Abstract
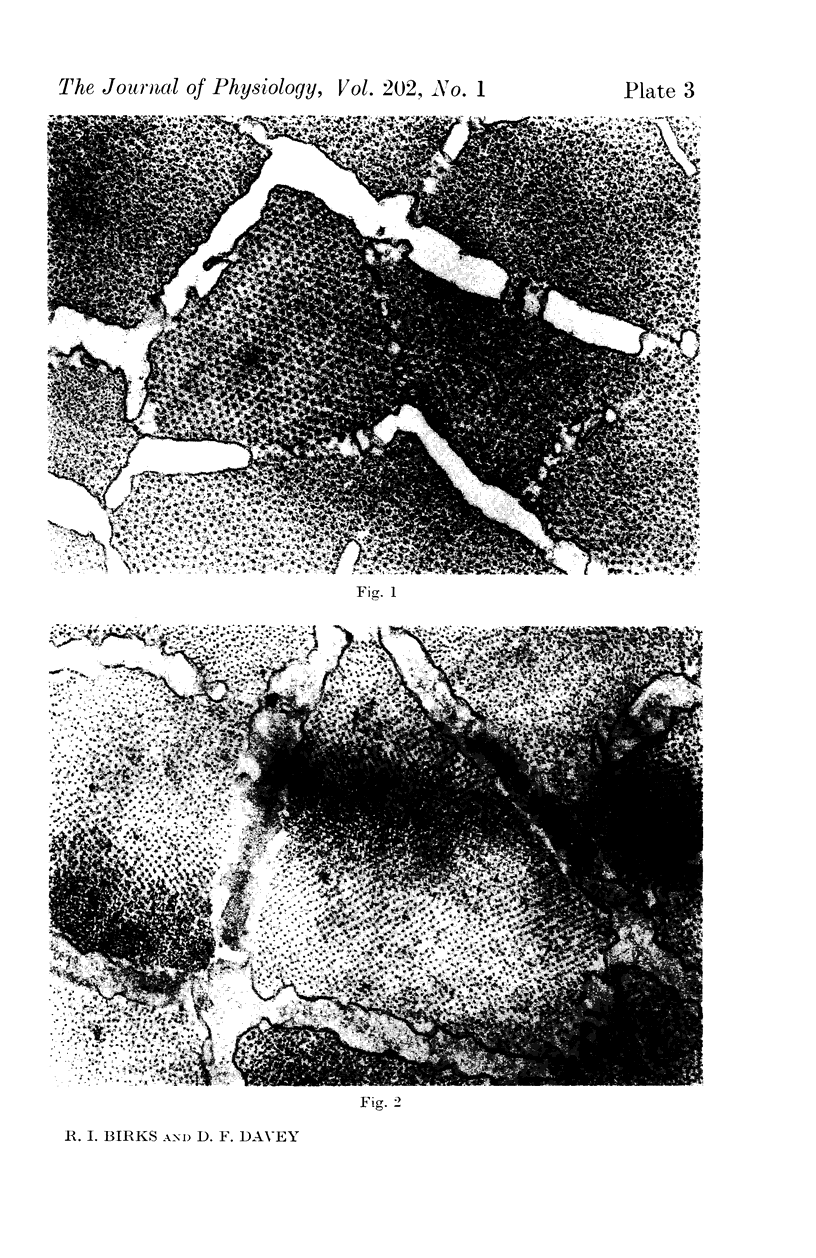
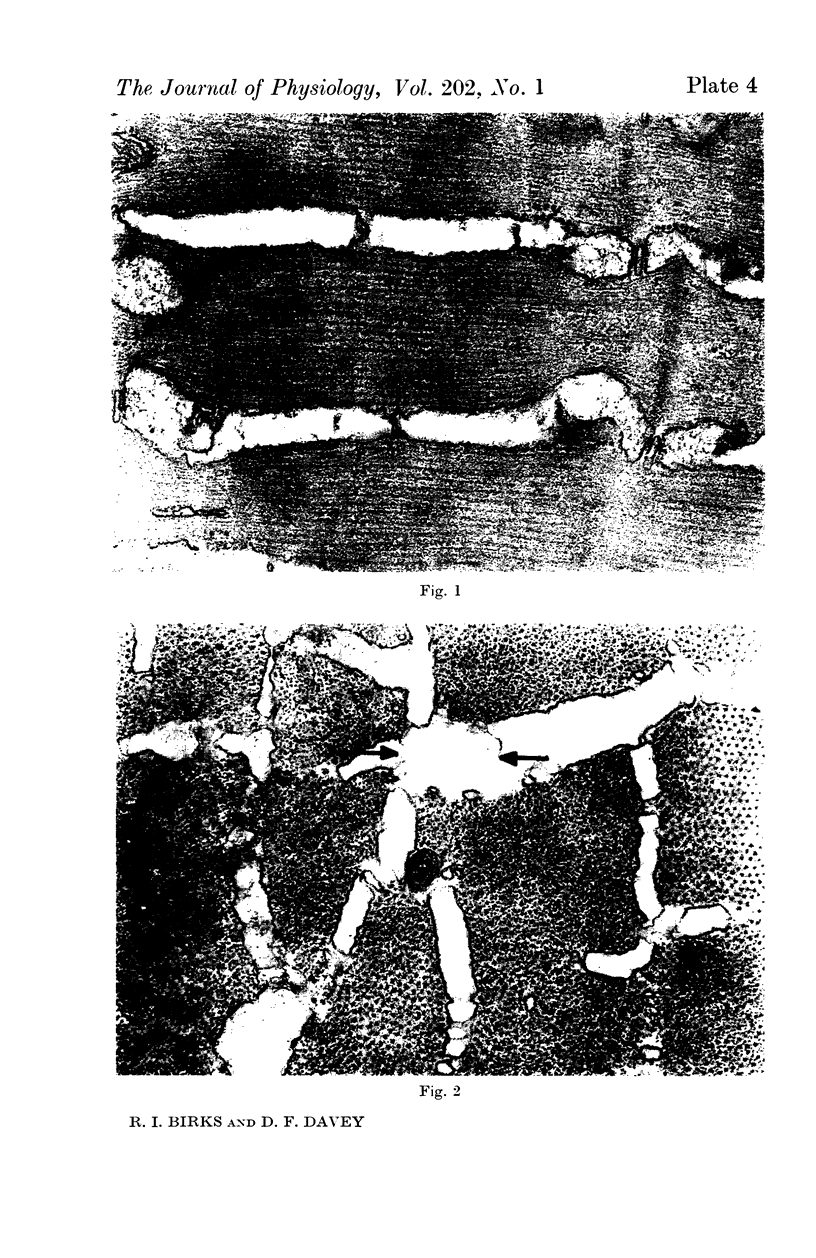
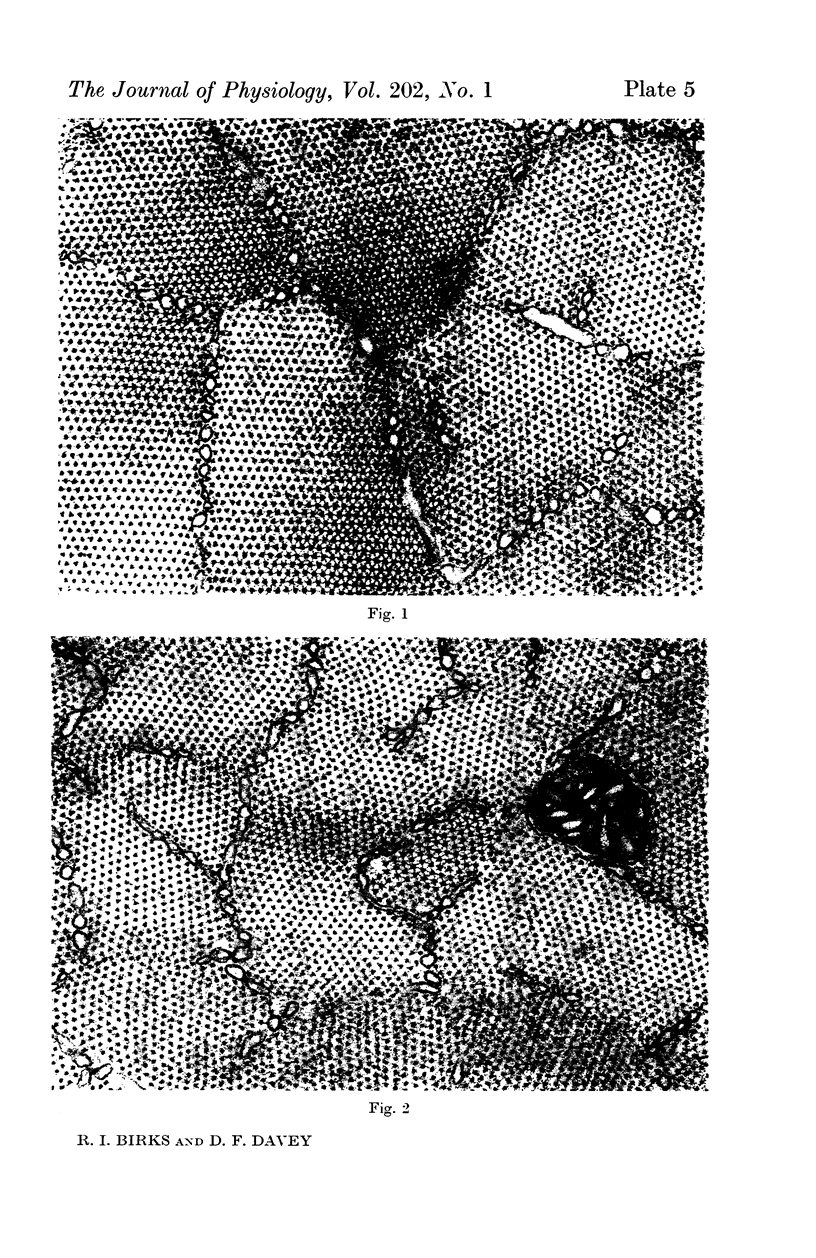
1. Changes in the dimensions of the sarcoplasmic reticulum in frog sartorius muscles exposed to hypertonic and hypotonic solutions have been studied with the electron microscope.
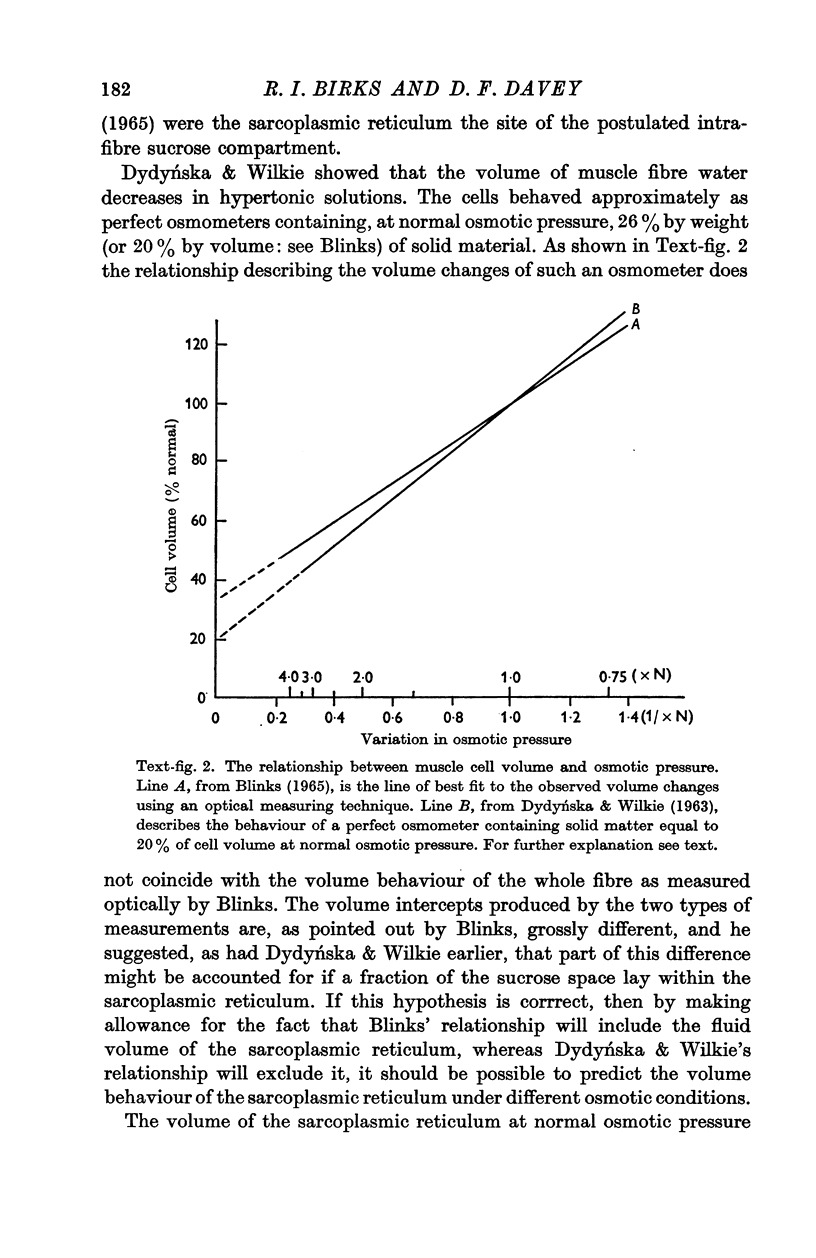
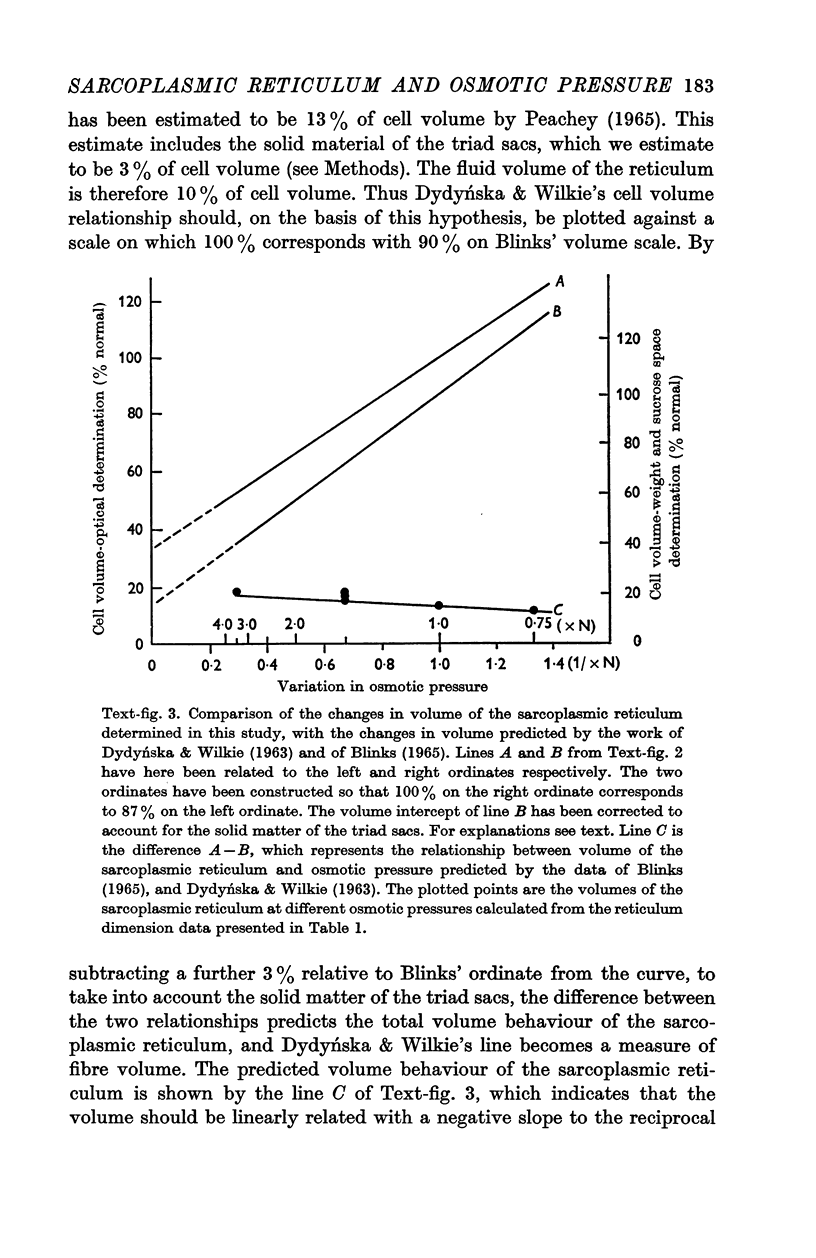
2. The volume of the sarcoplasmic reticulum has been found to be linearly related with a negative slope to the reciprocal of the osmotic pressure. Over the range 0·75 to 3·5 × normal osmotic pressure the reticulum volume has been calculated to change from 11·5 to 18·5% of normal cell volume.
3. These changes in sarcoplasmic reticulum volume correspond to the calculated changes in the volume of the intra-fibre sucrose compartment, postulated by earlier workers on the basis of studies on changes in cell volume with changes in osmotic pressure in living muscles.
4. To explain these and other related findings on the distribution of electrolytes in muscle, it is proposed that the sarcoplasmic reticulum of skeletal muscle is an extracellular compartment.
5. The significance of this hypothesis for the mechanism of excitation-contraction coupling is discussed.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTANTIN L. L., FRANZINI-ARMSTRONG C., PODOLSKY R. J. LOCALIZATION OF CALCIUM-ACCUMULATING STRUCTURES IN STRIATED MUSCLE FIBERS. Science. 1965 Jan 8;147(3654):158–160. doi: 10.1126/science.147.3654.158. [DOI] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J. Evidence for depolarization of the internal membrane system in activation of frog semitendinosus muscle. Nature. 1966 Apr 30;210(5035):483–486. doi: 10.1038/210483a0. [DOI] [PubMed] [Google Scholar]
- DYDYNSKA M., WILKIE D. R. THE OSMOTIC PROPERTIES OF STRIATED MUSCLE FIBERS IN HYPERTONIC SOLUTIONS. J Physiol. 1963 Nov;169:312–329. doi: 10.1113/jphysiol.1963.sp007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAHRENBACH W. H. SARCOPLASMIC RETICULUM: ULTRASTRUCTURE OF THE TRIADIC JUNCTION. Science. 1965 Mar 12;147(3663):1308–1309. doi: 10.1126/science.147.3663.1308. [DOI] [PubMed] [Google Scholar]
- FRANZINI-ARMSTRONG C., PORTER K. R. SARCOLEMMAL INVAGINATIONS CONSTITUTING THE T SYSTEM IN FISH MUSCLE FIBERS. J Cell Biol. 1964 Sep;22:675–696. doi: 10.1083/jcb.22.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J. Distribution and movement of muscle chloride. J Physiol. 1963 Apr;166:87–109. doi: 10.1113/jphysiol.1963.sp007092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASSELBACH W., MAKINOSE M. [The calcium pump of the "relaxing granules" of muscle and its dependence on ATP-splitting]. Biochem Z. 1961;333:518–528. [PubMed] [Google Scholar]
- HASSELBACH W. RELAXATION AND THE SARCOTUBULAR CALCIUM PUMP. Fed Proc. 1964 Sep-Oct;23:909–912. [PubMed] [Google Scholar]
- HOWARTH J. V. The behaviour of frog muscle in hypertonic solutions. J Physiol. 1958 Nov 10;144(1):167–175. doi: 10.1113/jphysiol.1958.sp006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., TAYLOR R. E. Local activation of striated muscle fibres. J Physiol. 1958 Dec 30;144(3):426–441. doi: 10.1113/jphysiol.1958.sp006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY H. E. EVIDENCE FOR CONTINUITY BETWEEN THE CENTRAL ELEMENTS OF THE TRIADS AND EXTRACELLULAR SPACE IN FROG SARTORIUS MUSCLE. Nature. 1964 Jun 13;202:1067–1071. doi: 10.1038/2021067b0. [DOI] [PubMed] [Google Scholar]
- JOHNSON J. A., SIMONDS M. A. Chemical and histological space determinations in rabbit heart. Am J Physiol. 1962 Mar;202:589–592. doi: 10.1152/ajplegacy.1962.202.3.589. [DOI] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., KANNO Y. STUDIES ON AN EPITHELIAL (GLAND) CELL JUNCTION. I. MODIFICATIONS OF SURFACE MEMBRANE PERMEABILITY. J Cell Biol. 1964 Sep;22:565–586. doi: 10.1083/jcb.22.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Ladinsky H., Choi S. J., Kasuya Y. Studies on the in vitro interaction of electrical stimulation and Ca++ movement in sarcoplasmic reticulum. J Gen Physiol. 1966 Mar;49(4):689–715. doi: 10.1085/jgp.49.4.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALADE G. E. A study of fixation for electron microscopy. J Exp Med. 1952 Mar;95(3):285–298. doi: 10.1084/jem.95.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PODOLSKY R. J. THE MAXIMUM SARCOMERE LENGTH FOR CONTRACTION OF ISOLATED MYOFIBRILS. J Physiol. 1964 Jan;170:110–123. doi: 10.1113/jphysiol.1964.sp007317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peachey L. D. The sarcoplasmic reticulum and transverse tubules of the frog's sartorius. J Cell Biol. 1965 Jun;25(3 Suppl):209–231. doi: 10.1083/jcb.25.3.209. [DOI] [PubMed] [Google Scholar]
- Potter D. D., Furshpan E. J., Lennox E. S. Connections between cells of the developing squid as revealed by electrophysiological methods. Proc Natl Acad Sci U S A. 1966 Feb;55(2):328–336. doi: 10.1073/pnas.55.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON S. E., SHAW F. H., BENNETT S., MULLER M. The relationship between sodium, potassium, and chloride in amphibian muscle. J Gen Physiol. 1957 May 20;40(5):753–777. doi: 10.1085/jgp.40.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickholm A. Local sarcomere contraction in fast muscle fibres. Nature. 1966 Nov 19;212(5064):835–836. doi: 10.1038/212835a0. [DOI] [PubMed] [Google Scholar]
- TASKER P., SIMON S. E., JOHNSTONE B. M., SHANKLY K. H., SHAW F. H. The dimensions of the extracellular space in sartorius muscle. J Gen Physiol. 1959 Sep;43:39–53. doi: 10.1085/jgp.43.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. The use of carbon films to support tissue sections for electron microscopy. J Biophys Biochem Cytol. 1955 Mar;1(2):183–184. doi: 10.1083/jcb.1.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A., HERZ R., REISS I. On the mechanism of the relaxing effect of fragmented sarcoplasmic reticulum. J Gen Physiol. 1963 Mar;46:679–702. doi: 10.1085/jgp.46.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINEGRAD S. AUTORADIOGRAPHIC STUDIES OF INTRACELLULAR CALCIUM IN FROG SKELETAL MUSCLE. J Gen Physiol. 1965 Jan;48:455–479. doi: 10.1085/jgp.48.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad S. The location of muscle calcium with respect to the myofibrils. J Gen Physiol. 1965 Jul;48(6):997–1002. doi: 10.1085/jgp.48.6.997. [DOI] [PMC free article] [PubMed] [Google Scholar]