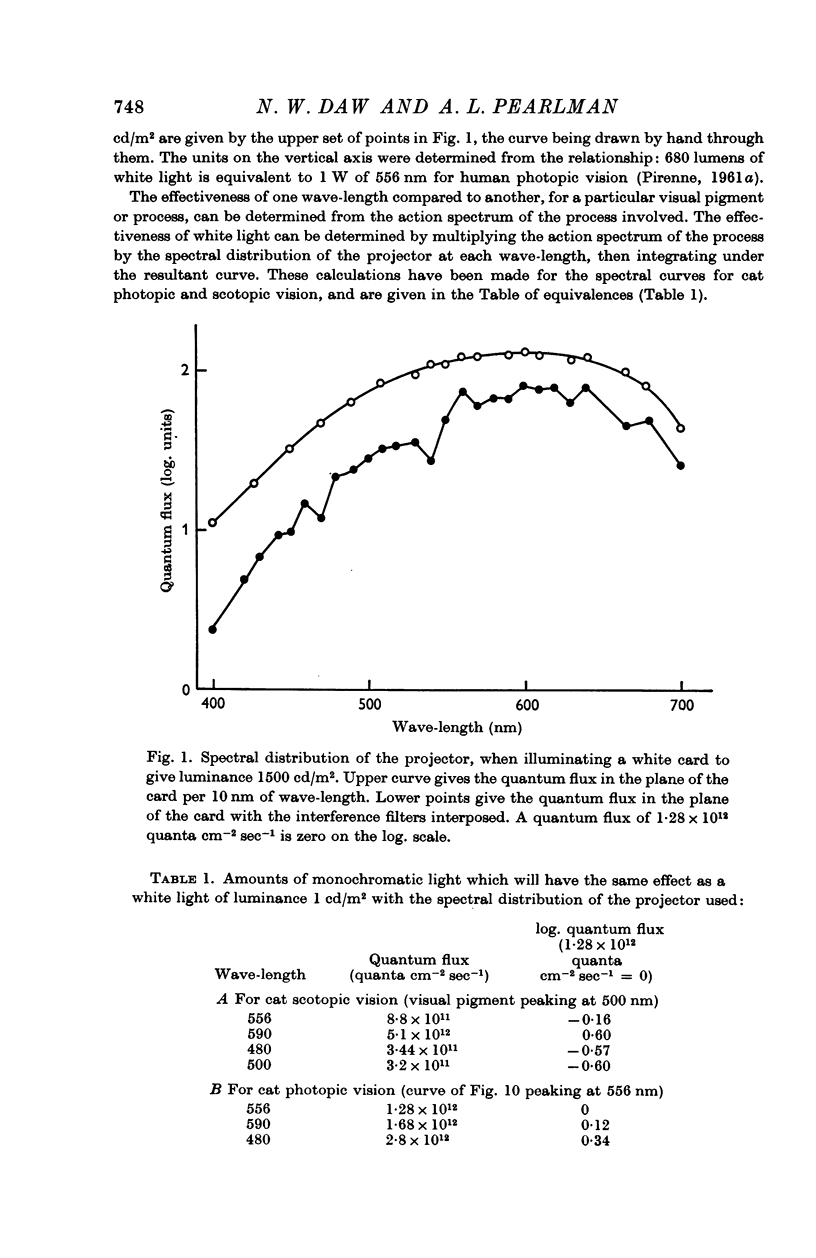
Abstract
1. Peripheral mechanisms that might contribute to colour vision in the cat have been investigated by recording from single units in the lateral geniculate and optic tract. Evidence is presented that the input to these cells comes from a single class of cones with a single spectral sensitivity.
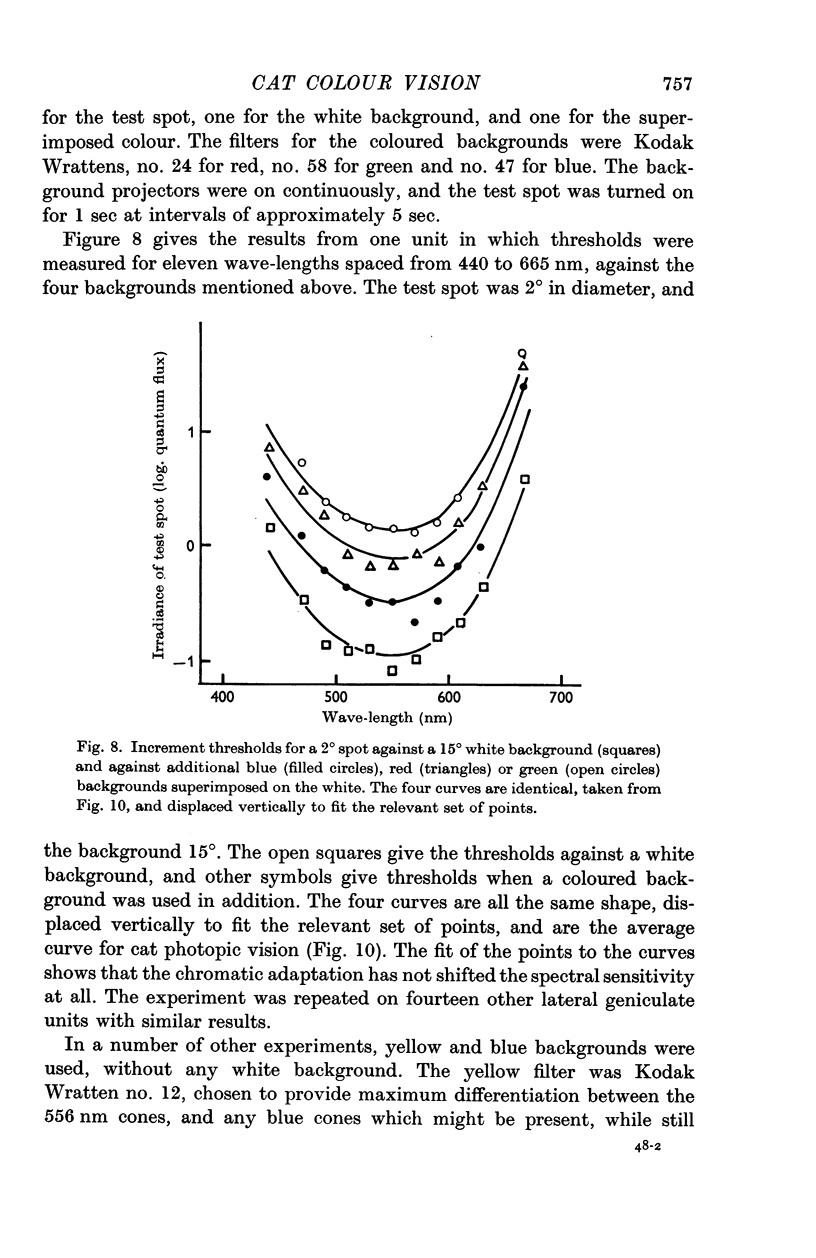
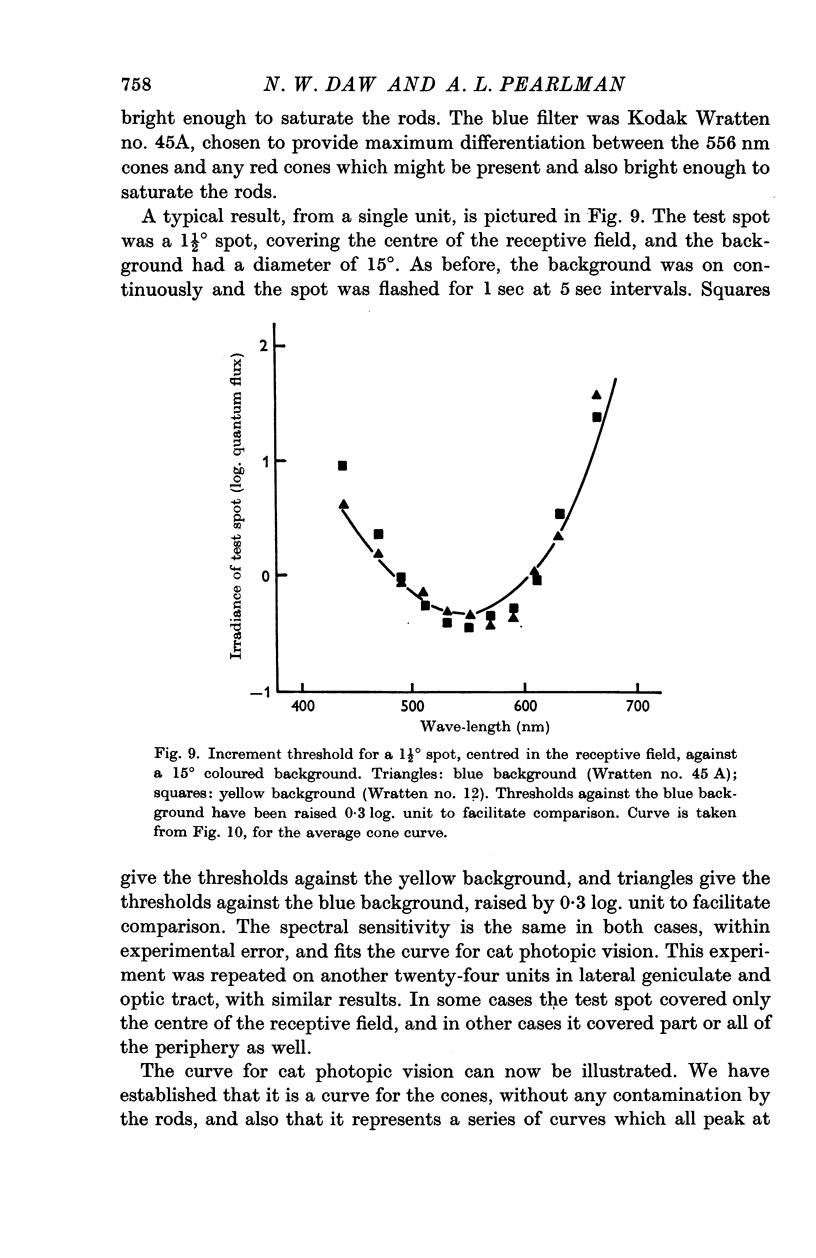
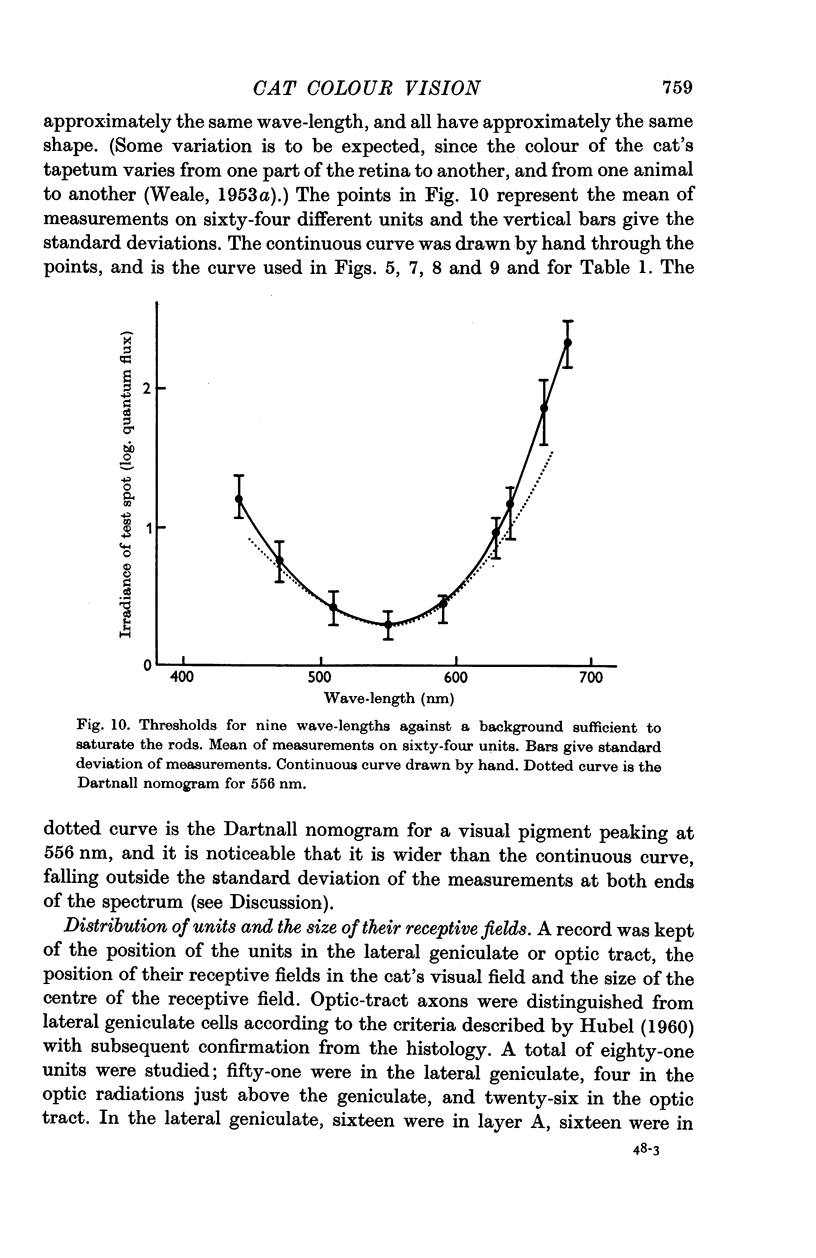
2. In cats with pupils dilated a background level of 10-30 cd/m2 was sufficient to saturate the rod system for all units. When the rods were saturated, the spectral sensitivity of all units peaked at 556 nm; this was true both for centre and periphery of the receptive field. The spectral sensitivity curve was slightly narrower than the Dartnall nomogram. It could not be shifted by chromatic adaptation with red, green, blue or yellow backgrounds.
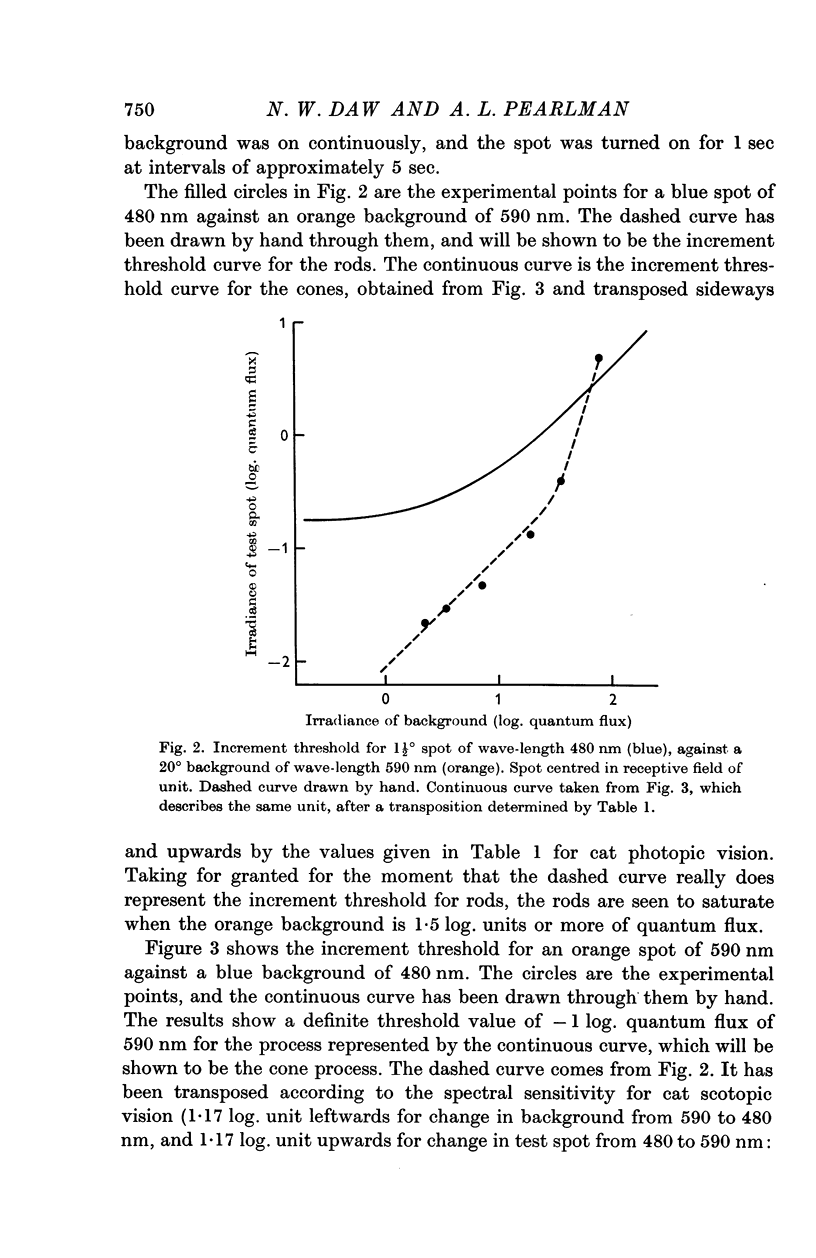
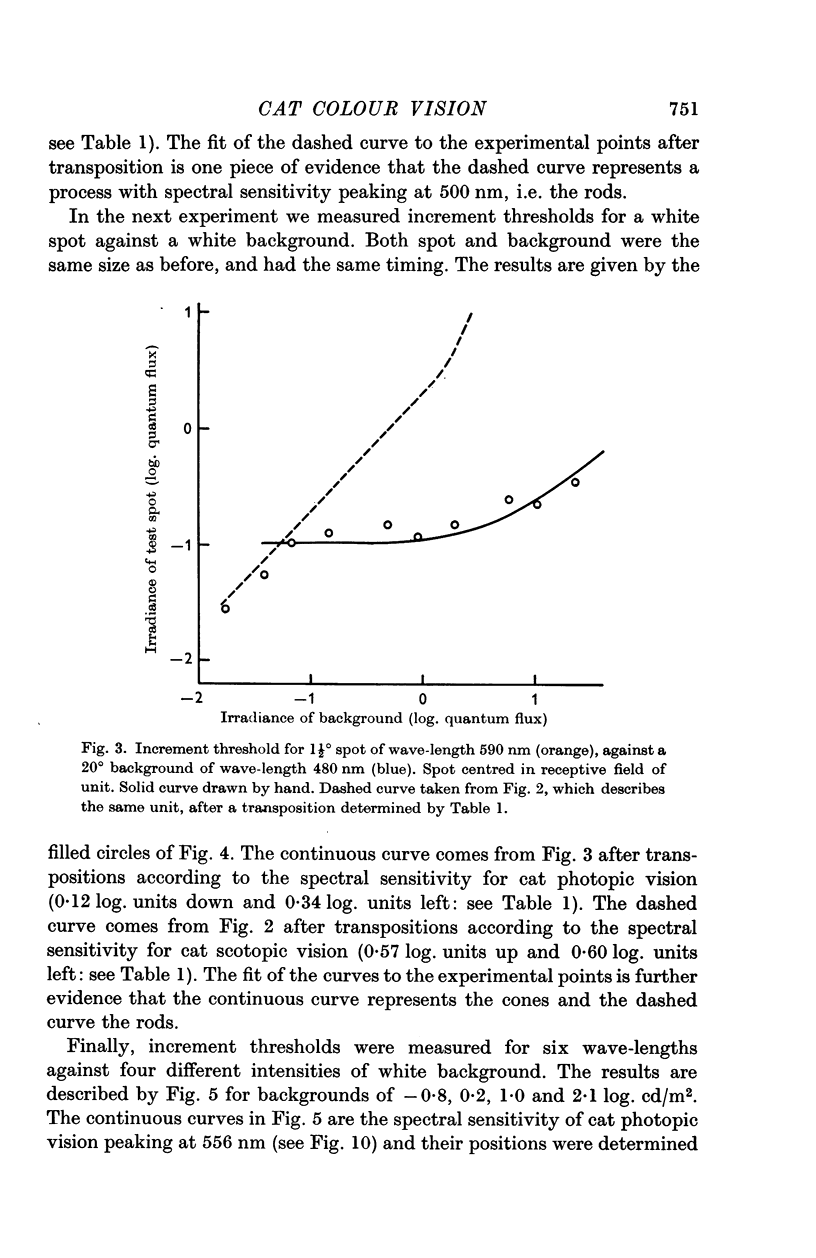
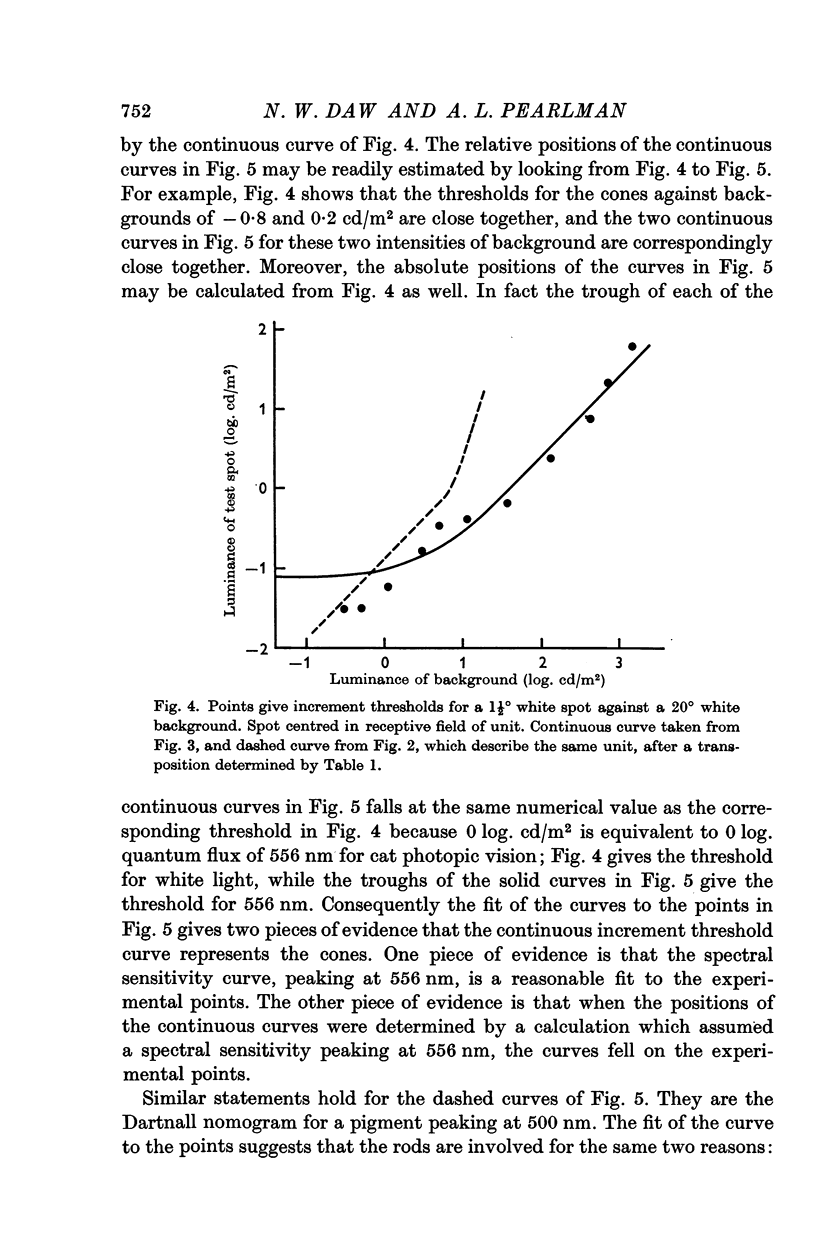
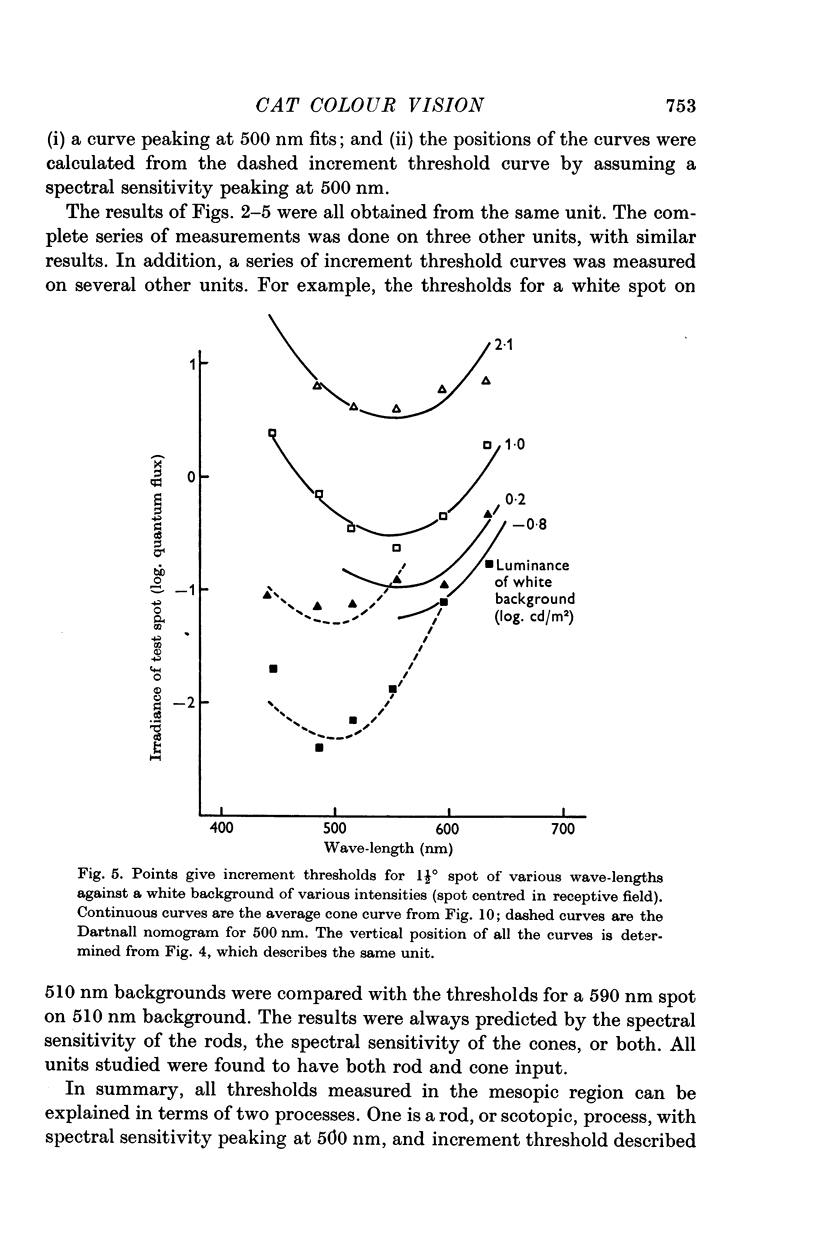
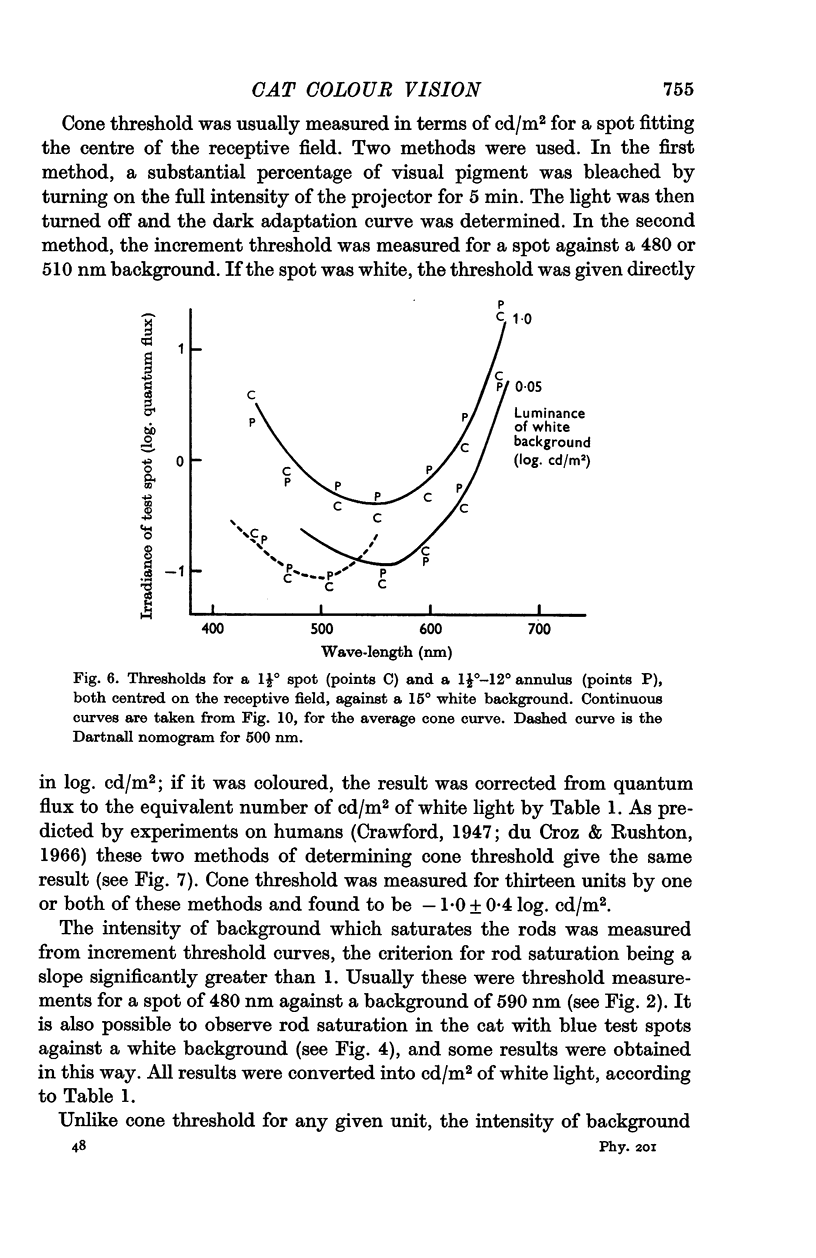
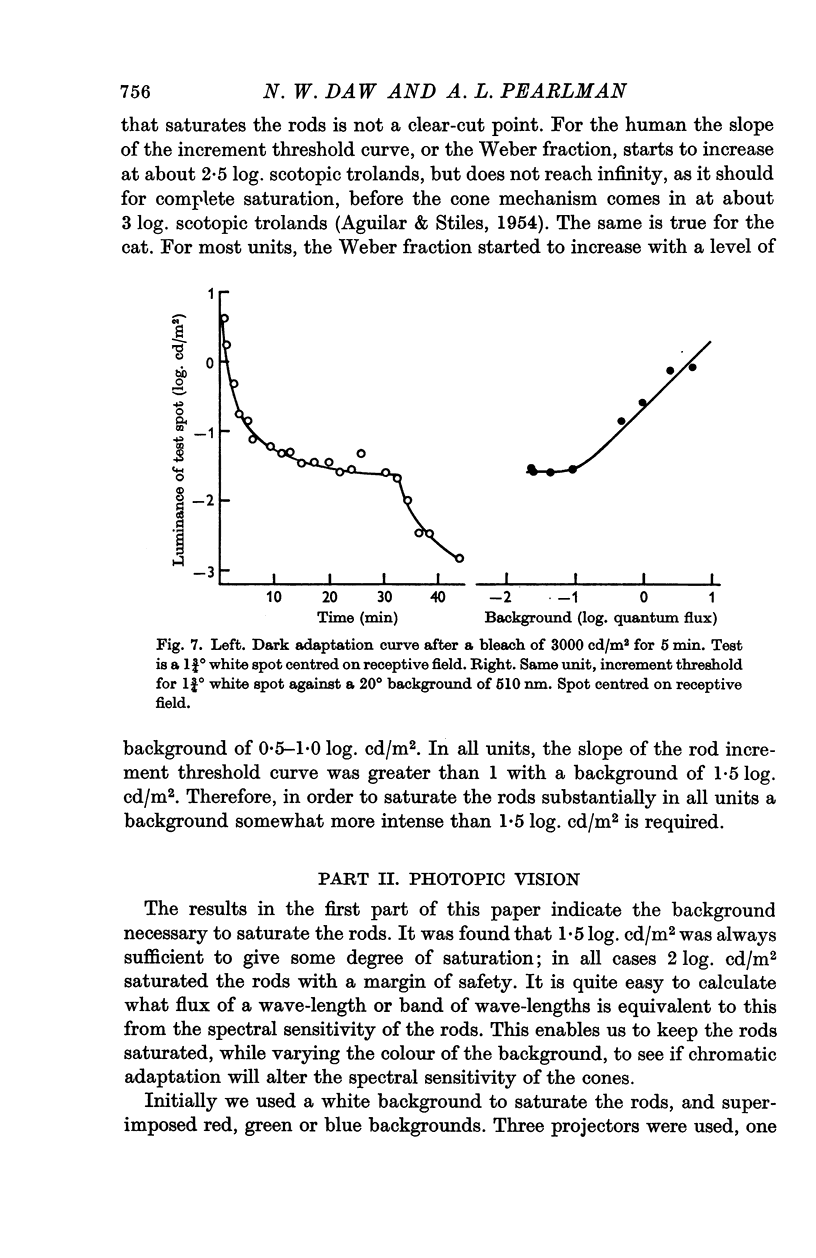
3. In the mesopic range (0·1-10 cd/m2), the threshold could be predicted in terms of two mechanisms, a cone mechanism with spectral sensitivity peaking at 556 nm, and a rod mechanism with spectral sensitivity at 500 nm. The mechanisms were separated and their increment threshold curves measured by testing with one colour against a background of another colour. All units had input from both rods and cones. The changeover from rods to cones occurred at the same level of adaptation in both centre and periphery of the receptive field. Threshold for the cones was between 0·04 and 0·25 cd/m2 with the pupil dilated, for a spot covering the centre of the receptive field.
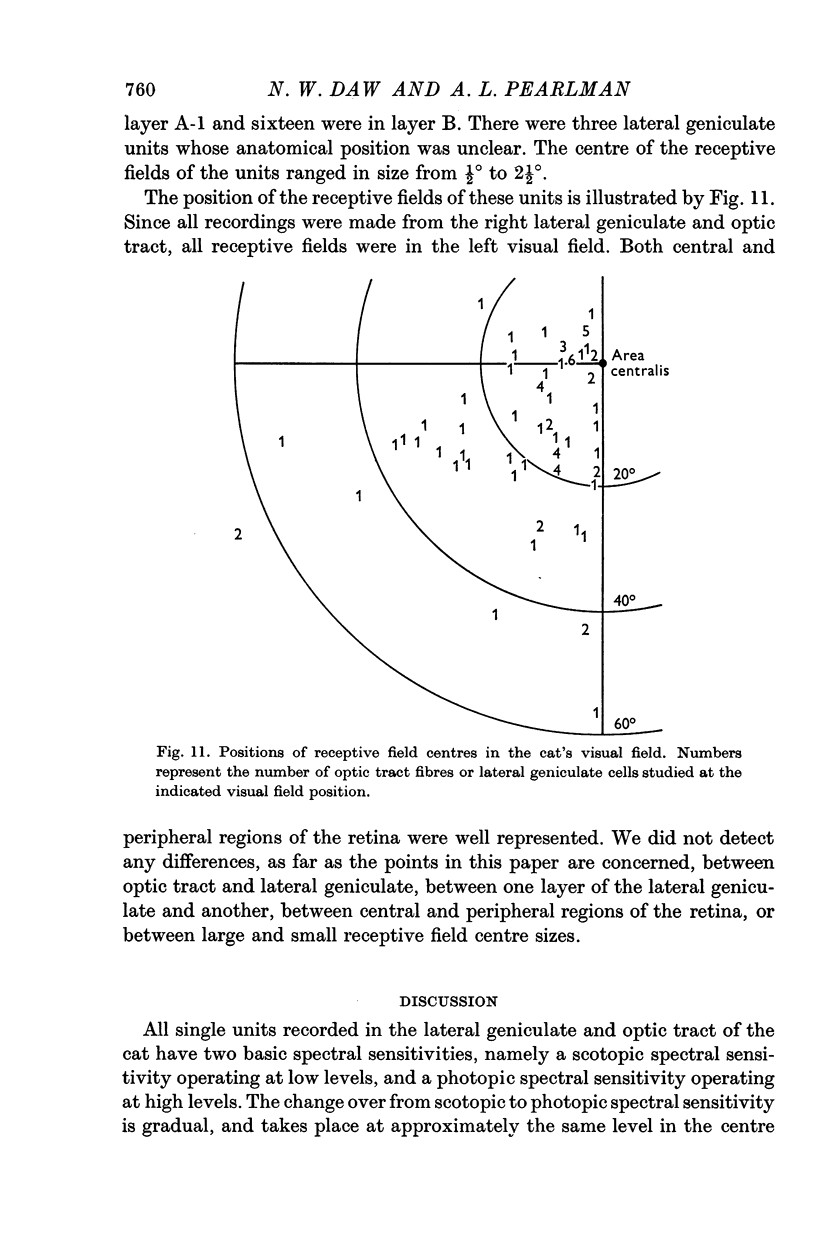
4. None of the results was found to vary between lateral geniculate and optic tract, with layer in the lateral geniculate, or with distance from area centralis in the visual field.
5. The lack of evidence for more than one cone type suggests that colour discrimination in the cat may be a phenomenon of mesopic vision, based on differences in spectral sensitivity of the rods and a single class of cones.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARDEN G. B., TANSLEY K. The spectral sensitivity of the pure-cone retina of the grey squirrel (Sciurus carolinensis leucotis). J Physiol. 1955 Mar 28;127(3):592–602. doi: 10.1113/jphysiol.1955.sp005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol. 1957 Aug 6;137(3):338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B., FITZHUGH R., KUFFLER S. W. Dark adaptation, absolute threshold and Purkinje shift in single units of the cat's retina. J Physiol. 1957 Aug 6;137(3):327–337. doi: 10.1113/jphysiol.1957.sp005816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIDGMAN C. S. The luminosity curve as affected by the relation between rod and cone adaptation. J Opt Soc Am. 1953 Sep;43(9):733–737. doi: 10.1364/josa.43.000733. [DOI] [PubMed] [Google Scholar]
- DODT E., ELENIUS V. Change of threshold during dark adaptation measured with orange and blue light in cats and rabbits. Experientia. 1960 Jul 15;16:313–314. doi: 10.1007/BF02157769. [DOI] [PubMed] [Google Scholar]
- DODT E., ENROTH C. Retinal flicker response in cat. Acta Physiol Scand. 1954 May 15;30(4):375–390. doi: 10.1111/j.1748-1716.1954.tb01076.x. [DOI] [PubMed] [Google Scholar]
- DODT E., WALTHER J. B. Der photopische Dominator im Flimmer-ERG der Katze. Pflugers Arch. 1958;266(2):175–186. doi: 10.1007/BF00363646. [DOI] [PubMed] [Google Scholar]
- DODT E., WALTHER J. B. Netzhautsensitivität, Linsenabsorption und physikalische Lichtstreuung; der skotopische Dominator der Katze im sichtbaren und ultravioletten Spektralbereich. Pflugers Arch. 1958;266(2):167–174. doi: 10.1007/BF00363645. [DOI] [PubMed] [Google Scholar]
- Du Croz J. J., Rushton W. A. The separation of cone mechanisms in dark adaptation. J Physiol. 1966 Mar;183(2):481–496. doi: 10.1113/jphysiol.1966.sp007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTER R. The discrimination between lights of different wave lengths in the cat. J Comp Physiol Psychol. 1954 Apr;47(2):169–172. doi: 10.1037/h0061325. [DOI] [PubMed] [Google Scholar]
- GUNTER R. The spectral sensitivity of dark-adapted cats. J Physiol. 1952 Nov;118(3):395–404. doi: 10.1113/jphysiol.1952.sp004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTER R. The spectral sensitivity of light-adapted cats. J Physiol. 1954 Feb 26;123(2):409–415. doi: 10.1113/jphysiol.1954.sp005061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- LENNOX M. A. Geniculate and cortical responses to colored light flash in cat. J Neurophysiol. 1956 May;19(3):271–279. doi: 10.1152/jn.1956.19.3.271. [DOI] [PubMed] [Google Scholar]
- MELLO N. K., PETERSON N. J. BEHAVIORAL EVIDENCE FOR COLOR DISCRIMINATION IN CAT. J Neurophysiol. 1964 May;27:323–333. doi: 10.1152/jn.1964.27.3.323. [DOI] [PubMed] [Google Scholar]
- MEYER D. R., MILES R. C., RATOOSH P. Absence of color vision in cat. J Neurophysiol. 1954 May;17(3):289–294. doi: 10.1152/jn.1954.17.3.289. [DOI] [PubMed] [Google Scholar]
- MUNTZ W. R. Microelectrode recordings from the diencephalon of the frog (Rana pipiens) and a blue-sensitive system. J Neurophysiol. 1962 Nov;25:699–711. doi: 10.1152/jn.1962.25.6.699. [DOI] [PubMed] [Google Scholar]
- Michael C. R. Receptive fields of single optic nerve fibers in a mammal with an all-cone retina. 3. Opponent color units. J Neurophysiol. 1968 Mar;31(2):268–282. doi: 10.1152/jn.1968.31.2.268. [DOI] [PubMed] [Google Scholar]
- OKUDA J., TAIRA N., MOTOKAWA K. Spectral response curves of postgeniculate neurons in the cat. Tohoku J Exp Med. 1962 Nov 25;78:147–157. doi: 10.1620/tjem.78.147. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Pettigrew J. D., Bishop P. O., Nikara T. Residual eye movements in receptive-field studies of paralyzed cats. Vision Res. 1967 Jan;7(1):107–110. doi: 10.1016/0042-6989(67)90031-4. [DOI] [PubMed] [Google Scholar]
- SECHZER J. A., BROWN J. L. COLOR DISCRIMINATION IN THE CAT. Science. 1964 Apr 24;144(3617):427–429. doi: 10.1126/science.144.3617.427. [DOI] [PubMed] [Google Scholar]
- SUZUKI H., TAIRA N., MOTOKAWA K. Spectral response curves and receptive fields of pre- and postgeniculate fibres of the cat. Tohoku J Exp Med. 1960 Mar 25;71:401–415. doi: 10.1620/tjem.71.401. [DOI] [PubMed] [Google Scholar]
- WALL P. D. Repetitive discharge of neurons. J Neurophysiol. 1959 May;22(3):305–320. doi: 10.1152/jn.1959.22.3.305. [DOI] [PubMed] [Google Scholar]
- WEALE R. A. Bleaching experiments on eyes of living grey squirrels (Sciurus carolinensis leucotis). J Physiol. 1955 Mar 28;127(3):587–591. doi: 10.1113/jphysiol.1955.sp005279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEALE R. A. Observations on photo-chemical reactions in living eyes. Br J Ophthalmol. 1957 Aug;41(8):461–474. doi: 10.1136/bjo.41.8.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEALE R. A. Photochemical reactions in the living cat's retina. J Physiol. 1953 Nov 28;122(2):322–331. doi: 10.1113/jphysiol.1953.sp005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEALE R. A. The spectral reflectivity of the cat's tapetum measured in situ. J Physiol. 1953 Jan;119(1):30–42. doi: 10.1113/jphysiol.1953.sp004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Spatial and chromatic interactions in the lateral geniculate body of the rhesus monkey. J Neurophysiol. 1966 Nov;29(6):1115–1156. doi: 10.1152/jn.1966.29.6.1115. [DOI] [PubMed] [Google Scholar]


