.1996.4513. [DOI] [PubMed] [Google Scholar]
- Tohya S, Mochizuki A, Iwasa Y. Difference in the retinal cone mosaic pattern between zebrafish and medaka: cell-rearrangement model. J Theor Biol. 2003;221:289–300. doi: 10.1006/jtbi.2003.3192. [DOI] [PubMed] [Google Scholar]
- Venkatesh B, Gilligan P, Brenner S. Fugu: A compact vertebrate reference genome. FEBS Lett. 2000;476:3–7. doi: 10.1016/s0014-5793(00)01659-8. [DOI] [PubMed] [Google Scholar]
- Vihtelic TS, Doro CJ, Hyde DR. Cloning and characterization of six zebrafish photoreceptor opsin cDNAs and immunolocalization of their corresponding proteins. Vis Neurosci. 1999;16:571–585. doi: 10.1017/s0952523899163168. [DOI] [PubMed] [Google Scholar]
- Wilkie SE, Stinton I, Cottrill P, Deery E, Newbold R, Warren MJ, Bhattacharya SS, Hunt DM. Characterisation of two genes for guanylate cyclase activator protein (GCAP1 and GCAP2) in the Japanese pufferfish, Fugu rubripes. Biochim Biophys Acta. 2002;1577:73–80. doi: 10.1016/s0167-4781(02)00413-x. [DOI] [PubMed] [Google Scholar]
- Wilkie SE, Li Y, Deery EC, Newbold RJ, Garibaldi D, Bateman JB, Zhang H, Lin W, Zack DJ, Bhattacharya SS, Warren MJ, Hunt DM, Zhang K. Identification and functional consequences of a new mutation (E155G) in the gene for GCAP1 that causes autosomal dominant cone dystrophy. Am J Hum Genet. 2001;69:471–480. doi: 10.1086/323265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittbrodt J, Shima A, Schartl M. Medaka—A model organism from the far East. Nat Rev Genet. 2002;3:53–64. doi: 10.1038/nrg704. [DOI] [PubMed] [Google Scholar]
- Yang RB, Garbers DL. Two eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J Biol Chem. 1997;272:13738–13742. doi: 10.1074/jbc.272.21.13738. [DOI] [PubMed] [Google Scholar]


