Abstract
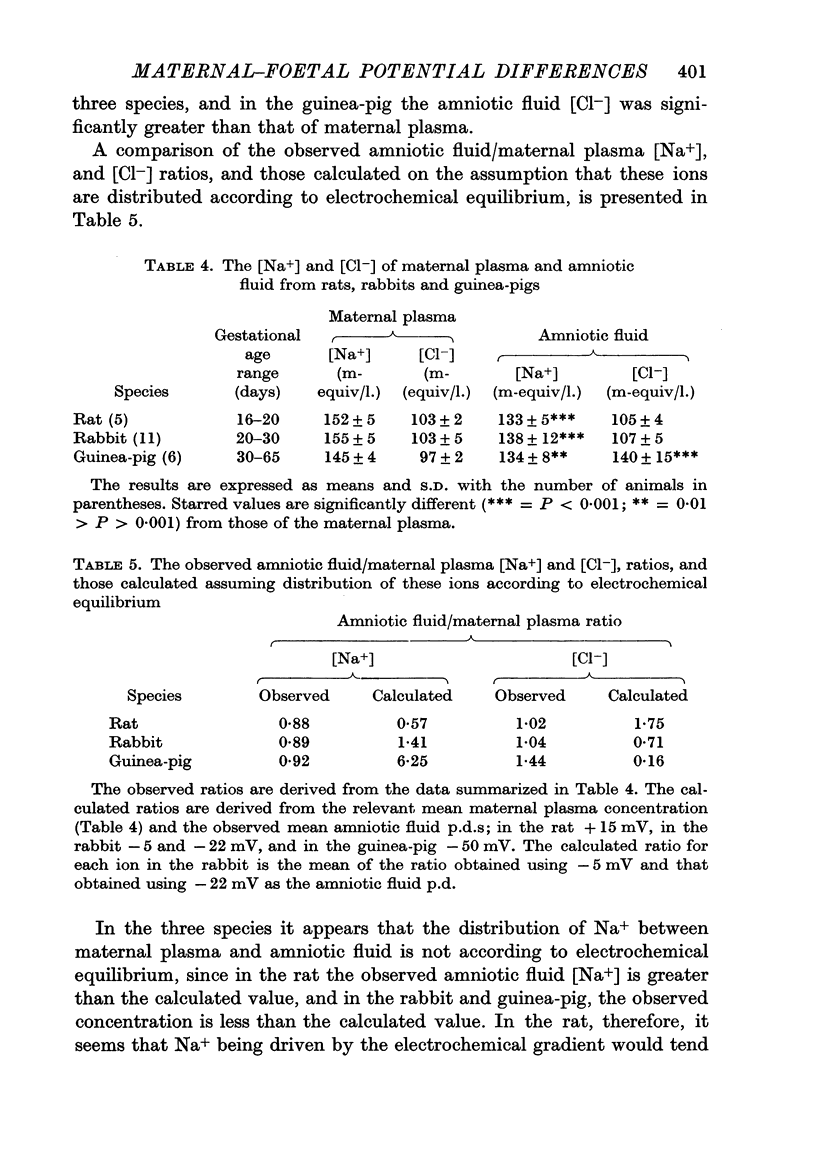
1. Potential differences associated with fluid compartments of rat, rabbit and guinea-pig conceptuses have been measured. [Na+] and [Cl-] in maternal plasma and amniotic fluid from these three species were also determined.
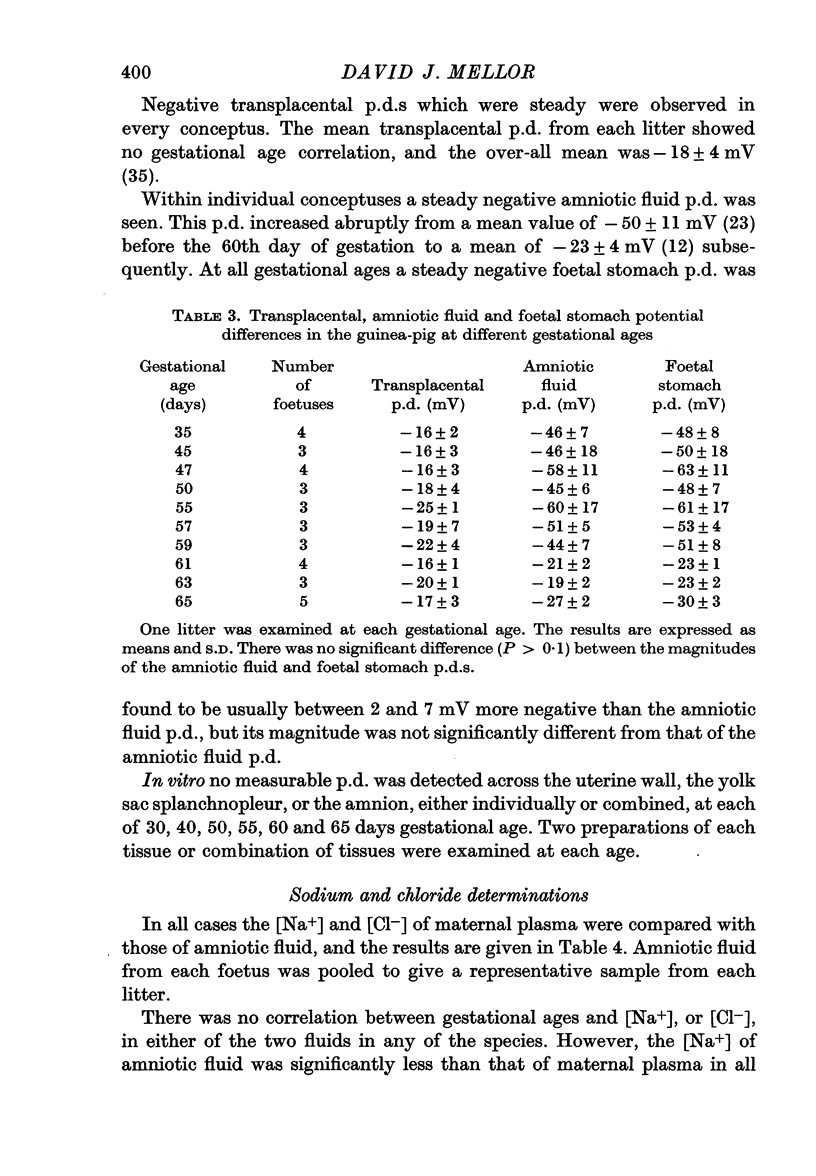
2. Transplacental potential differences of about 15 mV (foetus positive) were found in the rat, of approximately 0 mV in the rabbit, and of about 18 mV (foetus negative) in the guinea-pig.
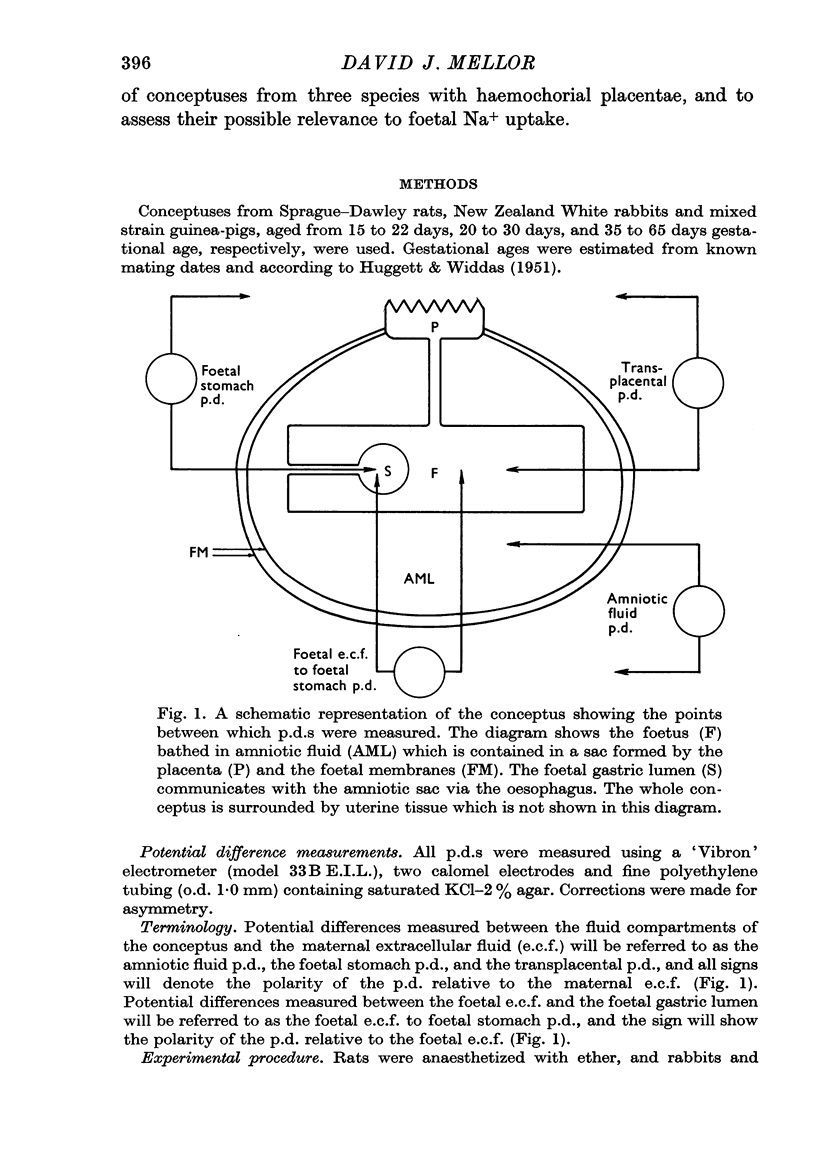
3. Amniotic fluid potential differences appeared to arise indirectly from the transplacental potential difference in the rat, from the foetal gastric mucosa in the rabbit, and possibly from the foetal gastric mucosa and indirectly from the placenta in the guinea-pig.
4. The results are discussed in the context of Na+ transfer to the foetus, and on this basis tend to question the general assumption that almost all Na+ reaching the foetus passes across the placenta.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRAWFORD J. D., McCANCE R. A. Sodium transport by the chorioallantoic membrane of the pig. J Physiol. 1960 Jun;151:458–471. doi: 10.1113/jphysiol.1960.sp006451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDERS A. C. A COMPARATIVE STUDY OF THE FINE STRUCTURE OF THE TROPHOBLAST IN SEVERAL HEMOCHORIAL PLACENTAS. Am J Anat. 1965 Jan;116:29–67. doi: 10.1002/aja.1001160103. [DOI] [PubMed] [Google Scholar]
- Faber J. J., Hart F. M., Poutala A. C. Diffusional resistance of the innermost layer of the placental barrier of the rabbit. J Physiol. 1968 Jul;197(2):381–393. doi: 10.1113/jphysiol.1968.sp008565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber J. J., Hart F. M. Transfer of charged and uncharged molecules in the placenta of the rabbit. Am J Physiol. 1967 Oct;213(4):890–894. doi: 10.1152/ajplegacy.1967.213.4.890. [DOI] [PubMed] [Google Scholar]
- HUGGETT A. S. G., WIDDAS W. F. The relationship between mammalian foetal weight and conception age. J Physiol. 1951 Jul;114(3):306–317. doi: 10.1113/jphysiol.1951.sp004622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschia G., Wolkoff A. S., Barron D. H. DIFFERENCE IN ELECTRIC POTENTIAL ACROSS THE PLACENTA OF GOATS. Proc Natl Acad Sci U S A. 1958 May;44(5):483–485. doi: 10.1073/pnas.44.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- WIDDAS W. F. Transport mechanisms in the foetus. Br Med Bull. 1961 May;17:107–111. doi: 10.1093/oxfordjournals.bmb.a069882. [DOI] [PubMed] [Google Scholar]
- WRIGHT G. H. Net transfers of water, sodium, chloride and hydrogen ions across the gastric mucosa of the rabbit foetus. J Physiol. 1962 Sep;163:281–293. doi: 10.1113/jphysiol.1962.sp006974. [DOI] [PMC free article] [PubMed] [Google Scholar]


