Abstract
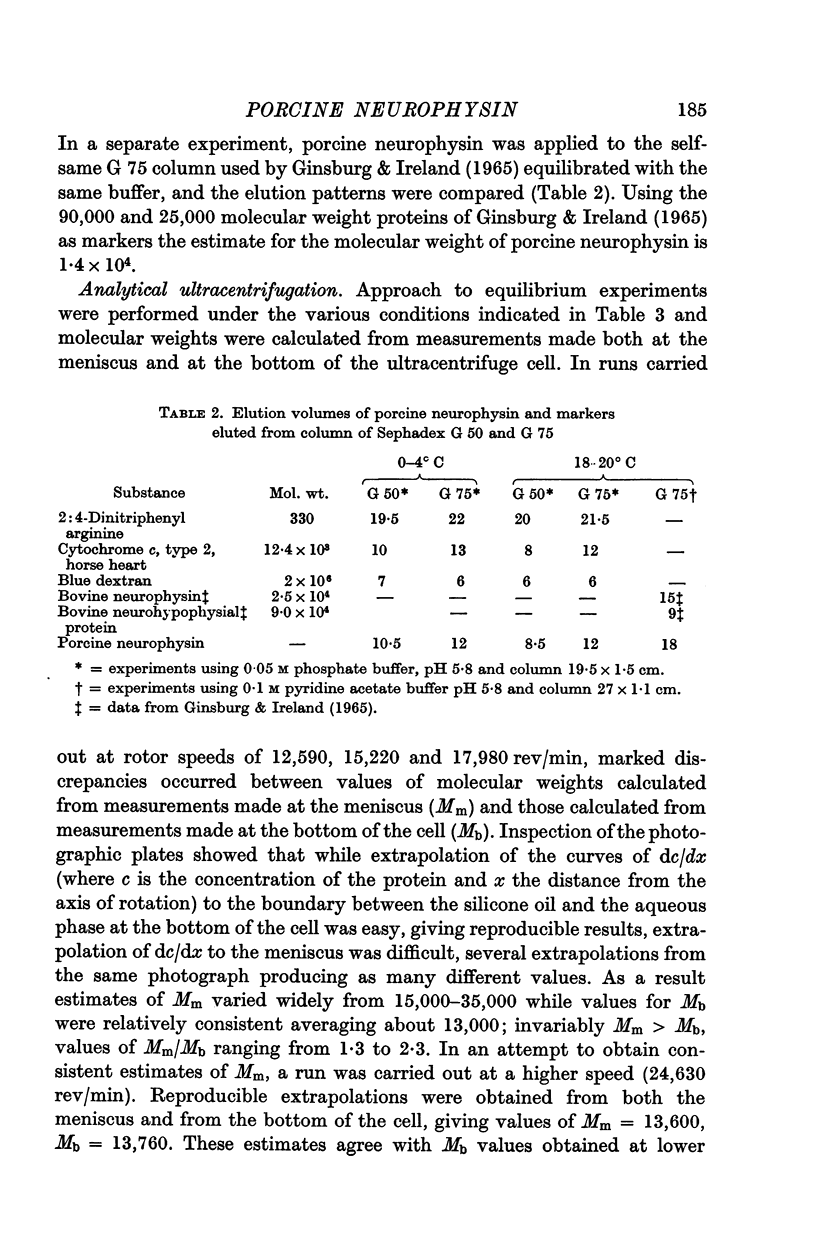
1. The molecular weight of porcine neurophysin was estimated by molecular sieve chromatography and by analytical ultracentrifugation and was found to be in the order of 13,000.
2. Internal evidence for the homogeneity of the preparation of neurophysin with respect to molecular weight was obtained in the ultracentrifugation experiments.
3. The frictional ratio of neurophysin was 1·1 which suggests that the molecular form of the protein approximates to a sphere.
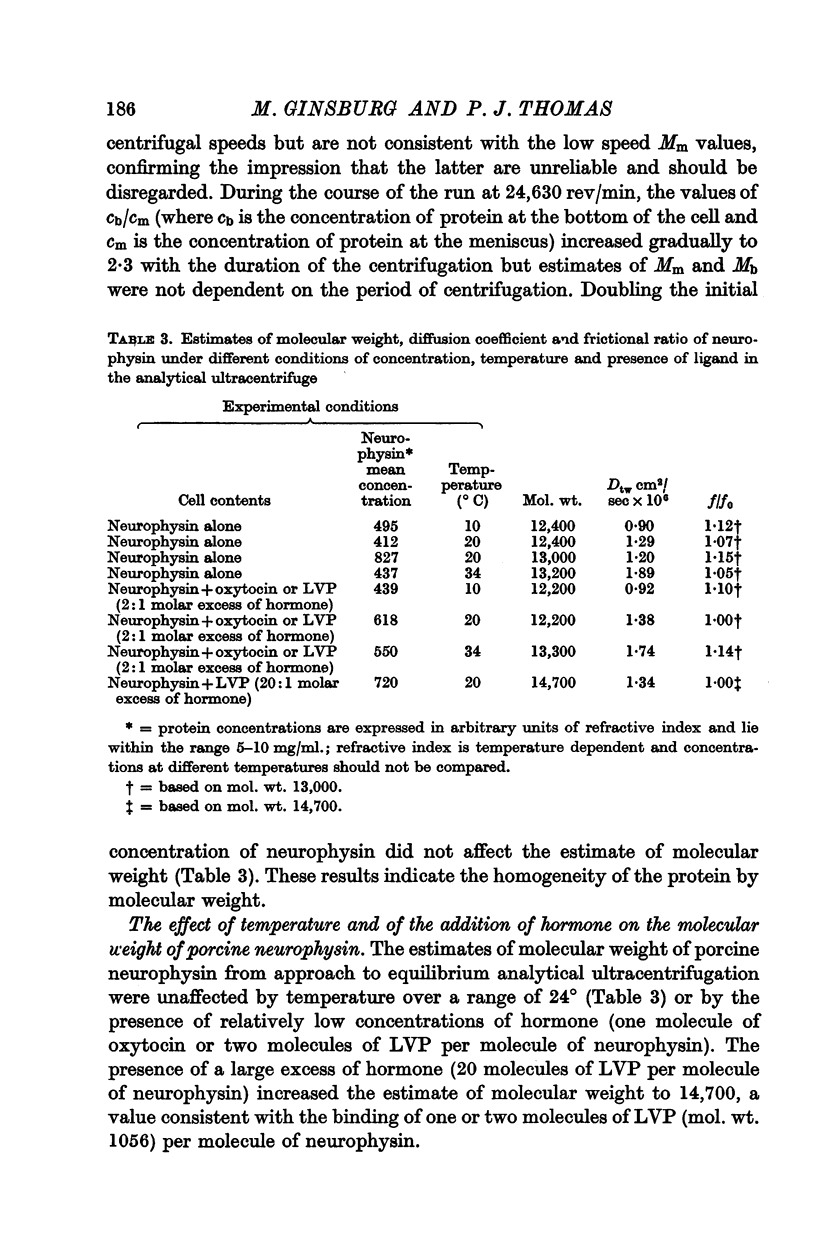
4. The molecular weight and frictional ratio were not affected by temperature change (10-34° C) or by twofold change in protein concentration.
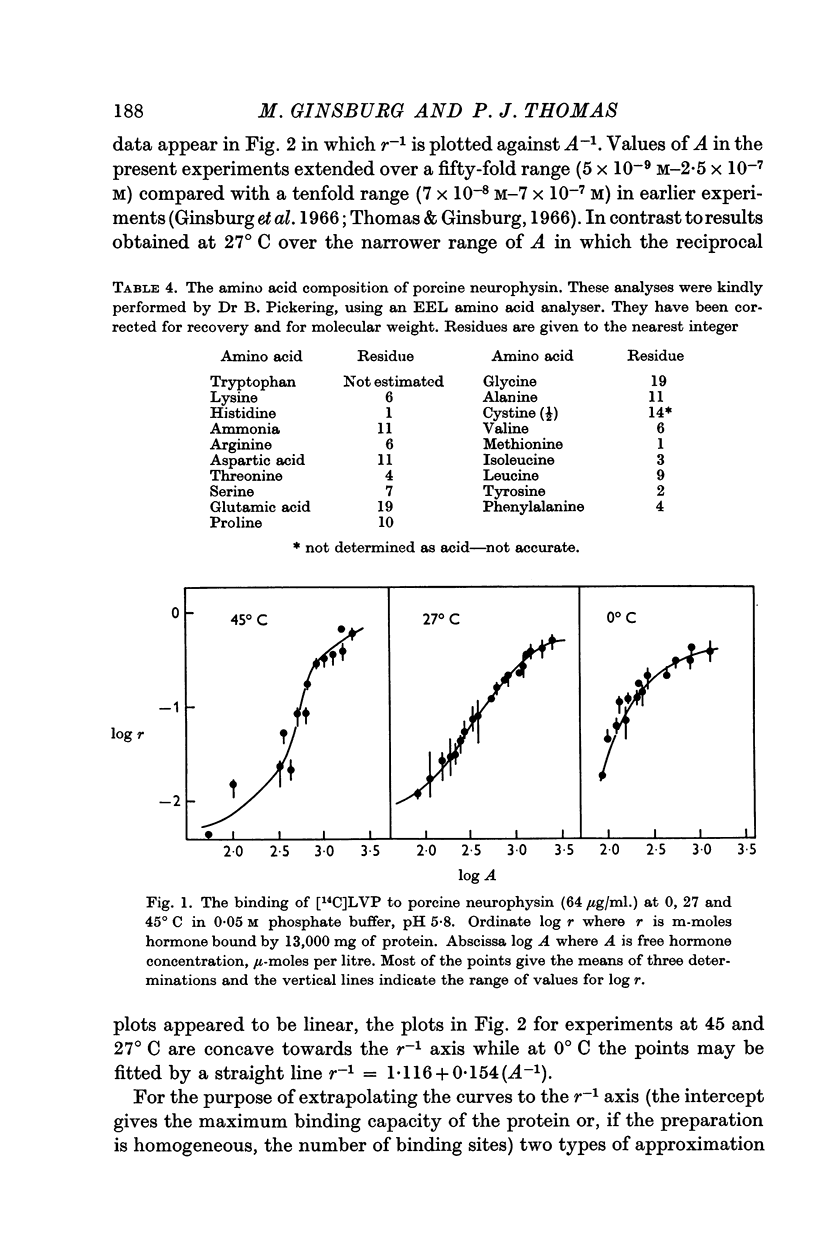
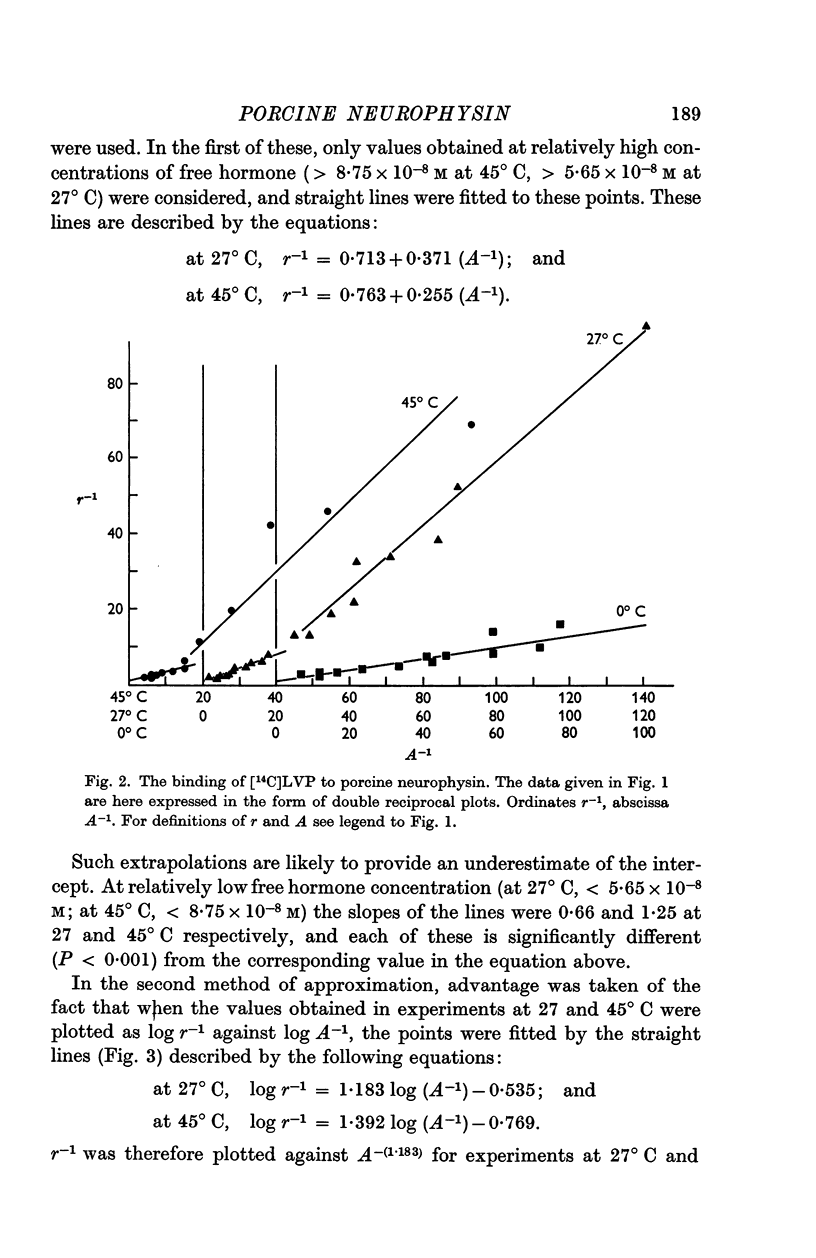
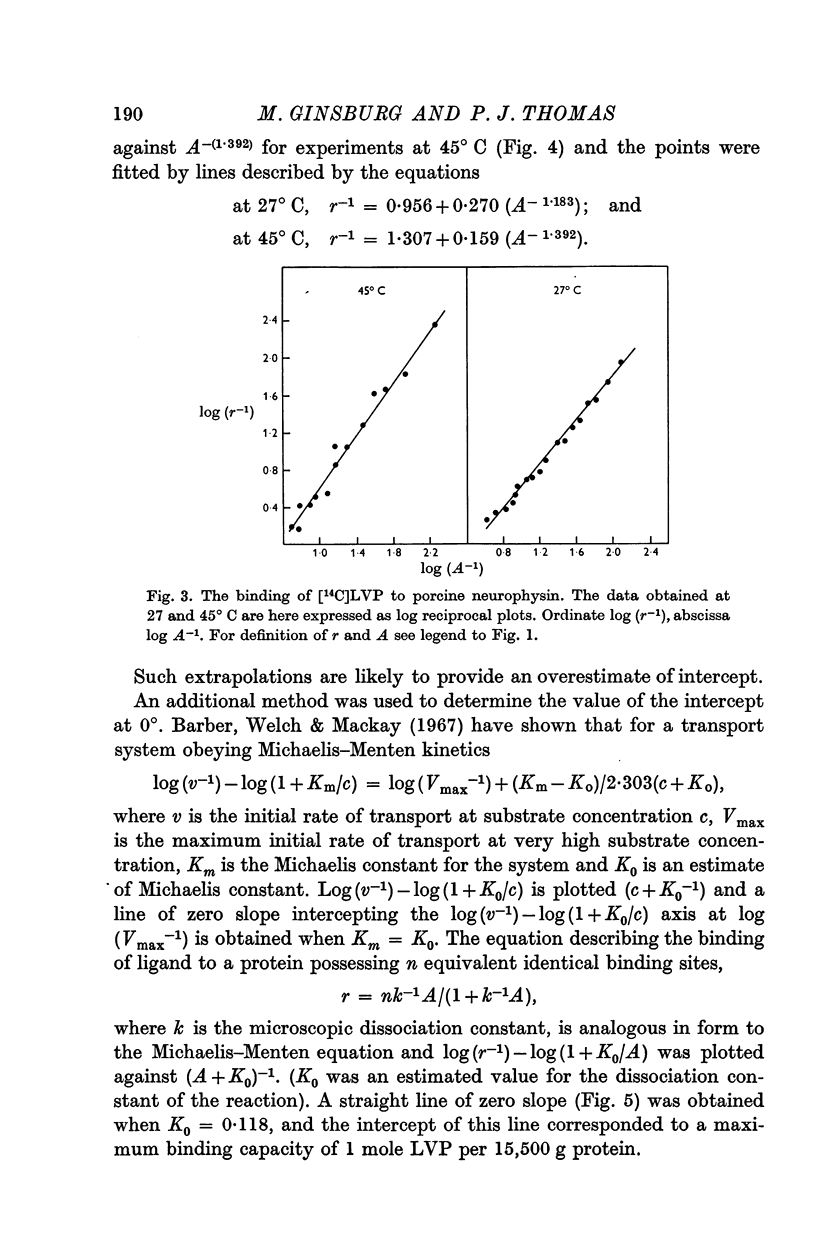
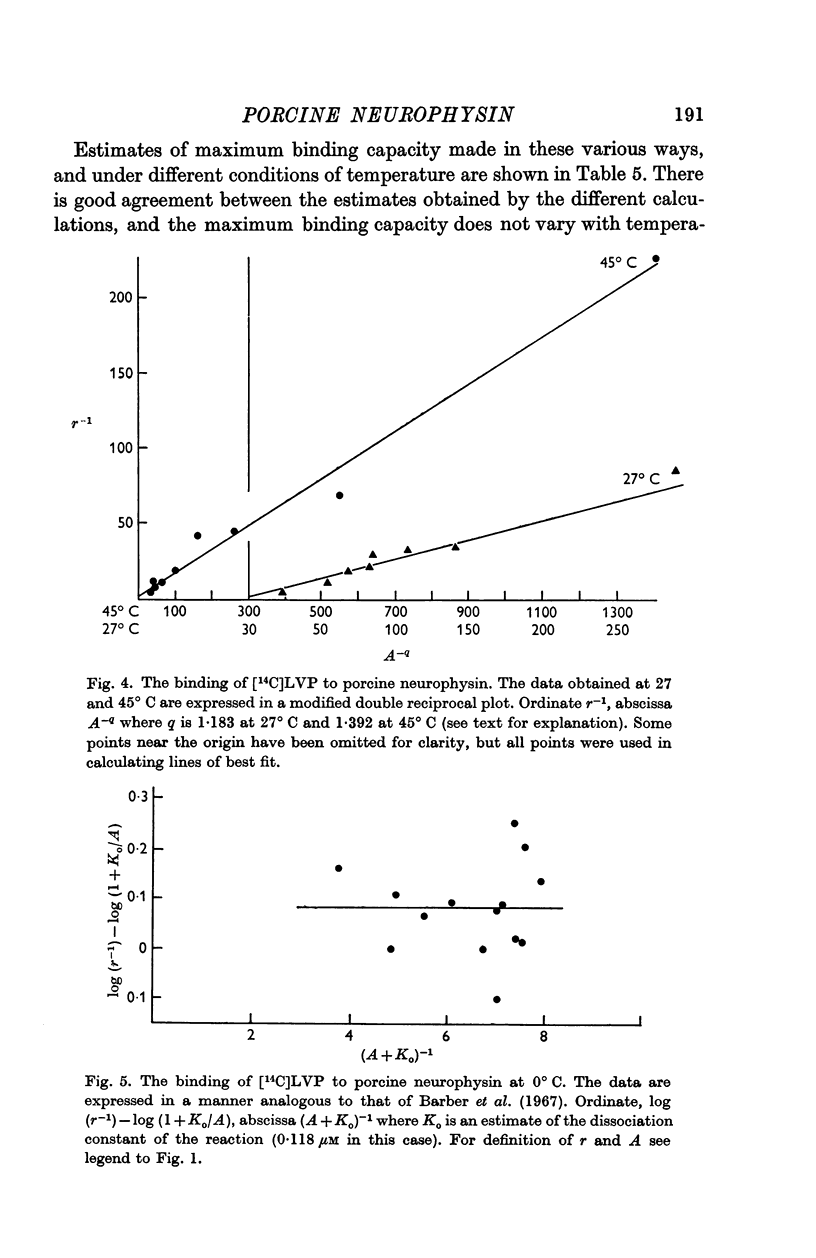
5. The binding of [14C]lysine vasopressin to porcine neurophysin was studied at 0, 27 and at 45° C, and double reciprocal plots of the binding were shown to be curvilinear at 27 and at 45° C and rectilinear at 0° C.
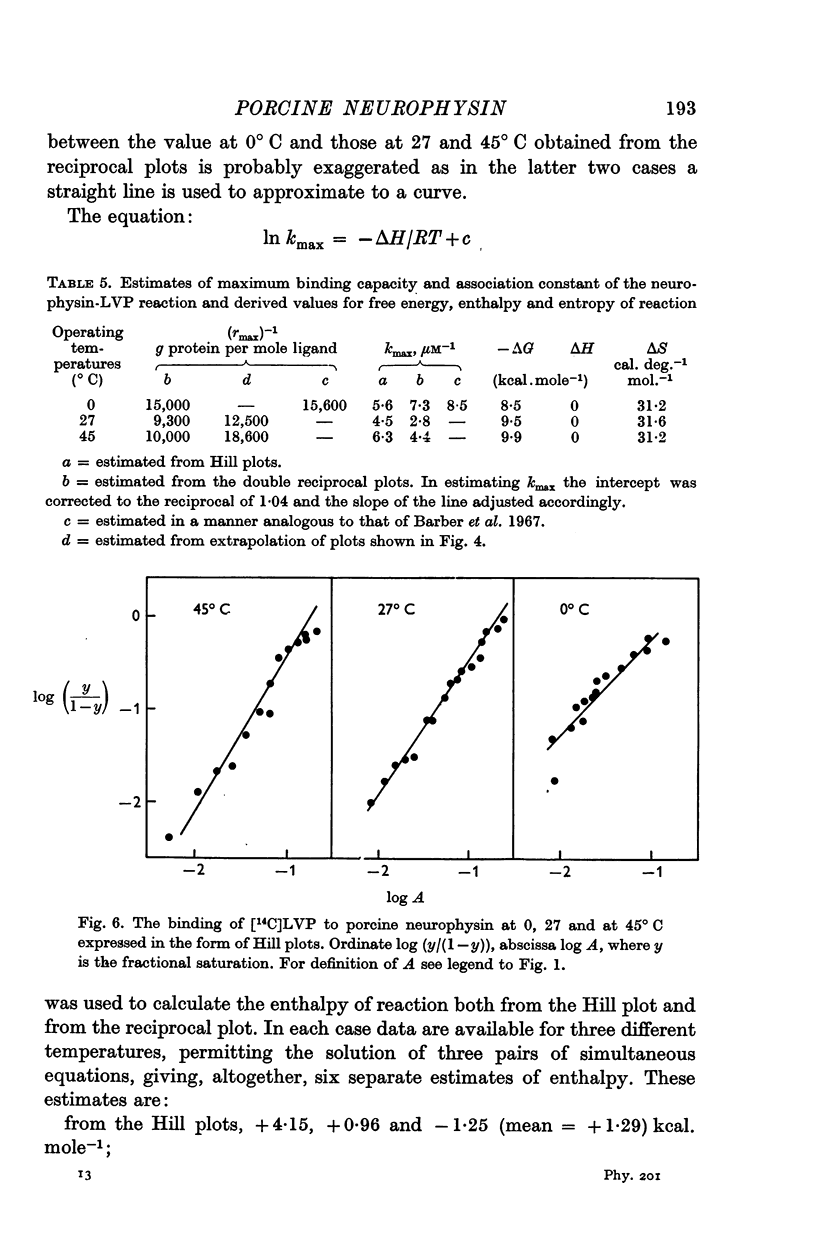
6. Concordant estimates for maximum binding capacity were obtained by extrapolations from the data at 27 and 45° C by applying two independent empirical methods of approximation; these agreed with the estimate obtained by extrapolation of the straight line, fitting data obtained at 0° C, being approximately 1 mole lysine vasopressin per 13,000 g protein.
7. The association constant and thermodynamic parameters of the reaction were estimated for near saturation conditions. The reaction is entropy driven.
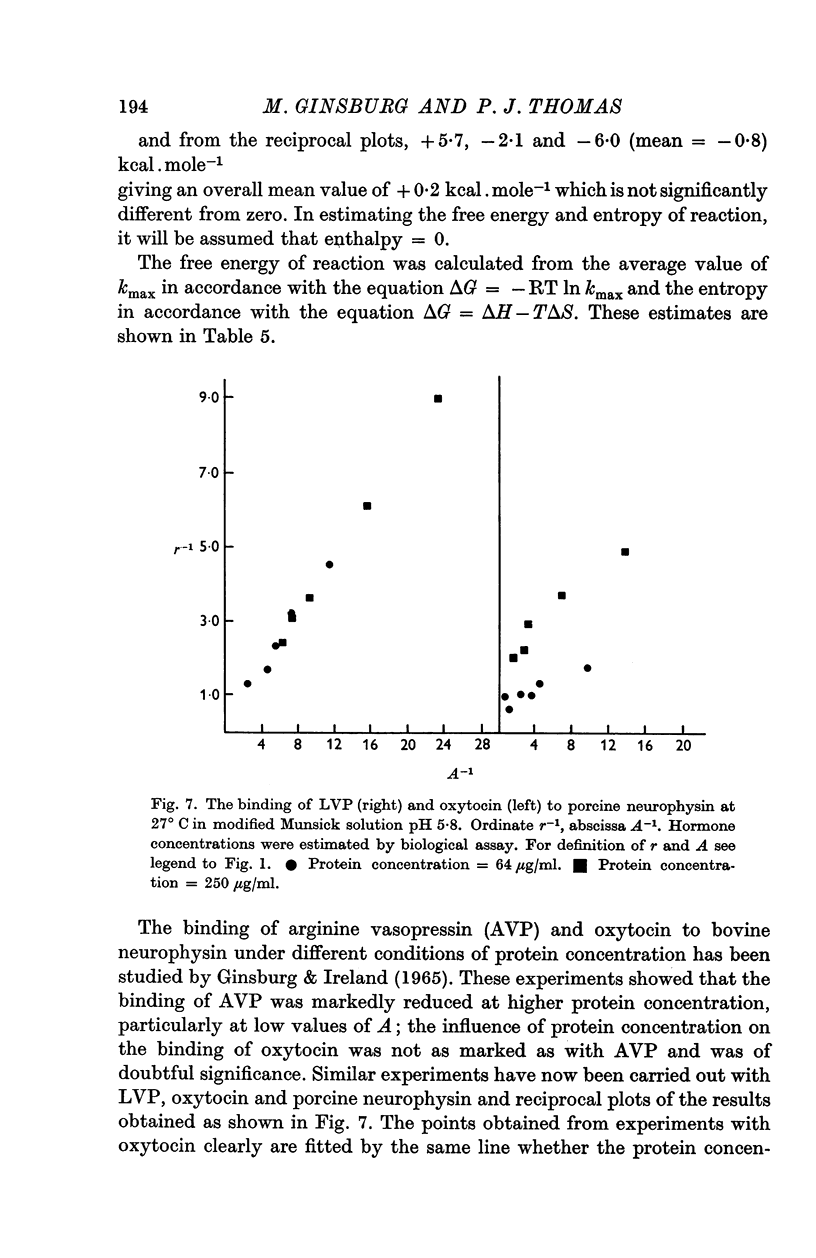
8. The binding of lysine vasopressin was found to be dependent on protein concentration. No dependence of oxytocin binding on protein concentration was apparent.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber H. E., Welch B. L., Mackay D. The use of the logarithmic transformation in the calculation of the transport parameters of a system that obeys Michaelis-Menten kinetics. Biochem J. 1967 Apr;103(1):251–255. doi: 10.1042/bj1030251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow C. C. On the average hydrophobicity of proteins and the relation between it and protein structure. J Theor Biol. 1967 Aug;16(2):187–211. doi: 10.1016/0022-5193(67)90004-5. [DOI] [PubMed] [Google Scholar]
- Breslow E., Abrash L. The binding of oxytocin and oxytocin analogues by purified bovine neurophysins. Proc Natl Acad Sci U S A. 1966 Aug;56(2):640–646. doi: 10.1073/pnas.56.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- GINSBURG M., IRELAND M. BINDING OF VASOPRESSIN AND OXYTOCIN TO PROTEIN IN EXTRACTS OF BOVINE AND RABBIT NEUROHYPOPHYSES. J Endocrinol. 1964 Aug;30:131–145. doi: 10.1677/joe.0.0300131. [DOI] [PubMed] [Google Scholar]
- GINSBURG M., IRELAND M. THE PREPARATION OF BOVINE NEUROPHYSIN AND THE ESTIMATION OF ITS MAXIMUM CAPACITY TO BIND OXYTOCIN AND ARGININE VASOPRESSIN. J Endocrinol. 1965 May;32:187–198. doi: 10.1677/joe.0.0320187. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Jayasena K., Thomas P. J. The preparation and properties of porcine neurophysin and the influence of calcium on the hormone-neurophysin complex. J Physiol. 1966 May;184(2):387–401. doi: 10.1113/jphysiol.1966.sp007921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. Fractionation of neurophysin by molecular-sieve and ion-exchange chromatography. Biochem J. 1967 Jul;104(1):122–127. doi: 10.1042/bj1040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Nichol L. W., Jackson W. J., Winzor D. J. A theoretical study of the binding of small molecules to a polymerizing protein system. A model for allosteric effects. Biochemistry. 1967 Aug;6(8):2449–2456. doi: 10.1021/bi00860a022. [DOI] [PubMed] [Google Scholar]


