Abstract
1. The hypothesis that noradrenaline (NA) may be a transmitter in the temperature regulating centre in the hypothalamus is based on the changes in rectal temperature induced by injection of large doses of NA into the brain. As an alternative approach, the effect of environmental temperature on the rate of turnover of endogenous NA in the hypothalamus has been studied.
2. Small amounts of tritium labelled noradrenaline [3H]NA were injected into the c.s.f. of rats in order to label radioactively the endogenous NA in the brain. The rats were then exposed to environmental temperatures of 9, 17, 24 and 32° C. The rates of disappearance of [3H]NA from discrete areas of brain were taken as indices of the rates of turnover of endogenous NA in those areas.
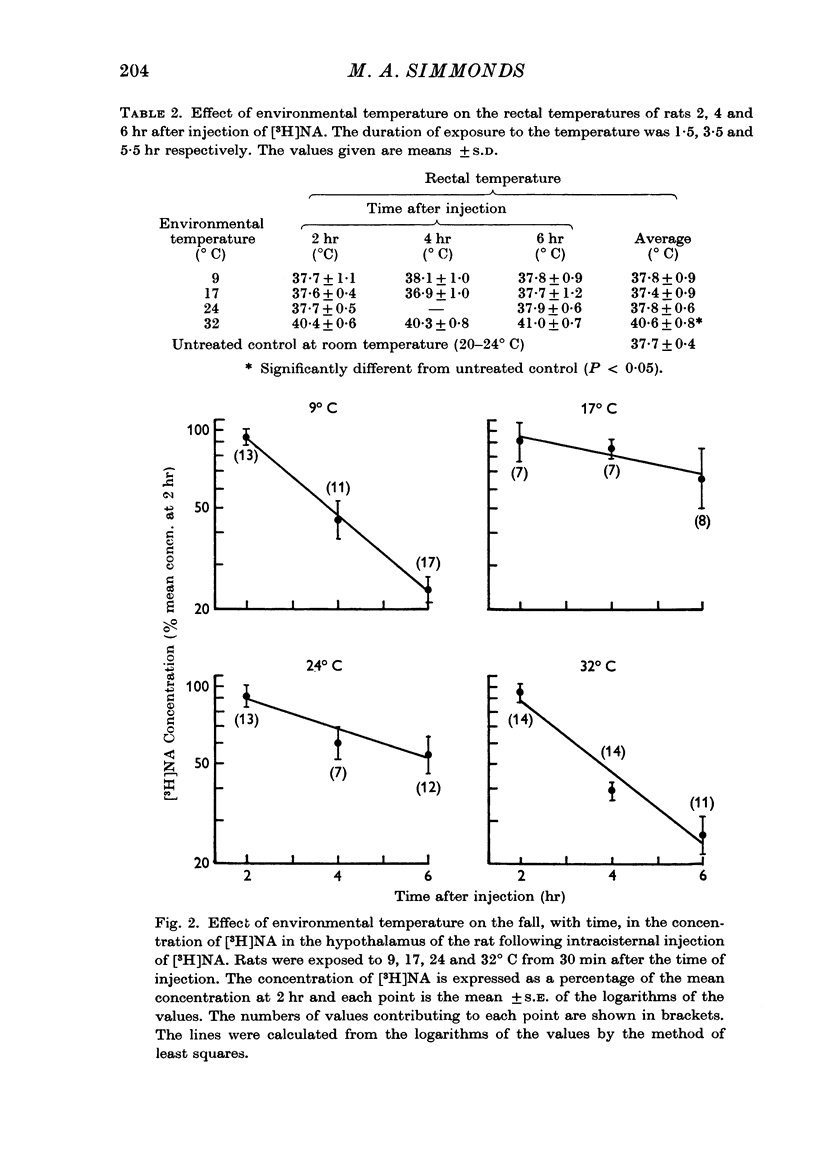
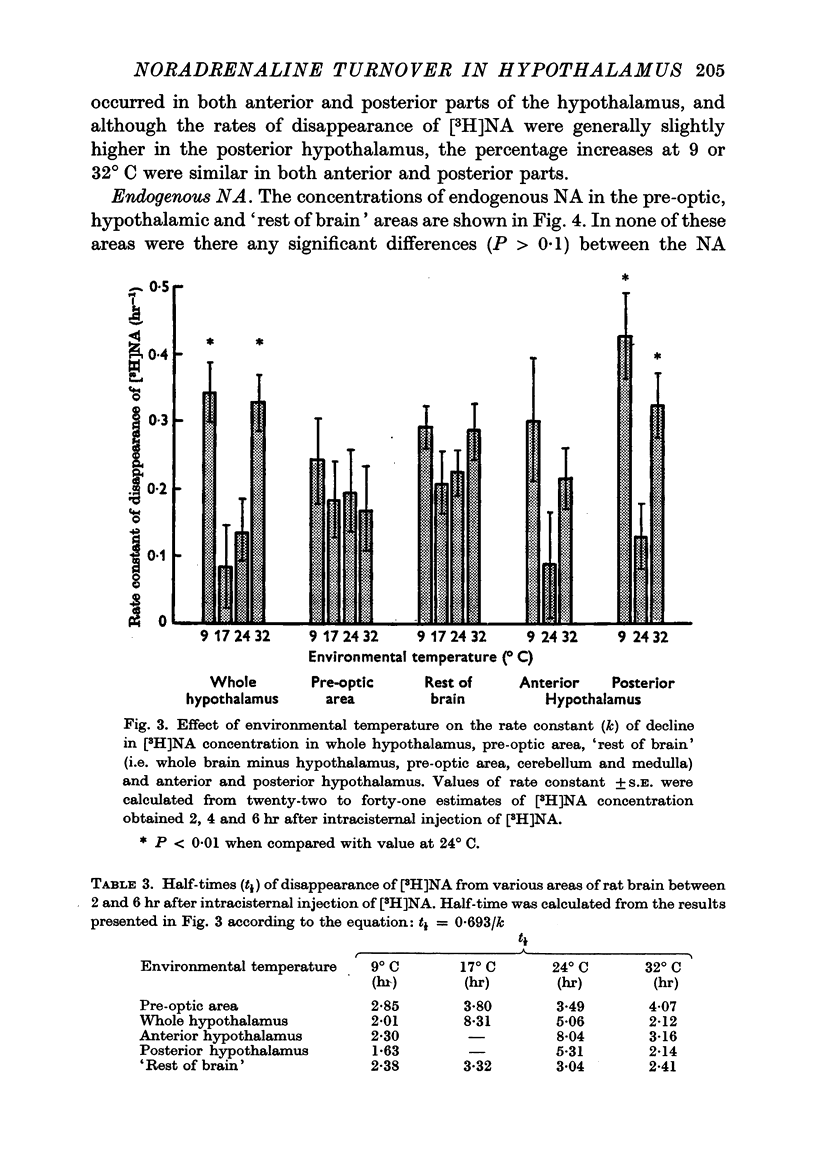
3. The rate of disappearance of [3H]NA from the hypothalamus was three times as fast at 9 and 32° C as at 17 or 24° C. There were no such significant differences from the pre-optic area or `rest of brain' (whole brain minus hypothalamus, pre-optic area, cerebellum and medulla).
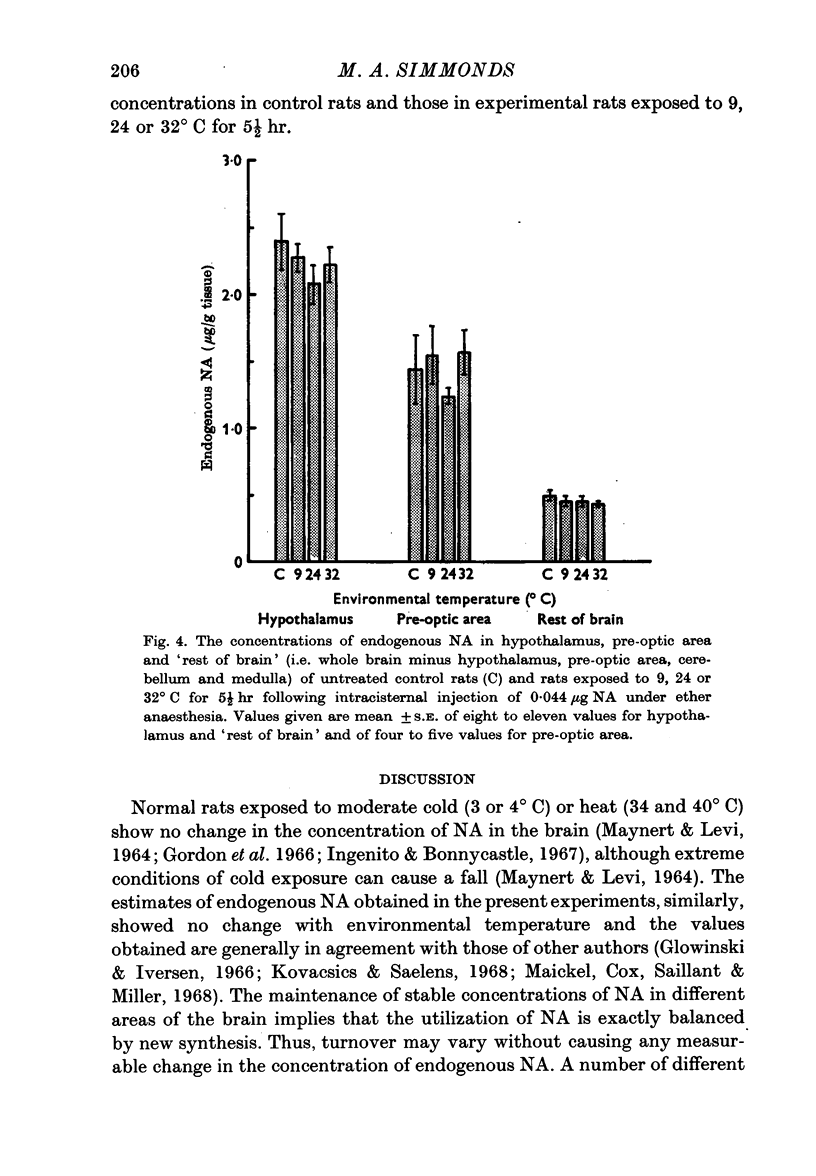
4. The endogenous concentrations of NA were not altered by the experimental procedures in any of the areas of brain studied.
5. The rats maintained normal rectal temperatures at environmental temperatures of 9, 17 and 24° C but became 2·8° C hyperthermic at 32° C.
6. It is concluded that mild conditions of both heat and cold resulted in an increased turnover of NA in specific nerve terminals in the hypothalamus. Since the rats were thermoregulating normally, the nerve terminals involved are regarded as forming a part of the central temperature regulating centre.
Full text
PDF


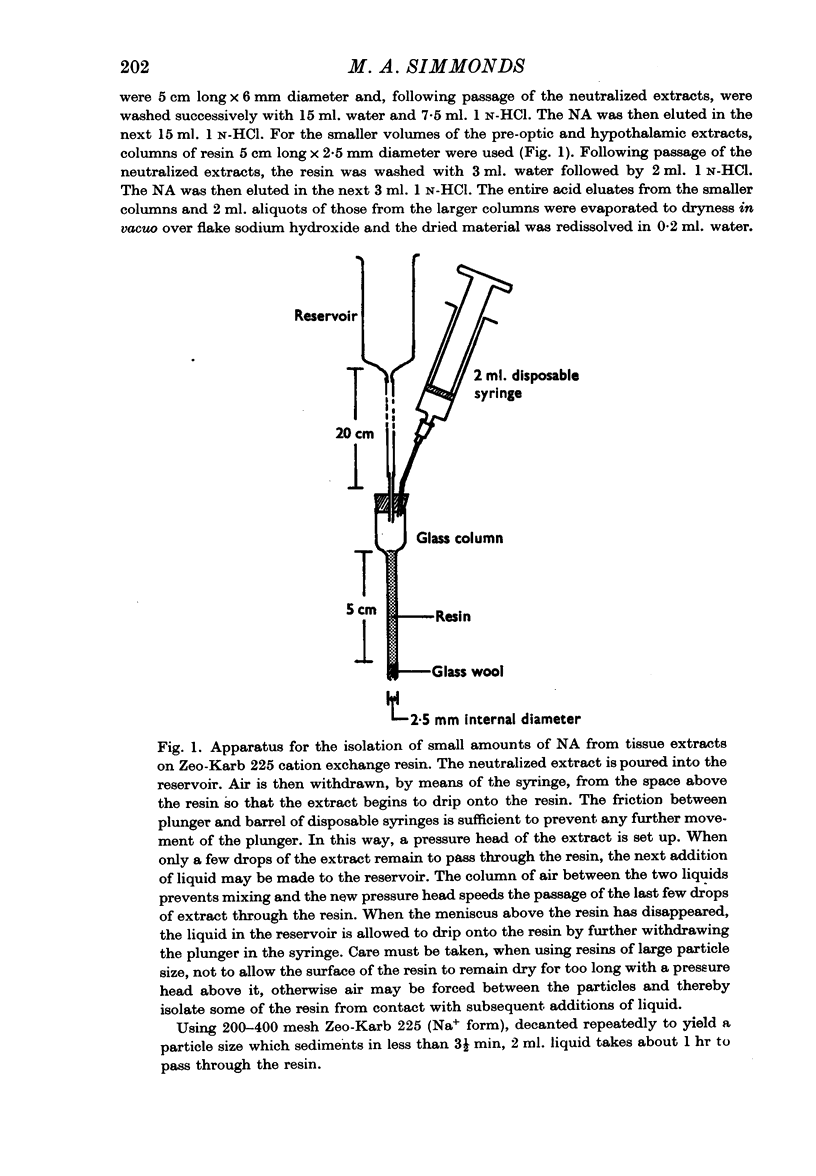





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Bloom F. E. Electron-microscopic localization of tritiated norepinephrine in rat brain: effect of drugs. J Pharmacol Exp Ther. 1967 Jun;156(3):407–416. [PubMed] [Google Scholar]
- Beauvallet M., Legrand M., Fugazza J. Hyperthermie et teneur en noradrénaline, dopamine et 5-hydroxytryptamine du cerveau chez le rat. C R Seances Soc Biol Fil. 1967;161(6):1291–1294. [PubMed] [Google Scholar]
- Bligh J. The thermosensitivity of the hypothalamus and thermoregulation in mammals. Biol Rev Camb Philos Soc. 1966 Aug;41(3):317–368. doi: 10.1111/j.1469-185x.1966.tb01496.x. [DOI] [PubMed] [Google Scholar]
- Brodie B. B., Costa E., Dlabac A., Neff N. H., Smookler H. H. Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J Pharmacol Exp Ther. 1966 Dec;154(3):493–498. [PubMed] [Google Scholar]
- Cabanac M., Stolwijk J. A., Hardy J. D. Effect of temperature and pyrogens on single-unit activity in the rabbit's brain stem. J Appl Physiol. 1968 May;24(5):645–652. doi: 10.1152/jappl.1968.24.5.645. [DOI] [PubMed] [Google Scholar]
- Cooper K. E., Cranston W. I., Honour A. J. Effects of intraventricular and intrahypothalamic injection of noradrenaline and 5-HT on body temperature in conscious rabbits. J Physiol. 1965 Dec;181(4):852–864. doi: 10.1113/jphysiol.1965.sp007801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrodi H., Fuxe K., Hökfelt T. A possible role played by central monoamine neurones in thermo-regulation. Acta Physiol Scand. 1967 Oct-Nov;71(2):224–232. doi: 10.1111/j.1748-1716.1967.tb03728.x. [DOI] [PubMed] [Google Scholar]
- Duce M., Crabai F., Vargiu L., Piras L., Adamo F., Gessa G. L. Effetto dell'alfa-metil-m-tirosina sulle catecolamine di animali esposti al freddo. Boll Soc Ital Biol Sper. 1967 Dec 15;43(23):1607–1609. [PubMed] [Google Scholar]
- FELDBERG W., MYERS R. D. A NEW CONCEPT OF TEMPERATURE REGULATION BY AMINES IN THE HYPOTHALAMUS. Nature. 1963 Dec 28;200:1325–1325. doi: 10.1038/2001325a0. [DOI] [PubMed] [Google Scholar]
- FELDBERG W., MYERS R. D. CHANGES IN TEMPERATURE PRODUCED BY MICRO-INJECTIONS OF AMINES INTO THE ANTERIOR HYPOTHALAMUS OF CATS. J Physiol. 1965 Mar;177:239–245. doi: 10.1113/jphysiol.1965.sp007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUXE K. EVIDENCE FOR THE EXISTENCE OF MONOAMINE NEURONS IN THE CENTRAL NERVOUS SYSTEM. IV. DISTRIBUTION OF MONOAMINE NERVE TERMINALS IN THE CENTRAL NERVOUS SYSTEM. Acta Physiol Scand Suppl. 1965:SUPPL 247–247:37+. [PubMed] [Google Scholar]
- Feldberg W., Hellon R. F., Lotti V. J. Temperature effects produced in dogs and monkeys by injections of monoamines and related substances into the third ventricle. J Physiol. 1967 Aug;191(3):501–515. doi: 10.1113/jphysiol.1967.sp008264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldberg W., Lotti V. J. Temperature responses to monoamines and an inhibitor of MAO injected into the cerebral ventricles of rats. Br J Pharmacol Chemother. 1967 Sep;31(1):152–161. doi: 10.1111/j.1476-5381.1967.tb01985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay J. D., Thompson G. E. The effect of intraventricular injections of noradrenaline, 5-hydroxytryptamine, acetylcholine and tranylcypromine on the ox (Bos taurus) at different environmental temperatures. J Physiol. 1968 Feb;194(3):809–816. doi: 10.1113/jphysiol.1968.sp008436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., Ungerstedt U. Localization of catecholamine uptake in rat brain after intraventricular injection. Life Sci. 1966 Oct;5(19):1817–1824. doi: 10.1016/0024-3205(66)90058-0. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Gordon R., Spector S., Sjoerdsma A., Udenfriend S. Increased synthesis of norepinephrine and epinephrine in the intact rat during exercise and exposure to cold. J Pharmacol Exp Ther. 1966 Sep;153(3):440–447. [PubMed] [Google Scholar]
- HAGGENDAL J. Fluorimetric determination of 3-O-methylated derivatives of adrenaline and noradrenaline in tissues and body fluids. Acta Physiol Scand. 1962 Nov-Dec;56:258–266. doi: 10.1111/j.1748-1716.1962.tb02503.x. [DOI] [PubMed] [Google Scholar]
- HAN P. W., BROBECK J. R. Deficits of temperature regulation in rats with hypothalamic lesions. Am J Physiol. 1961 Apr;200:707–710. doi: 10.1152/ajplegacy.1961.200.4.707. [DOI] [PubMed] [Google Scholar]
- Hellon R. F. Thermal stimulation of hypothalamic neurones in unanaesthetized rabbits. J Physiol. 1967 Nov;193(2):381–395. doi: 10.1113/jphysiol.1967.sp008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingenito A. J., Bonnycastle D. D. The effect of exposure to heat and cold upon rat brain catecholamine and 5-hydroxytryptamine levels. Can J Physiol Pharmacol. 1967 Jul;45(4):733–743. doi: 10.1139/y67-086. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Glowinski J. Regional studies of catecholamines in the rat brain. II. Rate of turnover of catecholamines in various brain regions. J Neurochem. 1966 Aug;13(8):671–682. doi: 10.1111/j.1471-4159.1966.tb09874.x. [DOI] [PubMed] [Google Scholar]
- Kovacsics G. B., Saelens J. K. Measurement of the levels and turnover of norepinephrine in discrete areas of rat brain using an enzymatic assay. Arch Int Pharmacodyn Ther. 1968 Aug;174(2):481–490. [PubMed] [Google Scholar]
- LAVERTY R., SHARMAN D. F. THE ESTIMATION OF SMALL QUANTITIES OF 3,4-DIHYDROXYPHENYLETHYLAMINE IN TISSUES. Br J Pharmacol Chemother. 1965 Apr;24:538–548. doi: 10.1111/j.1476-5381.1965.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYNERT E. W., LEVI R. STRESS-INDUCED RELEASE OF BRAIN NOREPINEPHRINE AND ITS INHIBITION BY DRUGS. J Pharmacol Exp Ther. 1964 Jan;143:90–95. [PubMed] [Google Scholar]
- Maickel R. P., Cox R. H., Jr, Saillant J., Miller F. P. A method for the determination of serotonin and norepinephrine in discrete areas of rat brain. Int J Neuropharmacol. 1968 May;7(3):275–281. doi: 10.1016/0028-3908(68)90034-8. [DOI] [PubMed] [Google Scholar]
- Reid W. D. Discussion of cerebral tryptamine receptors: functional considerations. Adv Pharmacol. 1968;6(Pt B):26–28. doi: 10.1016/s1054-3589(08)60292-6. [DOI] [PubMed] [Google Scholar]
- Reivich M., Glowinski J. An autoradiographic study of the distribution of C14-norepinephrine in the brain of the rat. Brain. 1967 Sep;90(3):633–646. doi: 10.1093/brain/90.3.633. [DOI] [PubMed] [Google Scholar]
- SATINOFF E. BEHAVIORAL THERMOREGULATION IN RESPONSE TO LOCAL COOLING OF THE RAT BRAIN. Am J Physiol. 1964 Jun;206:1389–1394. doi: 10.1152/ajplegacy.1964.206.6.1389. [DOI] [PubMed] [Google Scholar]
- Schanberg S. M., Schildkraut J. J., Kopin I. J. The effects of pentobarbital on the fate of intracisternally administered norepinephrine-H3. J Pharmacol Exp Ther. 1967 Aug;157(2):311–318. [PubMed] [Google Scholar]
- Sedvall G. C., Weise V. K., Kopin I. J. The rate of norepinephrine synthesis measured in vivo during short intervals; influence of adrenergic nerve impulse activity. J Pharmacol Exp Ther. 1968 Feb;159(2):274–282. [PubMed] [Google Scholar]
- Simmonds M. A., Iversen L. L. Thermoregulation: effects of environmental temperature on turnover of hypothalamic norepinephrine. Science. 1969 Jan 31;163(3866):473–474. doi: 10.1126/science.163.3866.473. [DOI] [PubMed] [Google Scholar]
- Thierry A. M., Javoy F., Glowinski J., Kety S. S. Effects of stress on the metabolism of norepinephrine, dopamine and serotonin in the central nervous system of the rat. I. Modifications of norepinephrine turnover. J Pharmacol Exp Ther. 1968 Sep;163(1):163–171. [PubMed] [Google Scholar]
- VOGT M. The concentration of sympathin in different parts of the central nervous system under normal conditions and after the administration of drugs. J Physiol. 1954 Mar 29;123(3):451–481. doi: 10.1113/jphysiol.1954.sp005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von EULER U., LISHAJKO F. Improved technique for the fluorimetric estimation of catecholamines. Acta Physiol Scand. 1961 Apr;51:348–355. doi: 10.1111/j.1748-1716.1961.tb02128.x. [DOI] [PubMed] [Google Scholar]


