Abstract
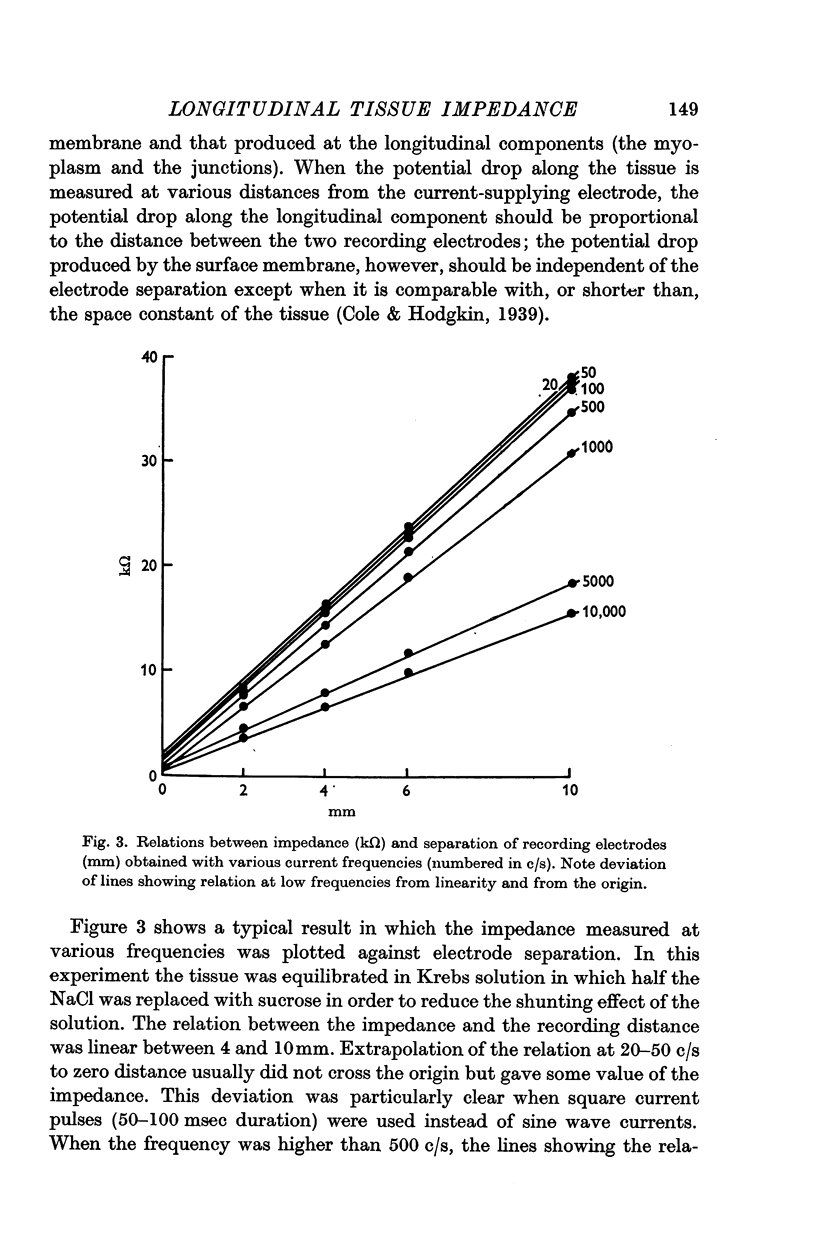
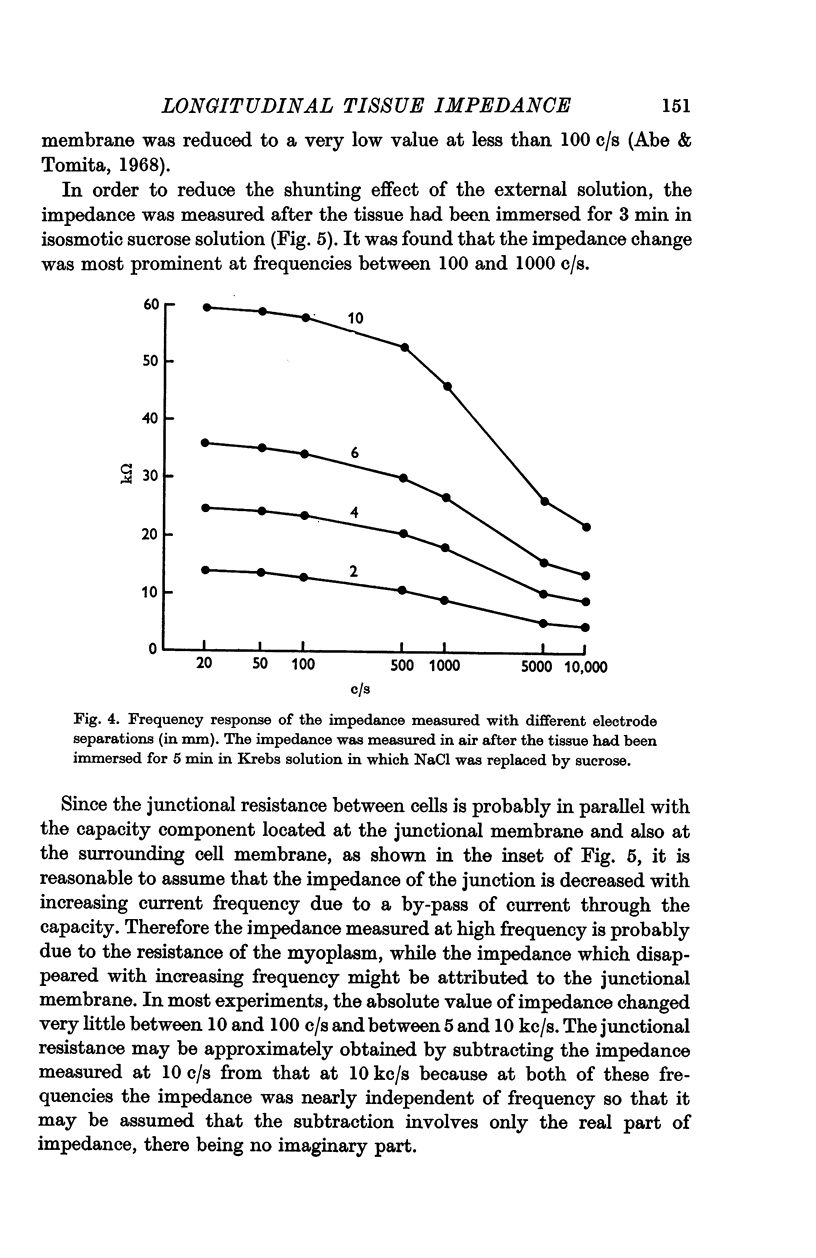
1. The absolute value of the tissue impedance of the guinea-pig taenia coli was measured in the longitudinal direction in air at 36° C. The relation between the tissue impedance and the separation of the recording electrodes indicated that the tissue has cable-like properties. The frequency dependence of the impedance, observed at various distances from the current supplying electrode, showed that the tissue has a capacity component in the longitudinal direction.
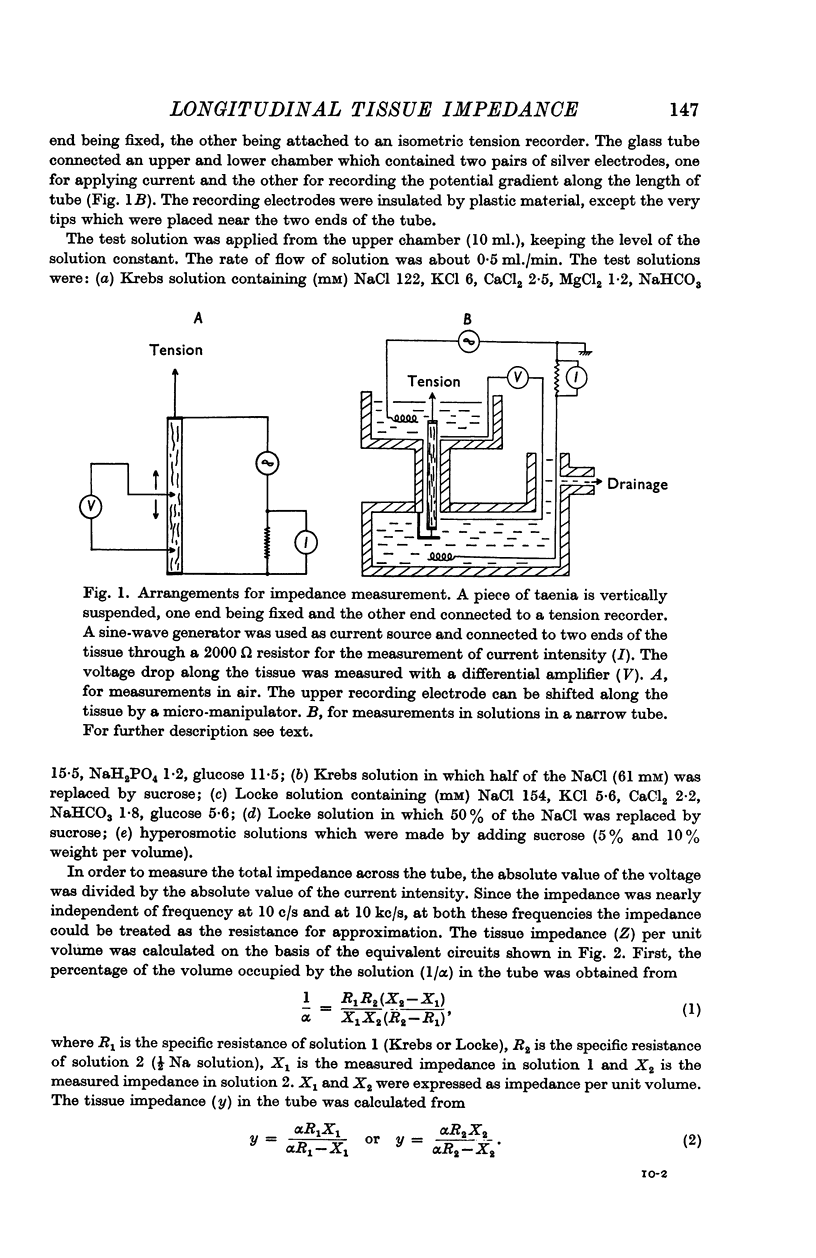
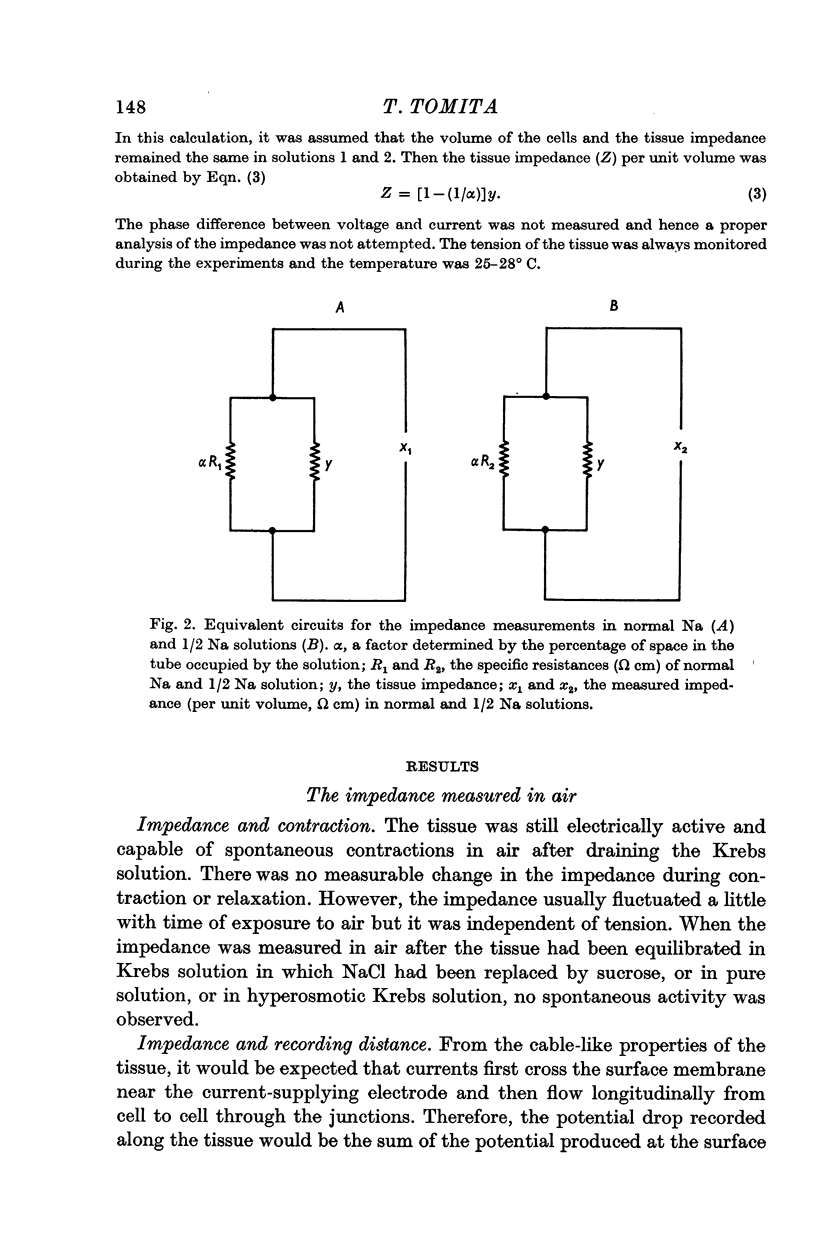
2. The tissue impedance in the longitudinal direction was also measured while the tissue was suspended in a narrow tube at 25-28° C. The tissue impedance was calculated on the basis of the difference between the total impedance in Krebs solution and that in Krebs solution containing half the normal Na concentration, knowing the specific resistance of both solutions.
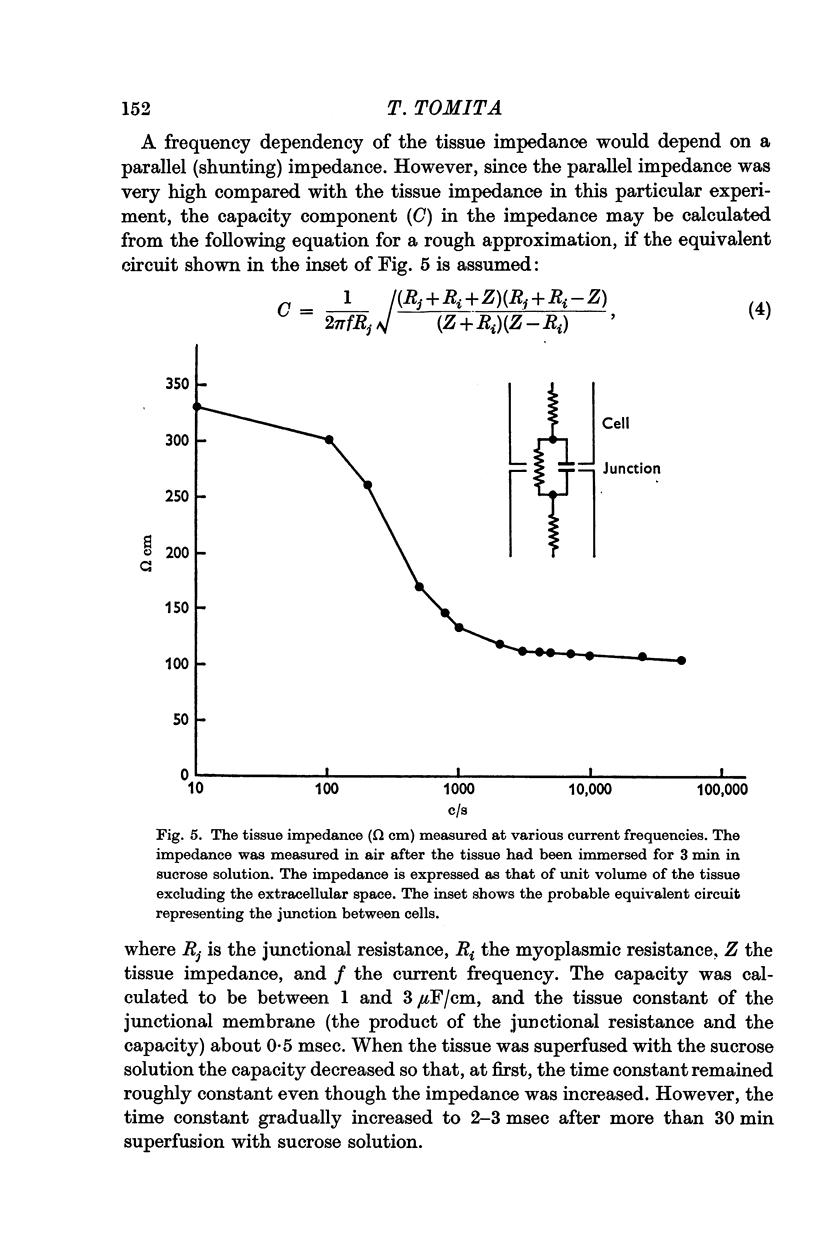
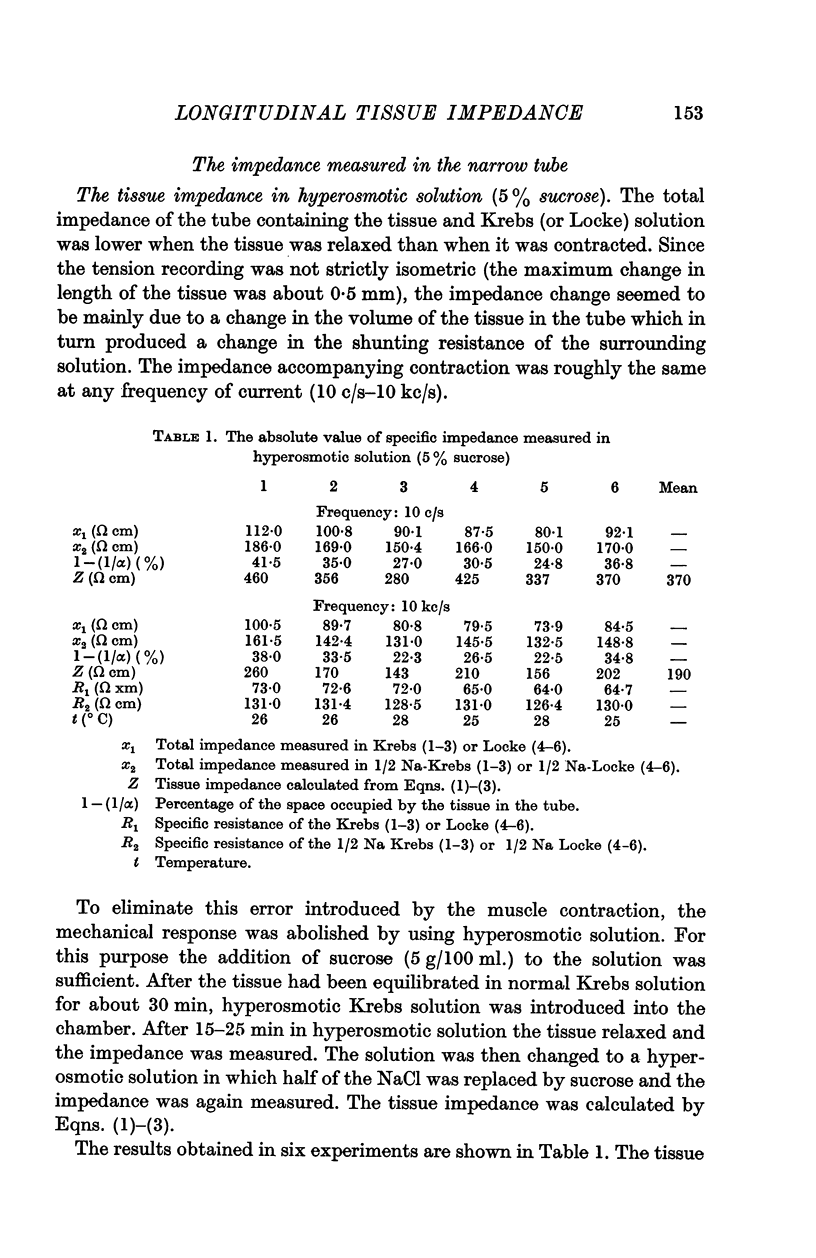
3. The impedance per unit volume of the tissue was 370 Ω cm at 10 c/s and 190 Ω cm at 10 kc/s; at these frequencies the impedance was nearly independent of current frequency. The difference between the impedances (370 - 190 = 180 Ω cm) was taken to be the impedance of the junctions between cells. The possibility of slightly lower values for the actual tissue impedance was discussed. The capacity located at or near the junction was calculated to be 1-3 μF/cm, the time constant of the junctional membrane being about 0·5 msec.
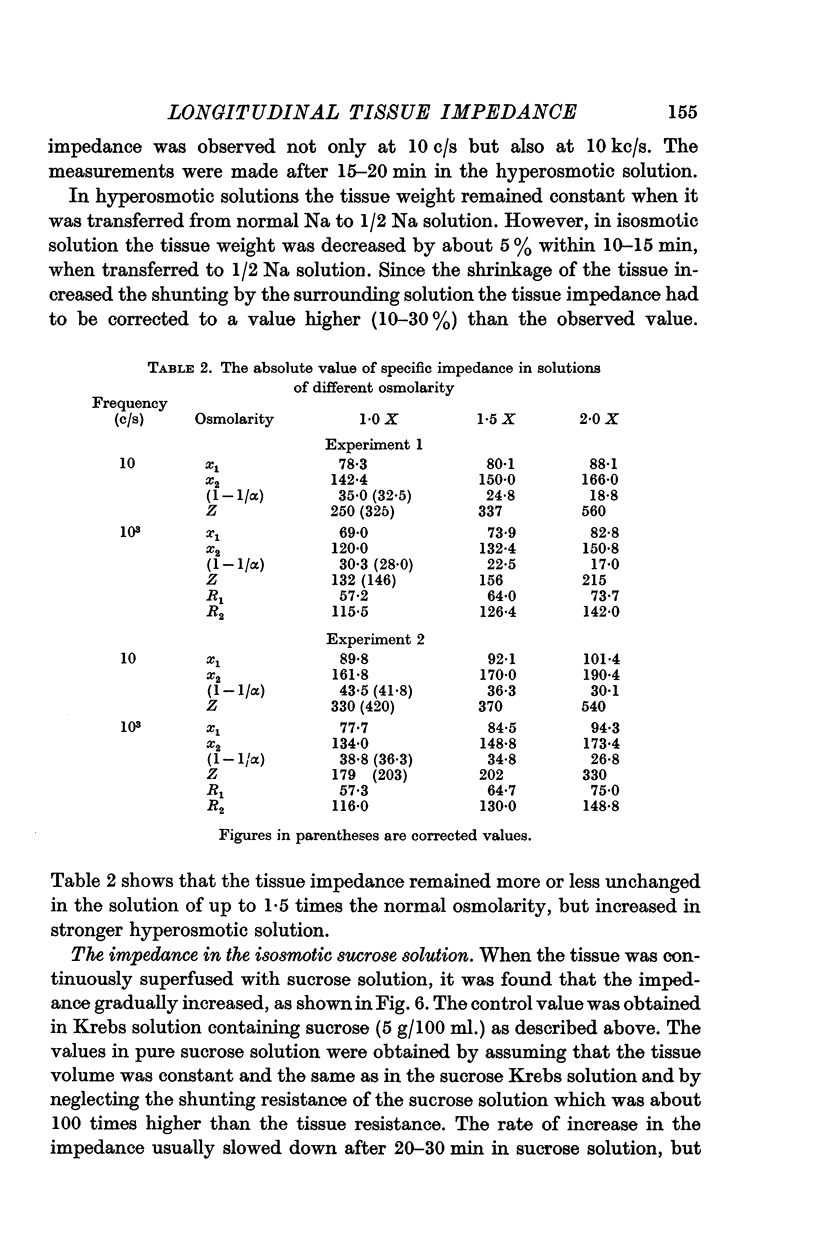
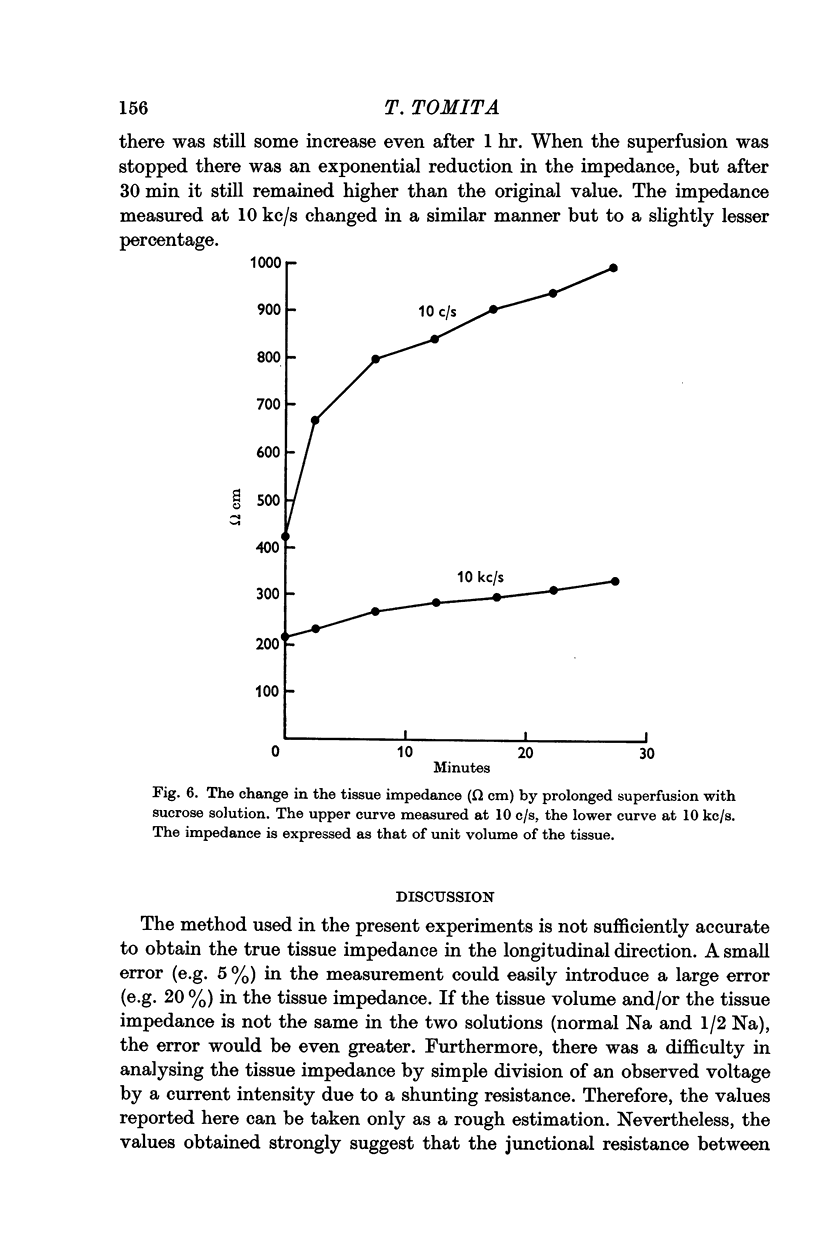
4. Ca ions (0-12·5 mM) and adrenaline (2 × 10-7 g/ml.) had no measurable effect on the longitudinal impedance within 10-15 min. In hyperosmotic solution of twice osmolarity (by adding sucrose), the tissue impedance was increased by about 50%. When the tissue was immersed in sucrose solution containing no ions, the impedance gradually increased up to nearly 10 times in the course of 1 hr.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARR L., DEWEY M. M., BERGER W. PROPAGATION OF ACTION POTENTIALS AND THE STRUCTURE OF THE NEXUS IN CARDIAC MUSCLE. J Gen Physiol. 1965 May;48:797–823. doi: 10.1085/jgp.48.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. Membrane constants of mammalian muscle fibres. J Physiol. 1959 Oct;147:450–457. doi: 10.1113/jphysiol.1959.sp006255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Membrane potentials of smooth muscle fibres of the taenia coli of the guinea-pig. J Physiol. 1954 Aug 27;125(2):302–315. doi: 10.1113/jphysiol.1954.sp005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr L., Berger W., Dewey M. M. Electrical transmission at the nexus between smooth muscle cells. J Gen Physiol. 1968 Mar;51(3):347–368. doi: 10.1085/jgp.51.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- Bülbring E., Tomita T. The effect of catecholamines on the membrane resistance and spike generation in the smooth muscle of guinea-pig taenia coli. J Physiol. 1968 Feb;194(2):74–6P. [PubMed] [Google Scholar]
- CORABOEUF E., WEIDMANN S. Temperature effects on the electrical activity of Purkinje fibres. Helv Physiol Pharmacol Acta. 1954;12(1):32–41. [PubMed] [Google Scholar]
- Dreifuss J. J., Girardier L. Etude de la propagation de l'excitation dans le ventricule de rat au moyen de solutions hypertoniques. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;292(1):13–33. [PubMed] [Google Scholar]
- Goodford P. J., Leach E. H. The extracellular space of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966 Sep;186(1):1–10. doi: 10.1113/jphysiol.1966.sp008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERELAKIS N., HOSHIKO T. Electrical impedance of cardiac muscle. Circ Res. 1961 Nov;9:1280–1283. doi: 10.1161/01.res.9.6.1280. [DOI] [PubMed] [Google Scholar]
- Tomita T. Electrical responses of smooth muscle to external stimulation in hypertonic solution. J Physiol. 1966 Mar;183(2):450–468. doi: 10.1113/jphysiol.1966.sp007876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T. Membrane capacity and resistance of mammalian smooth muscle. J Theor Biol. 1966 Nov;12(2):216–227. doi: 10.1016/0022-5193(66)90114-7. [DOI] [PubMed] [Google Scholar]
- Tomita T. Spike propagation in the smooth muscle of the guinea-pig taenia coli. J Physiol. 1967 Aug;191(3):517–527. doi: 10.1113/jphysiol.1967.sp008265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]


