Abstract
1. Intracellular records were obtained from ganglion cells of the pelvic plexus of male guinea-pigs.
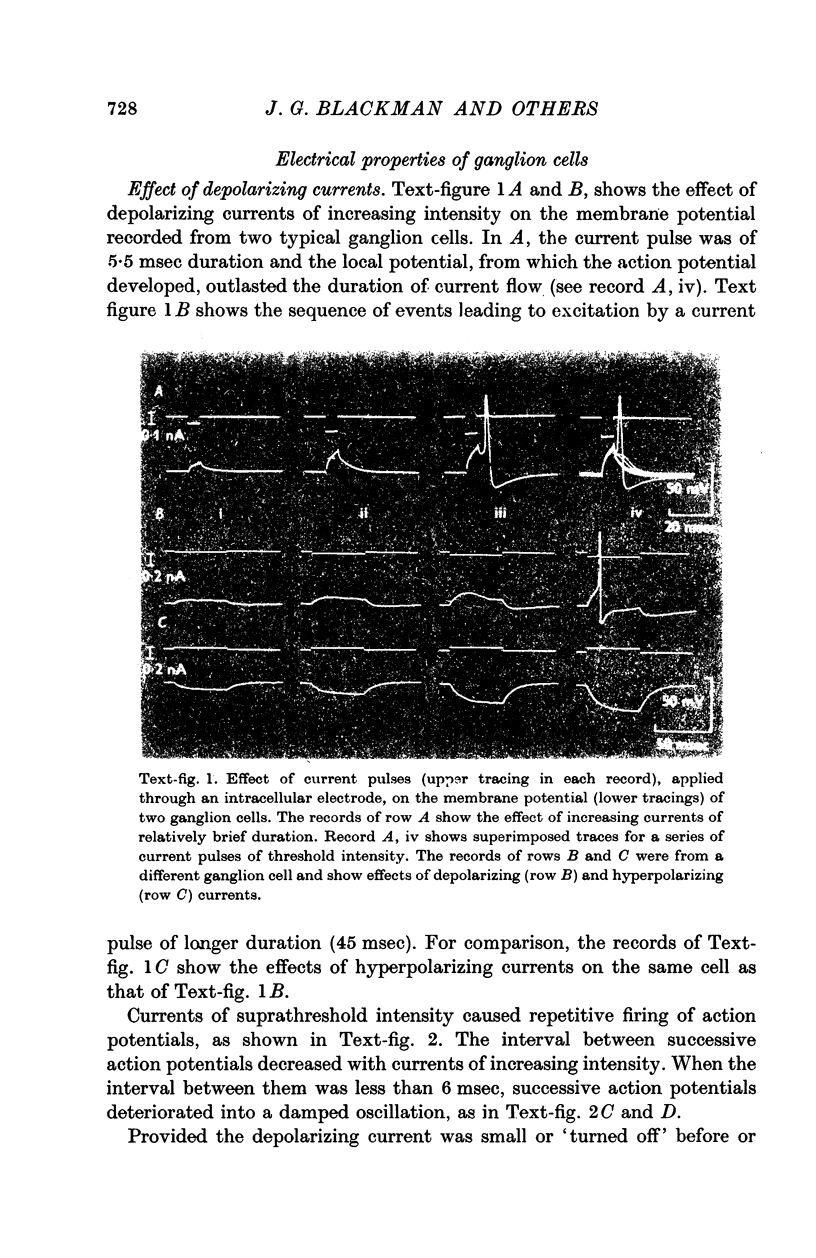
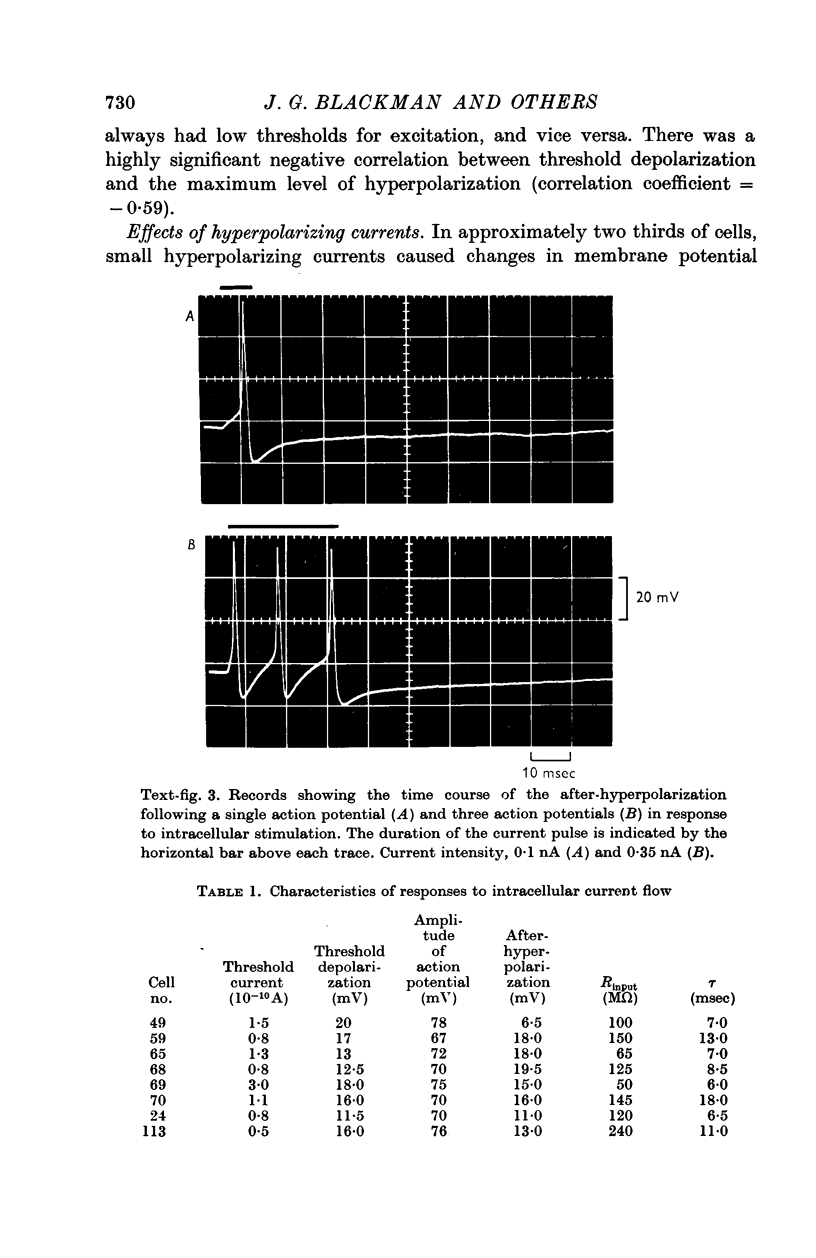
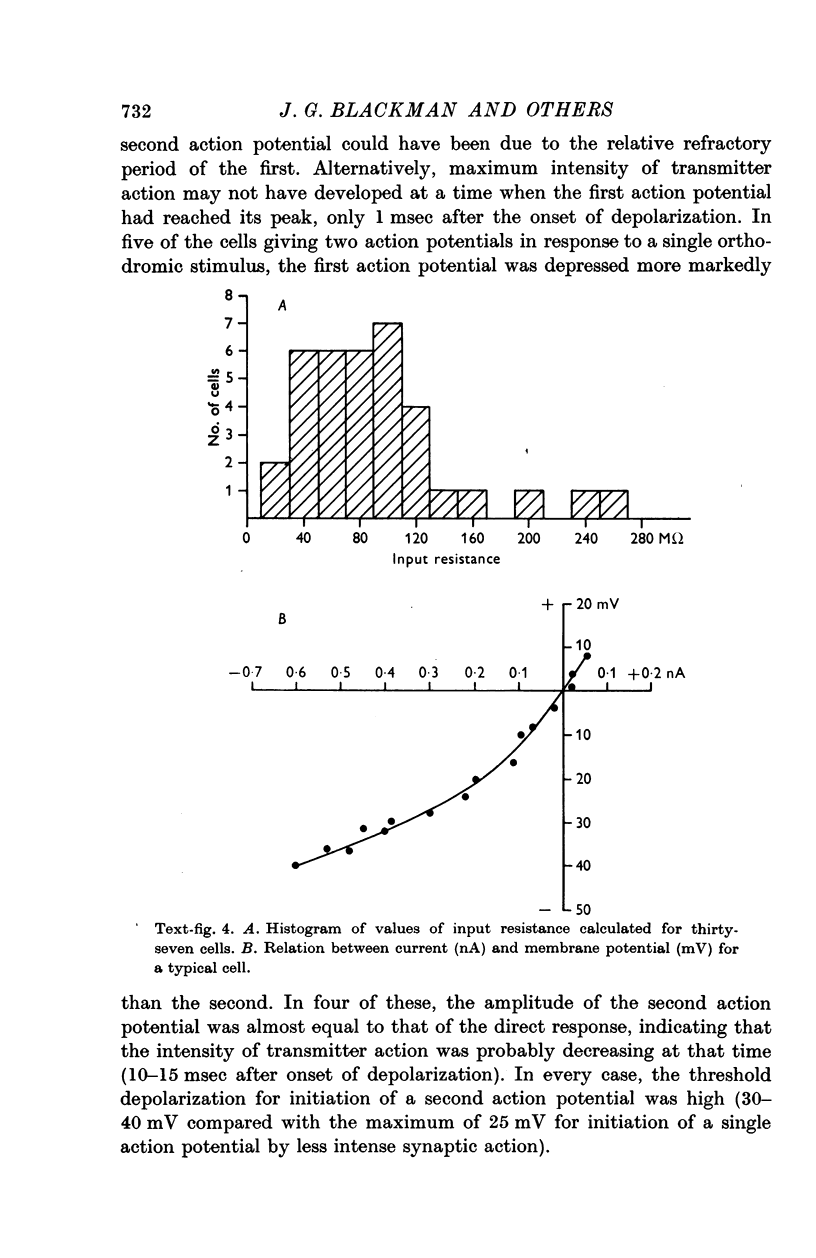
2. The input resistance of cells which responded to intracellular stimulation varied from 40 to 150 MΩ. Slope resistance decreased when the membrane was hyperpolarized. Time constants varied from 5 to 200 msec. Resting membrane potentials ranged from 40 to 70 mV.
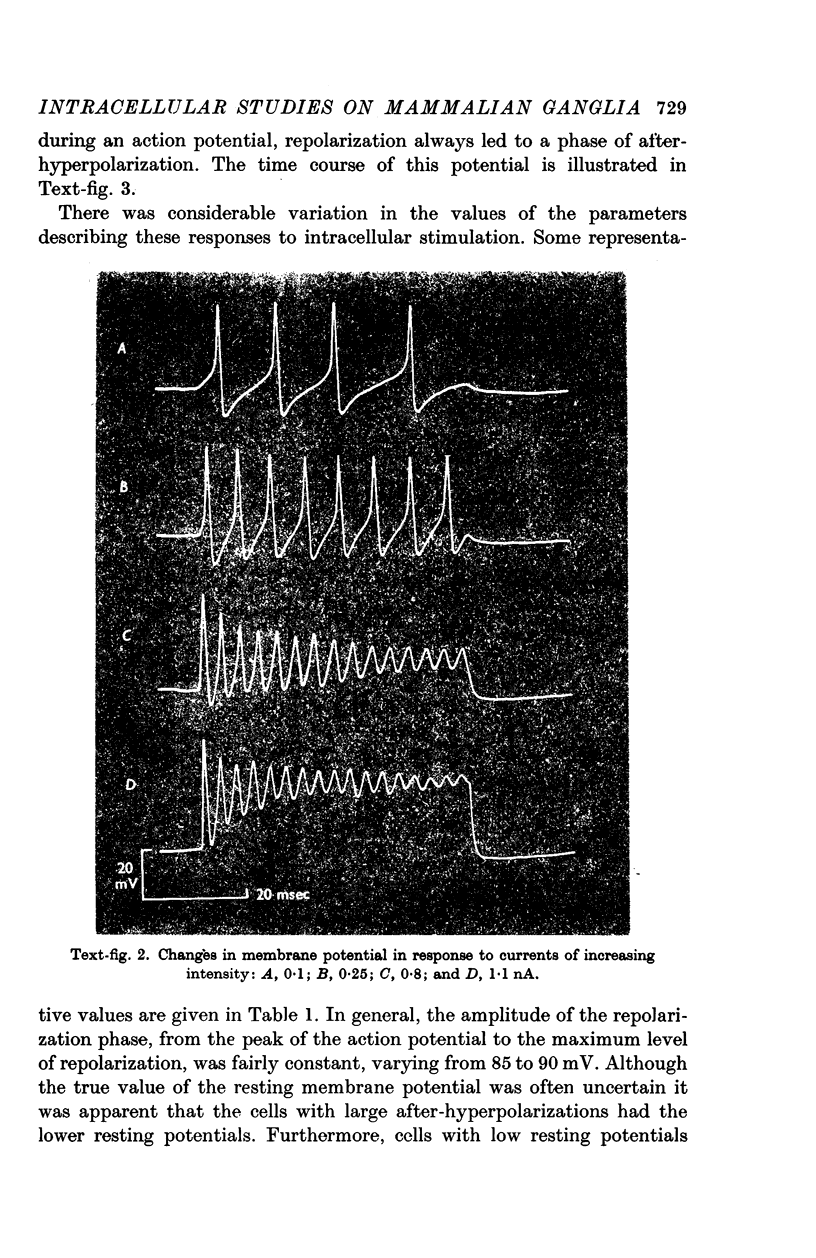
3. Action potentials in response to direct stimulation were followed by a prolonged phase of after-hyperpolarization.
4. A second type of cell was also impaled which did not respond to electrical stimulation. These cells had resting membrane potentials in the range 60-70 mV, input resistances of less than 20 MΩ and time constants of less than 3 msec.
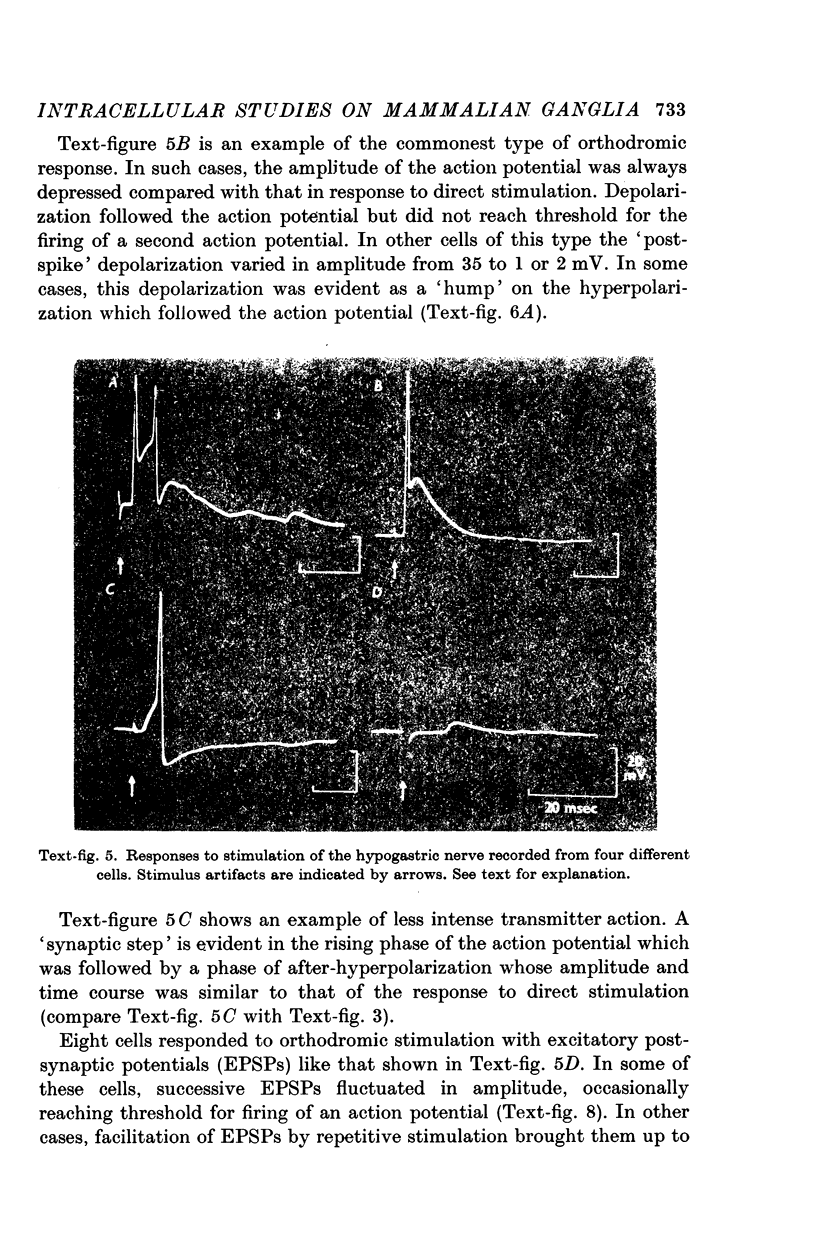
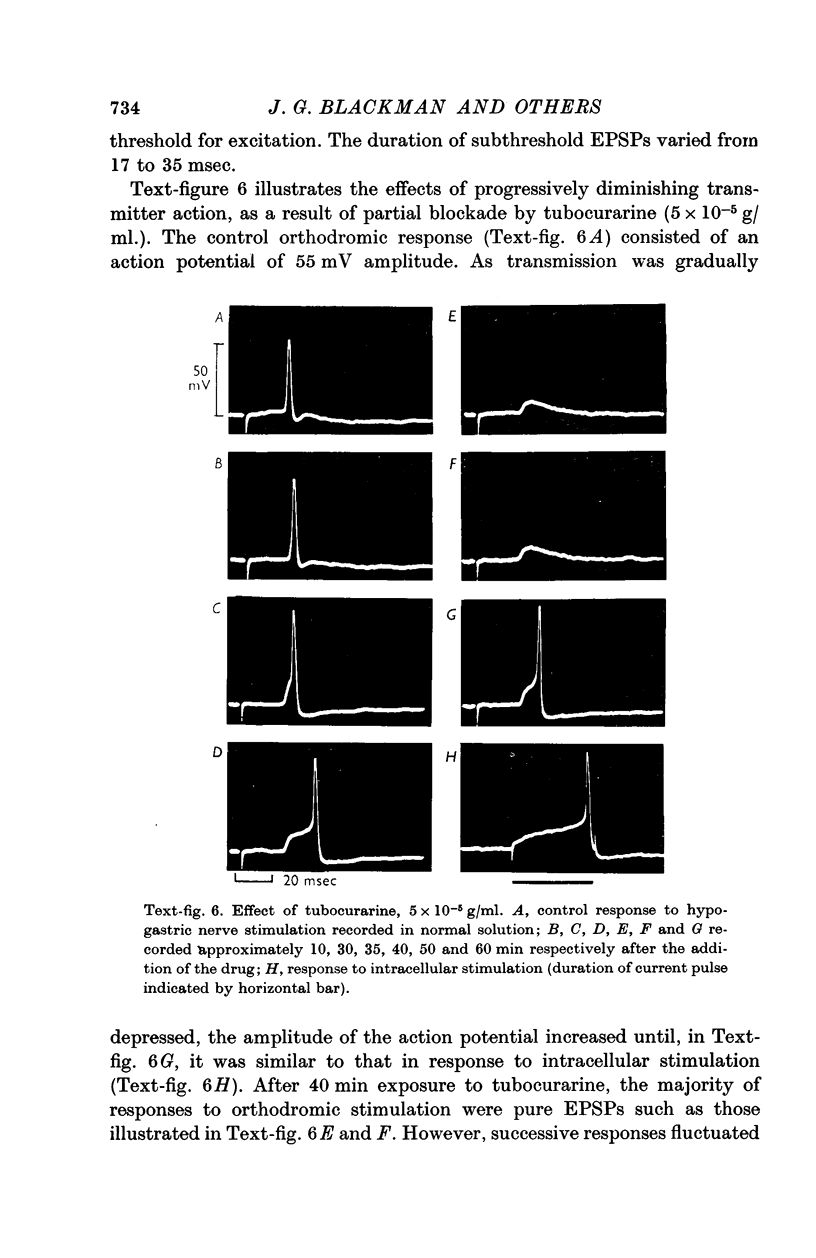
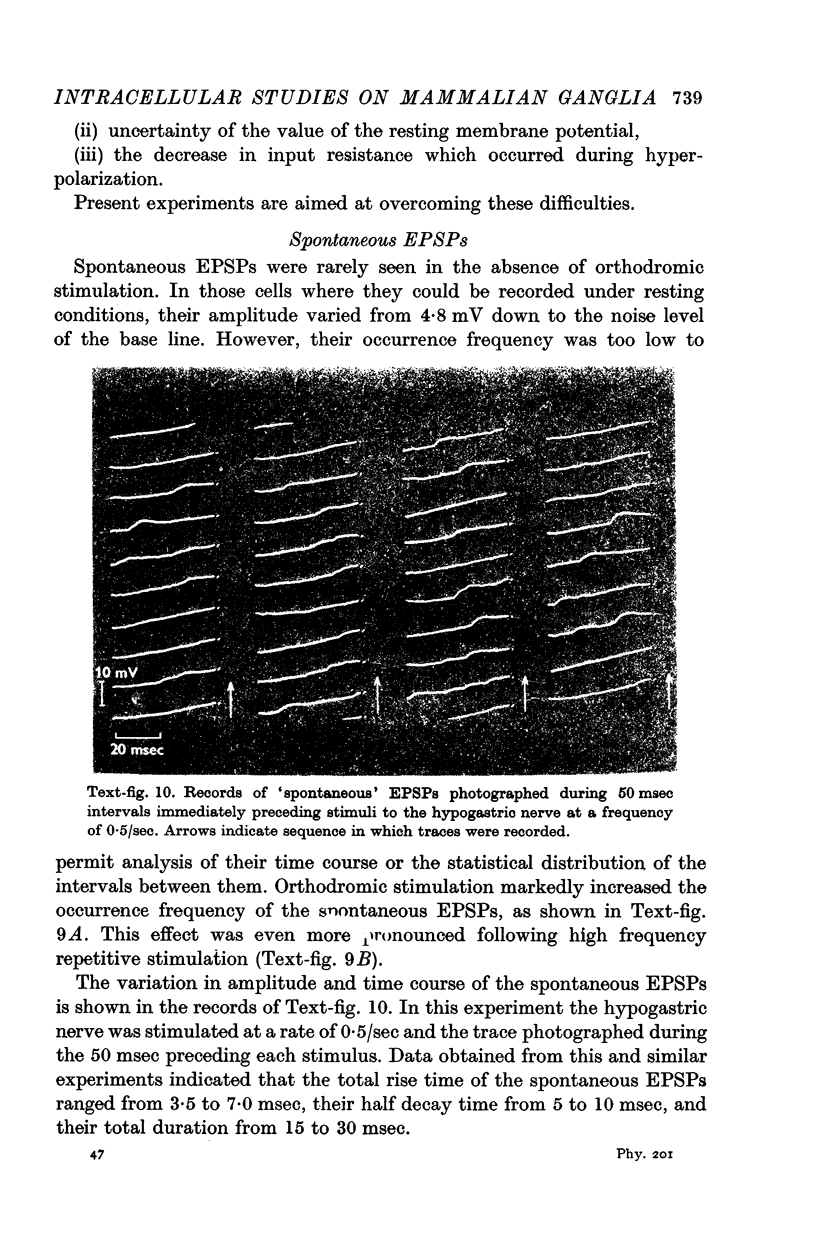
5. In most ganglion cells, stimulation of the hypogastric nerve evoked action potentials which were often followed by a secondary phase of depolarization indicating continuing transmitter action.
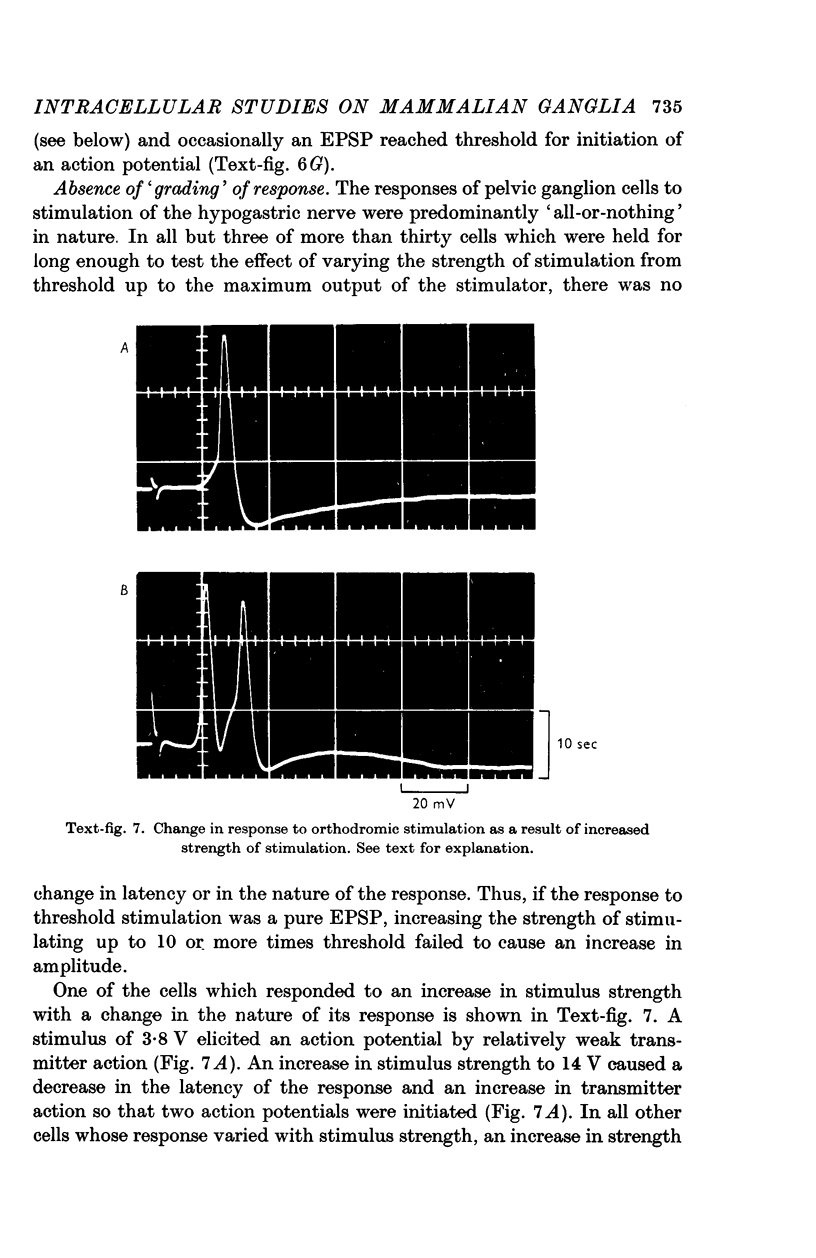
6. Orthodromic responses were generally `all-or-nothing' and could not be graded with changes in stimulus strength. The latency of orthodromic responses indicated that ganglion cells were innervated by both B and C fibres in the hypogastric nerve.
7. Orthodromic responses were blocked by tubocurarine, 5 × 10-5 g/ml., and dihydro-β-erythroidine, 10-5 g/ml.
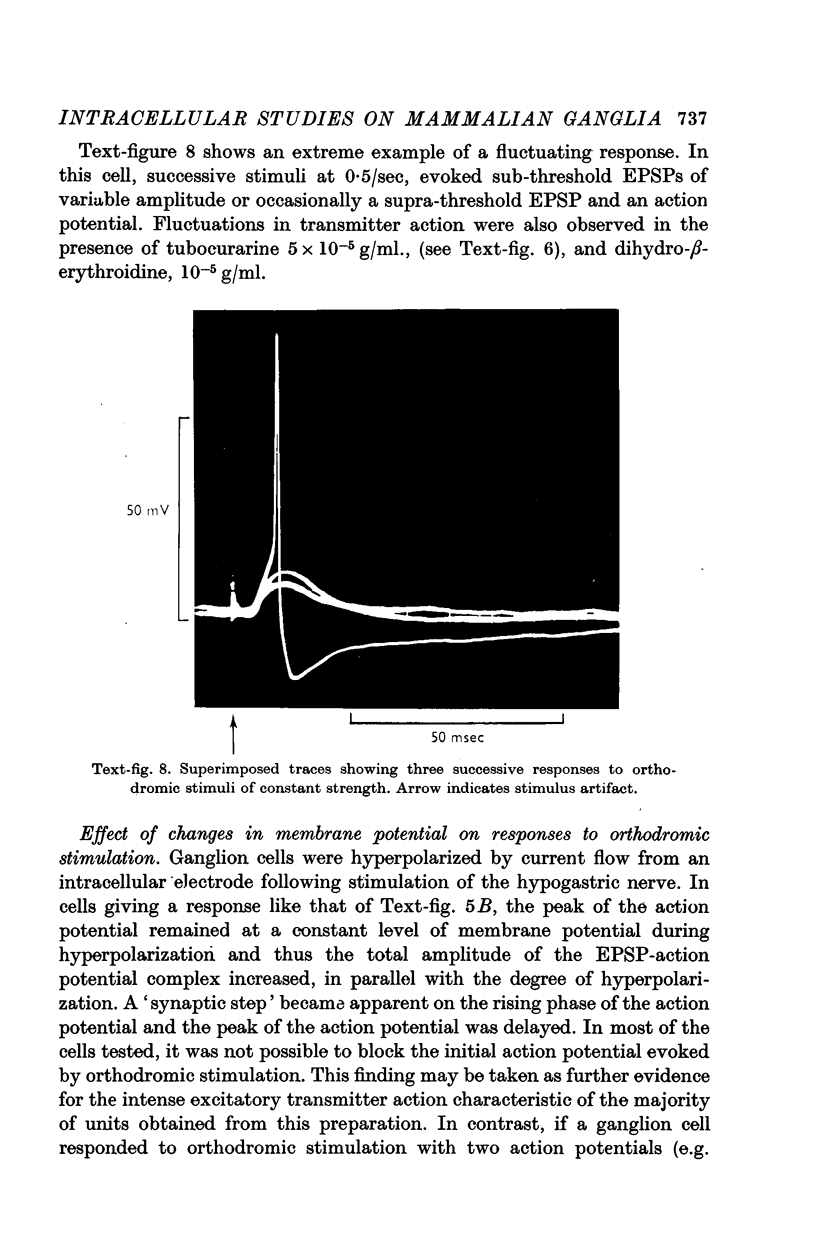
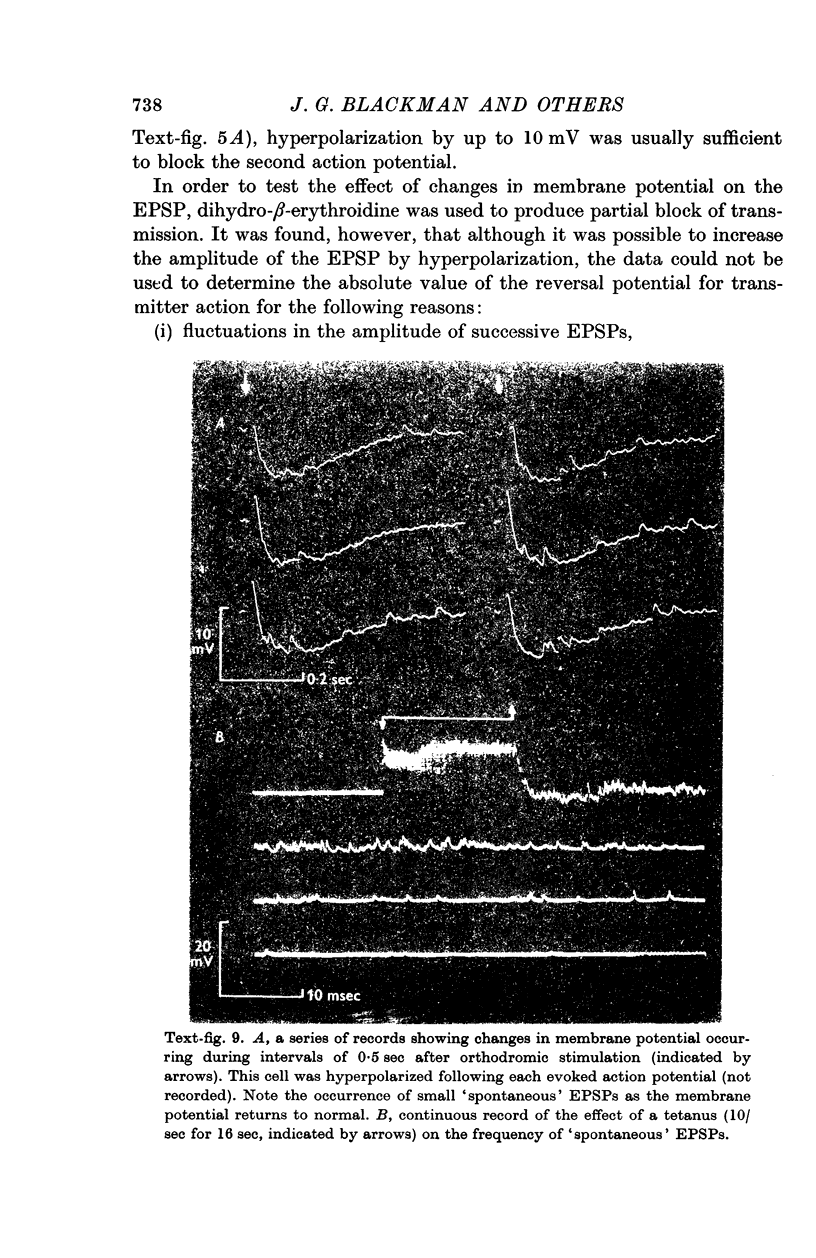
8. Spontaneous, excitatory post-synaptic potentials of up to 4·8 mV in amplitude were observed. The frequency of their discharge was greatly increased by repetitive stimulation of the hypogastric nerve.
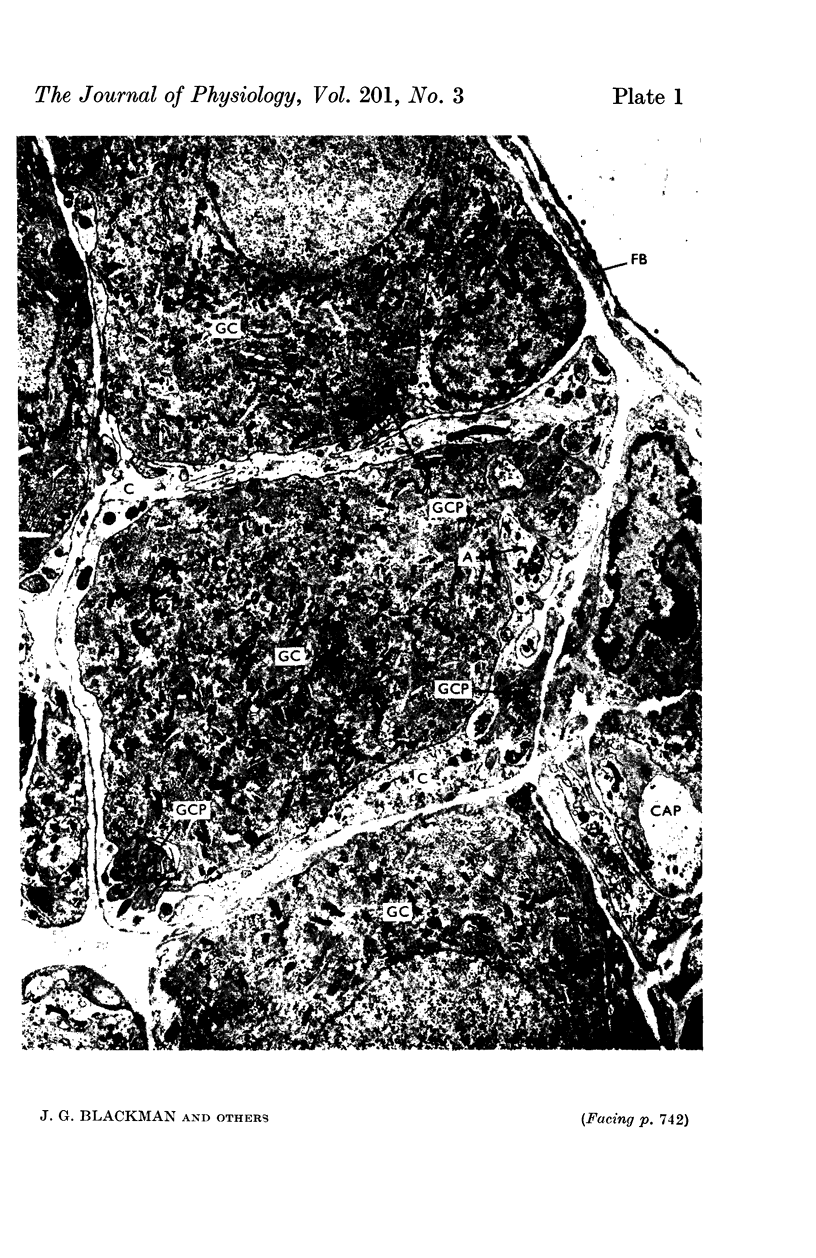
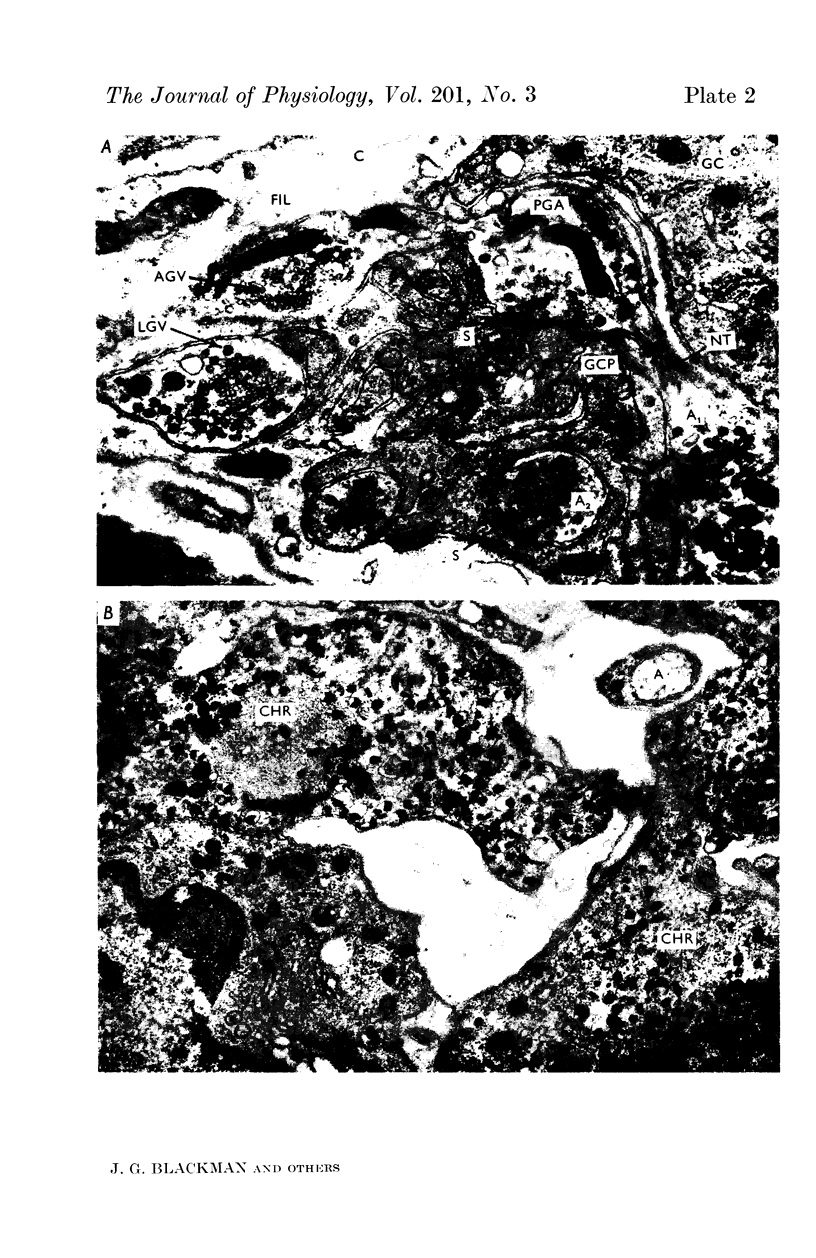
9. The ultrastructure of the pelvic ganglia was studied by electronmicroscopy. Two types of ganglion cell process were observed, fine (0·1 μ) branching tufts thrown up from the soma within the surrounding capsule and longer, thicker (1 μ) extracapsular processes. Synapses were found to occur most frequently between the varicose terminal segments of preganglionic axons and the small intracapsular processes.
10. Similarities between the properties of the pelvic ganglia innervated by the hypogastric nerve and those of the parasympathetic division of the autonomic nervous system are discussed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKMAN J. G., GINSBORG B. L., RAY C. Synaptic transmission in the sympathetic ganglion of the frog. J Physiol. 1963 Jul;167:355–373. doi: 10.1113/jphysiol.1963.sp007155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. The transmission of excitation from autonomic nerve to smooth muscle. J Physiol. 1961 Jan;155:115–133. doi: 10.1113/jphysiol.1961.sp006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES R. M. Intracellular potentials recorded from a mammalian sympathetic ganglion. J Physiol. 1955 Dec 29;130(3):572–584. doi: 10.1113/jphysiol.1955.sp005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMELIN N., MACINTOSH F. C. The release of acetylcholine from perfused sympathetic ganglia and skeletal muscles. J Physiol. 1956 Feb 28;131(2):477–496. doi: 10.1113/jphysiol.1956.sp005477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C. The nature of synaptic transmission in a sympathetic ganglion. J Physiol. 1944 Jun 15;103(1):27–54. doi: 10.1113/jphysiol.1944.sp004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles R. M. Orthodromic activation of single ganglion cells. J Physiol. 1963 Mar;165(3):387–391. doi: 10.1113/jphysiol.1963.sp007064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry C. B. The innervation of the vas deferens of the guinea-pig. J Physiol. 1967 Sep;192(2):463–478. doi: 10.1113/jphysiol.1967.sp008309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBERGER B., LEVI-MONTALCINI R., NORBERG K. A., SJOEQVIST F. MONOAMINES IN IMMUNOSYMPATHECTOMIZED RATS. Int J Neuropharmacol. 1965 Apr;4:91–95. doi: 10.1016/0028-3908(65)90032-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Holman M. E., Tille J. Electrical properties of the smooth muscle membrane of the guinea-pig vas deferens. J Physiol. 1966 Sep;186(1):27–41. doi: 10.1113/jphysiol.1966.sp008018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Oshima T. Electrical behaviour of the motoneurone membrane during intracellularly applied current steps. J Physiol. 1965 Oct;180(3):607–635. doi: 10.1113/jphysiol.1965.sp007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R., PILAR G. DUAL MODE OF SYNAPTIC TRANSMISSION IN THE AVIAN CILIARY GANGLION. J Physiol. 1963 Sep;168:443–463. doi: 10.1113/jphysiol.1963.sp007202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHI S., KOKETSU K. Electrical properties and activities of single sympathetic neurons in frogs. J Cell Comp Physiol. 1960 Feb;55:15–30. doi: 10.1002/jcp.1030550104. [DOI] [PubMed] [Google Scholar]
- Nishi S., Soeda H., Koketsu K. Studies on sympathetic B and C neurons and patterns of pregnaglionic innervation. J Cell Physiol. 1965 Aug;66(1):19–32. doi: 10.1002/jcp.1030660103. [DOI] [PubMed] [Google Scholar]
- OGSTON A. G. Removal of acetylcholine from a limited volume by diffusion. J Physiol. 1955 Apr 28;128(1):222–223. doi: 10.1113/jphysiol.1955.sp005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöstrand N. O., Swedin G. Effect of reserpine on the noradrenaline content of the vas deferens and the seminal vesicle compared with the submaxillary gland and the heart of the rat. Acta Physiol Scand. 1968 Mar;72(3):370–377. doi: 10.1111/j.1748-1716.1968.tb03859.x. [DOI] [PubMed] [Google Scholar]
- Tauc L. Transmission in invertebrate and vertebrate ganglia. Physiol Rev. 1967 Jul;47(3):521–593. doi: 10.1152/physrev.1967.47.3.521. [DOI] [PubMed] [Google Scholar]