Abstract
1. We have investigated the conductivity of neural pathways in slabs of unanaesthetized, isolated, cerebral cortex, cut from the isolated forebrains of twenty-five cats.
2. Neurones within the isolated area were indirectly excited, either by a small electrode thrust into the subcortical white matter, or by remote stimulation of the pial surface. Sometimes a small electrode was employed for intracortical stimulation.
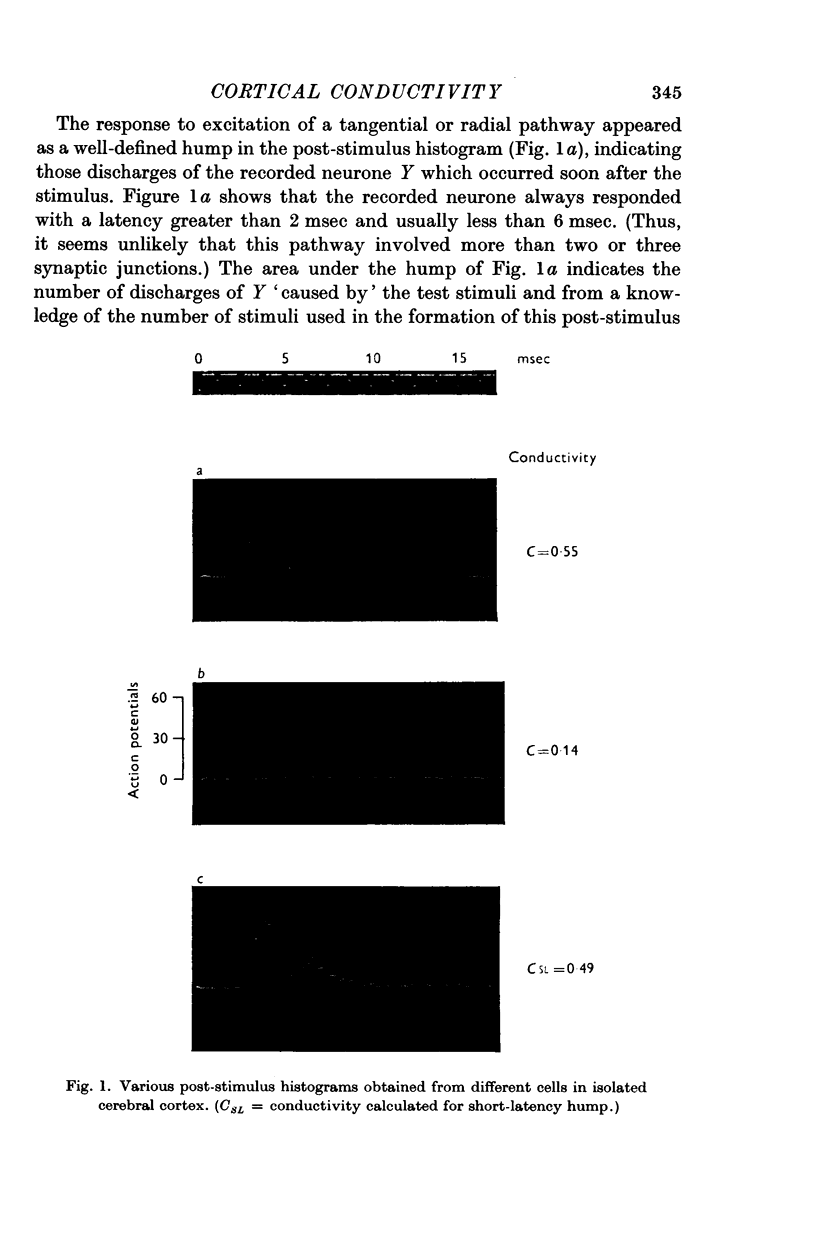
3. The response of single neurones to these stimuli was recorded with extracellular micropipettes. Submaximal stimuli produced a stochastic response which was measured from the post-stimulus histogram (PSH) and provided an estimate of the probability of discharge at various times after the stimulus.
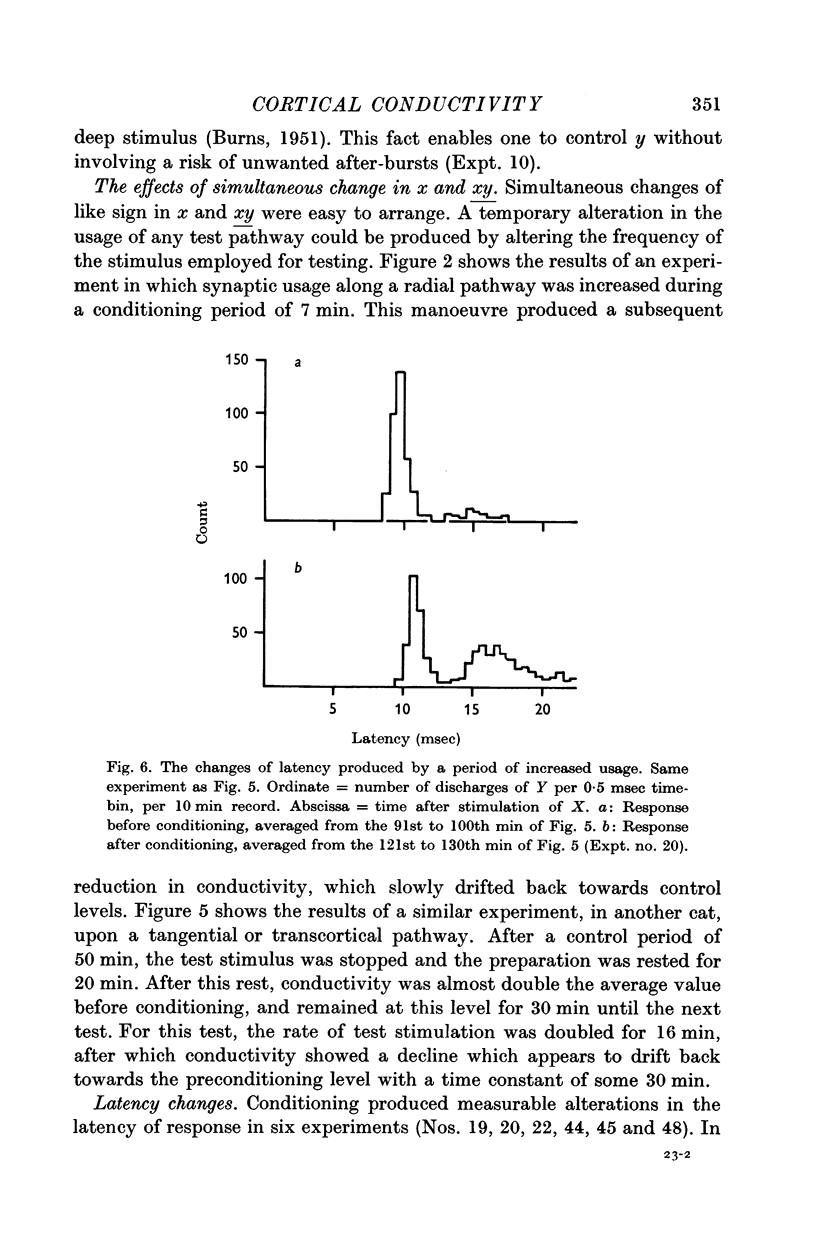
4. The PSH often displayed several discrete humps of different latencies, indicating several pathways between stimulated and recording point. Conductivity measurements were usually restricted to the pathway of shortest latency.
5. The conductivity of a pathway was defined as C = xy/x, averaged over 1 or 2 min, where
x = frequency of afferent test volleys,
xy = frequency of response: i.e. of those action potentials contained within a well-defined hump of the PSH,
(y = frequency of all discharges of the recorded neurone).
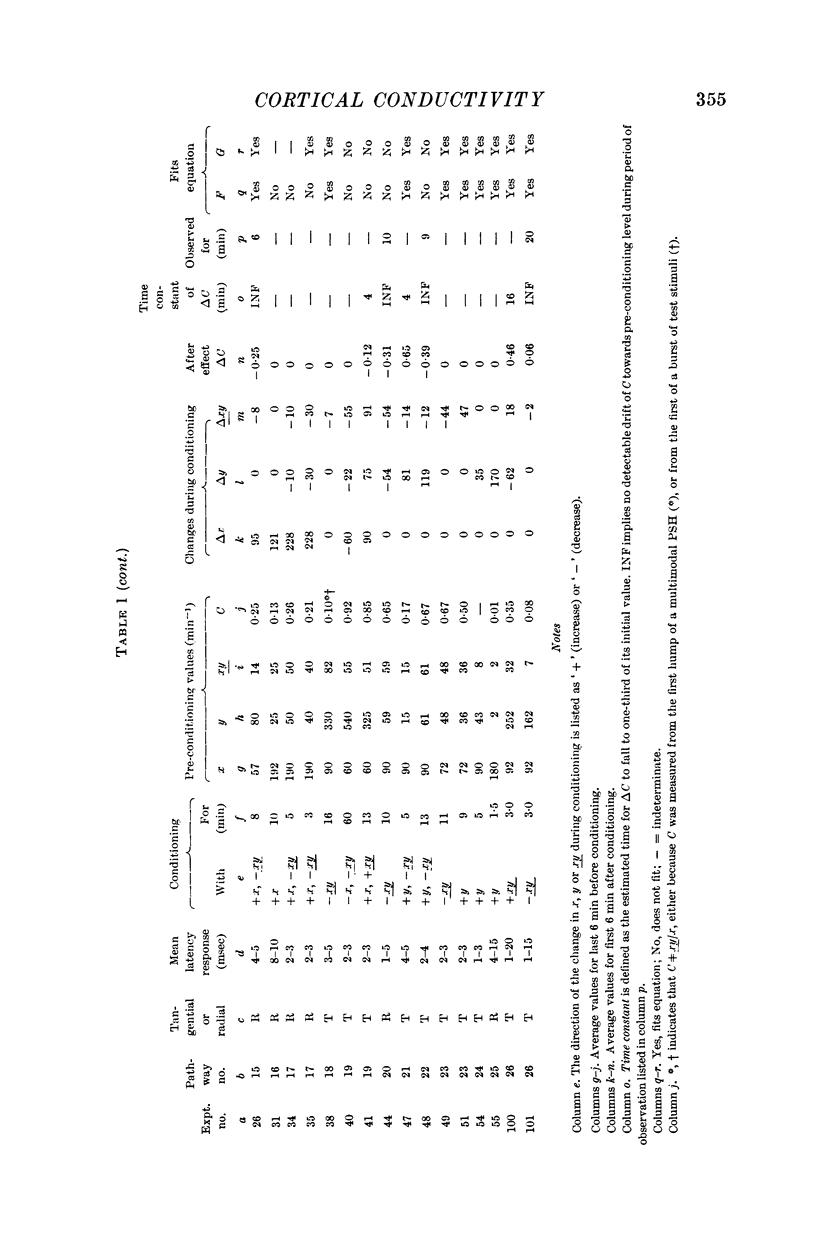
6. The progress of conductivity was tested with some constant form of cortical stimulation, repeated at regular intervals of 1-5 sec. Reliable results were obtained for twenty-six pathways subjected to thirty-eight experiments.
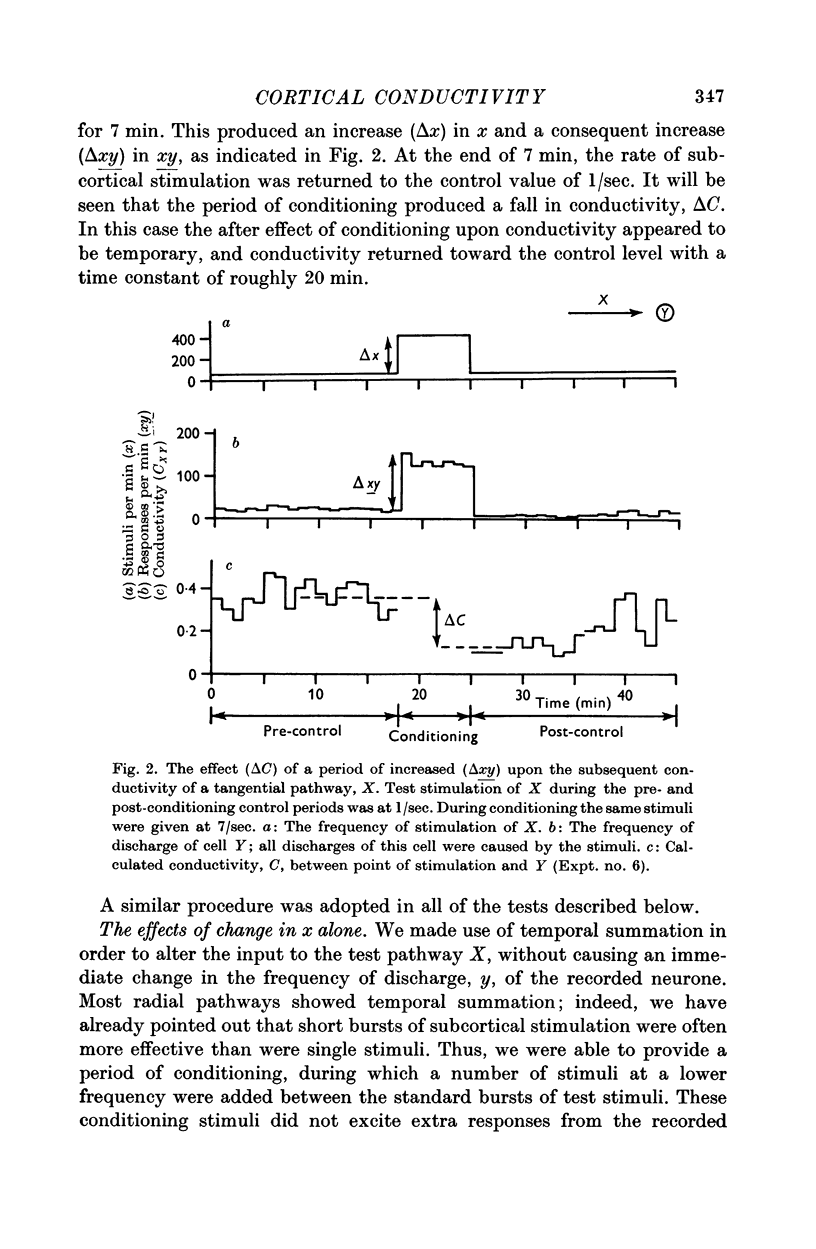
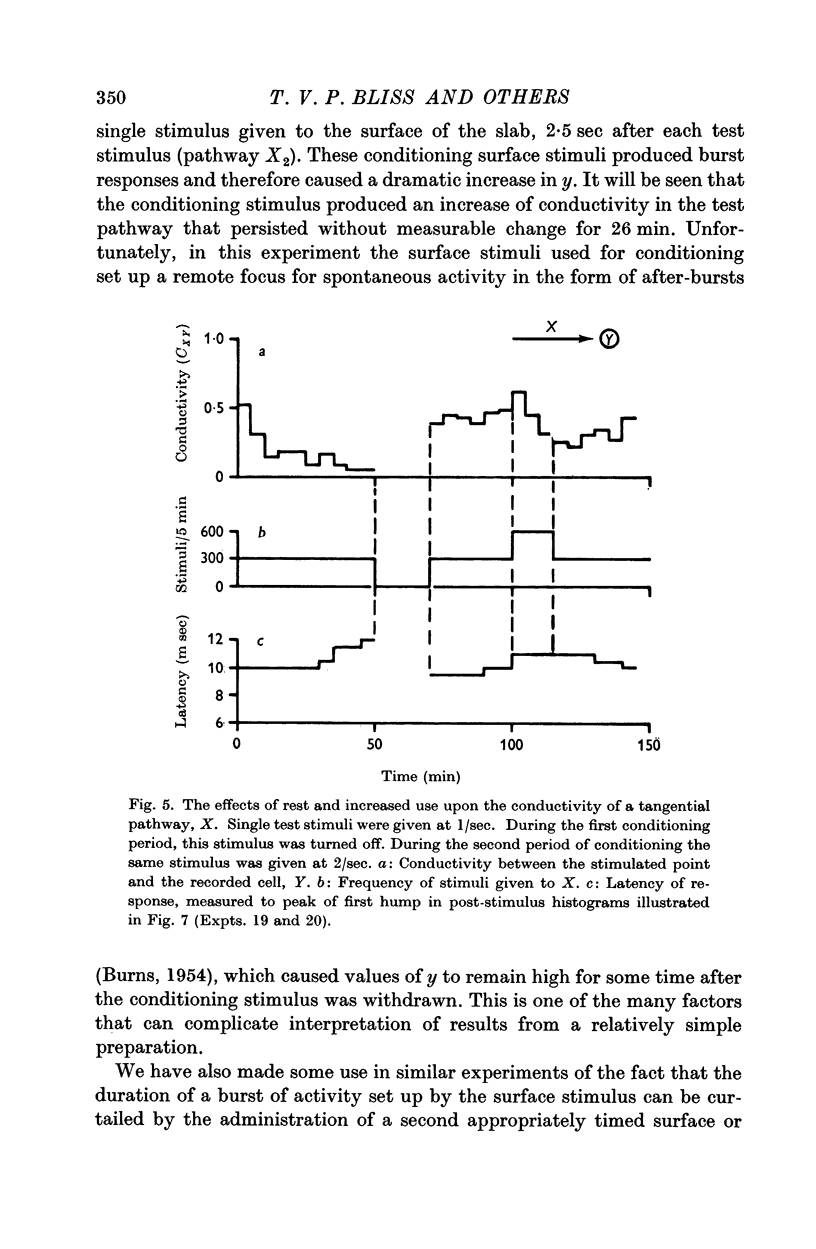
7. Temporary alteration of x, y or xy (conditioning with Δx, Δy or Δxy), for a period of 6-25 min, often caused a subsequent change in the conductivity (ΔC) of a pathway which sometimes attenuated with a time constant of about 10 min, but which could persist without detectable attenuation for 20-30 min.
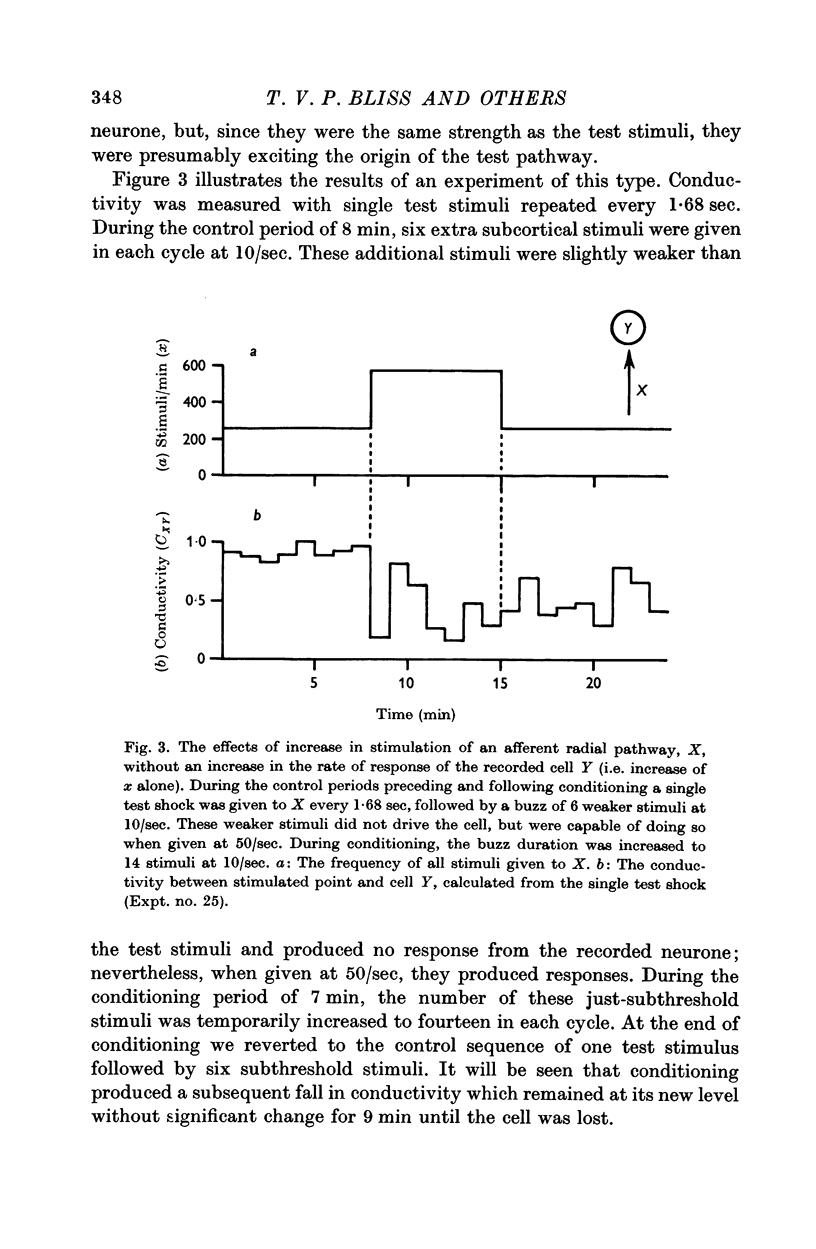
8. Conditioning periods less than 6 min rarely produced changes in conductivity; alterations of conductivity were more likely to be caused by conditioning periods longer than 17 min, than by periods of 6-16 min.
9. Changes in conductivity were usually correlated
negatively with temporary changes in xy,
negatively with temporary changes in x,
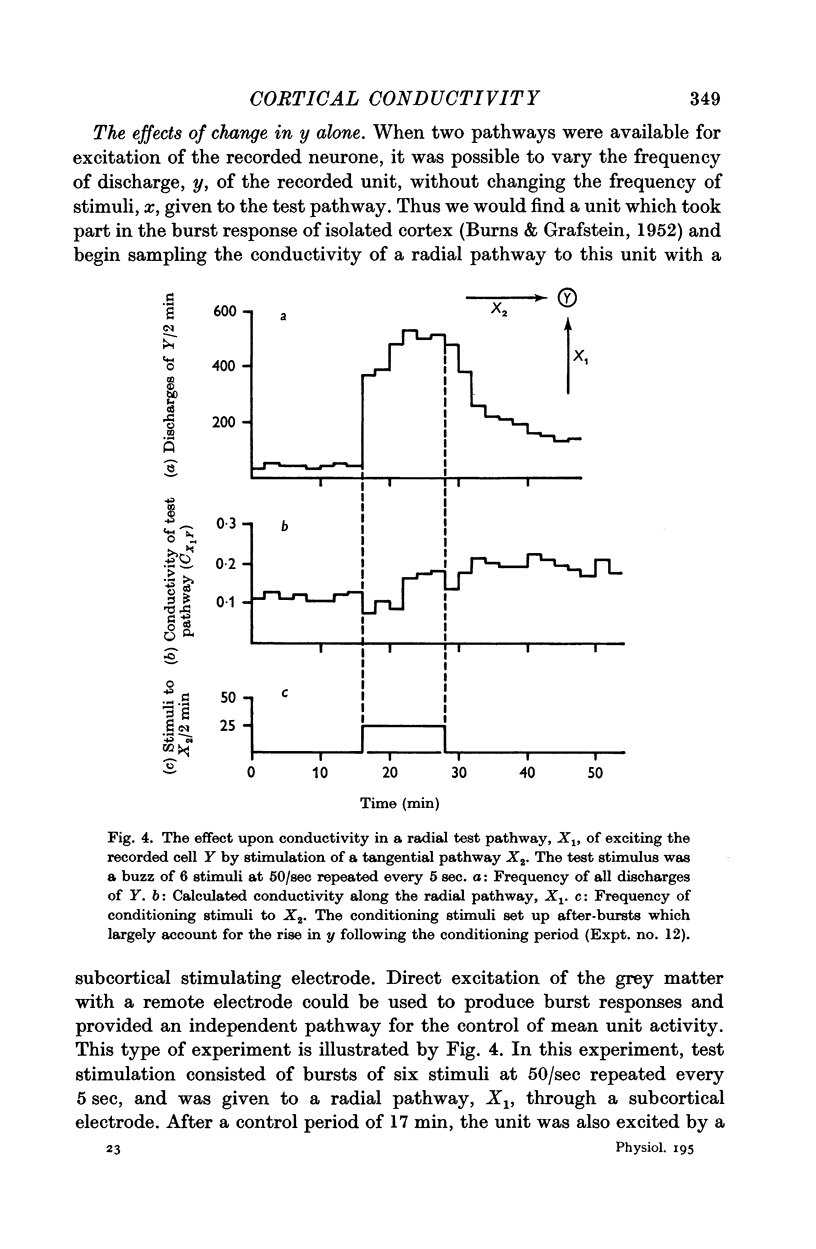
positively with temporary changes in y.
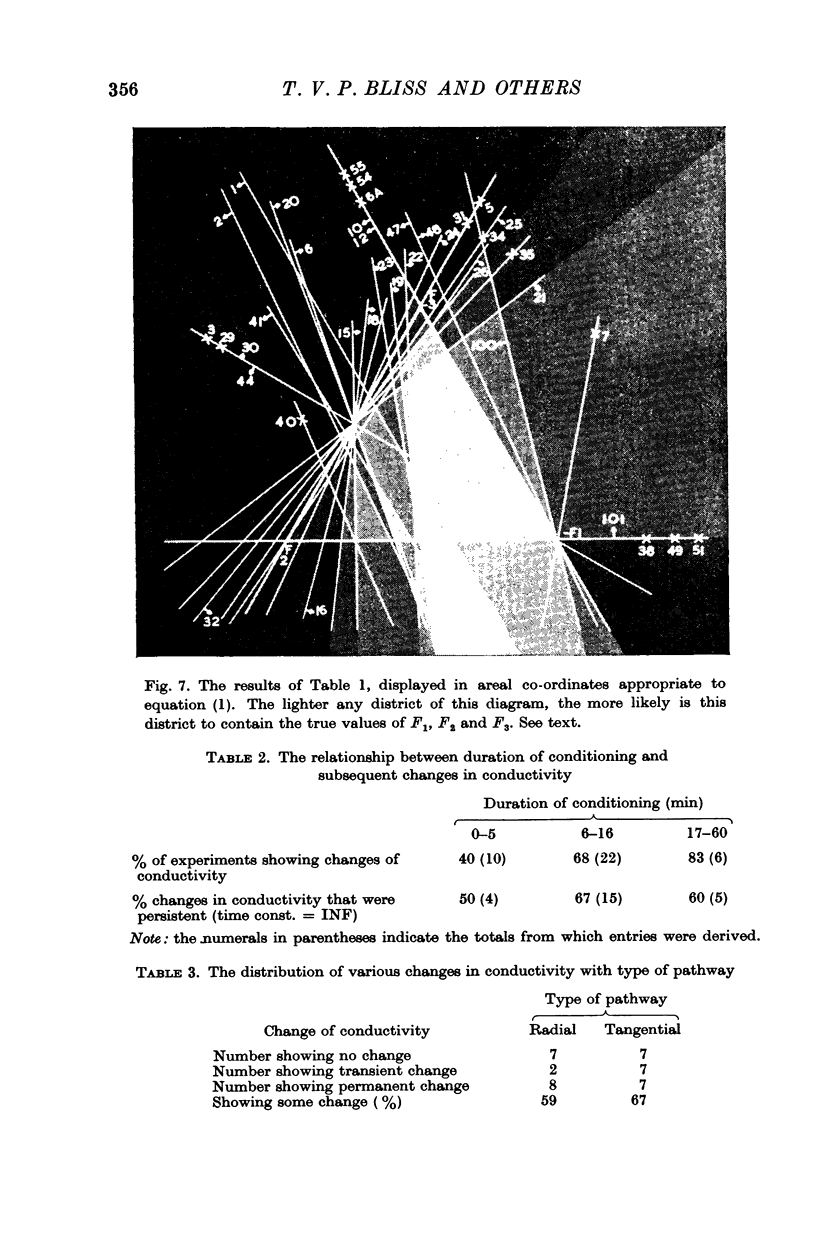
10. Nineteen of twenty-six pathways tested showed properties consistent with the formula [Formula: see text], where
G1 lies between -1·0 and -0·16, with mean value -0·50,
G2 lies between 0 and 0·42, with mean value +0·12,
G3 lies between -0·61 and 0 with mean value -0·38,
K is a coefficient which is usually different for each experiment.
Four out of twenty-six pathways so tested provided results which did not fit this formula; three out of twenty-six pathways did not give adequate information.
Full text
PDF









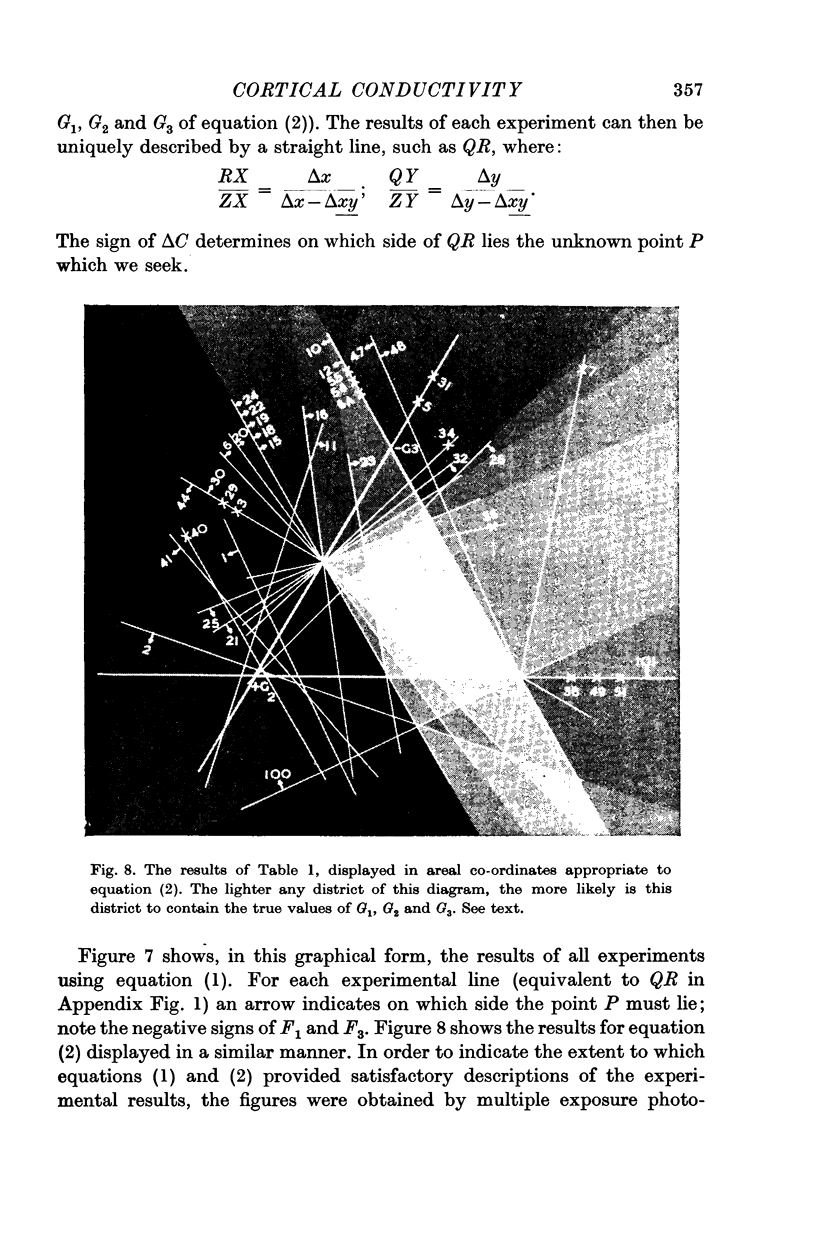



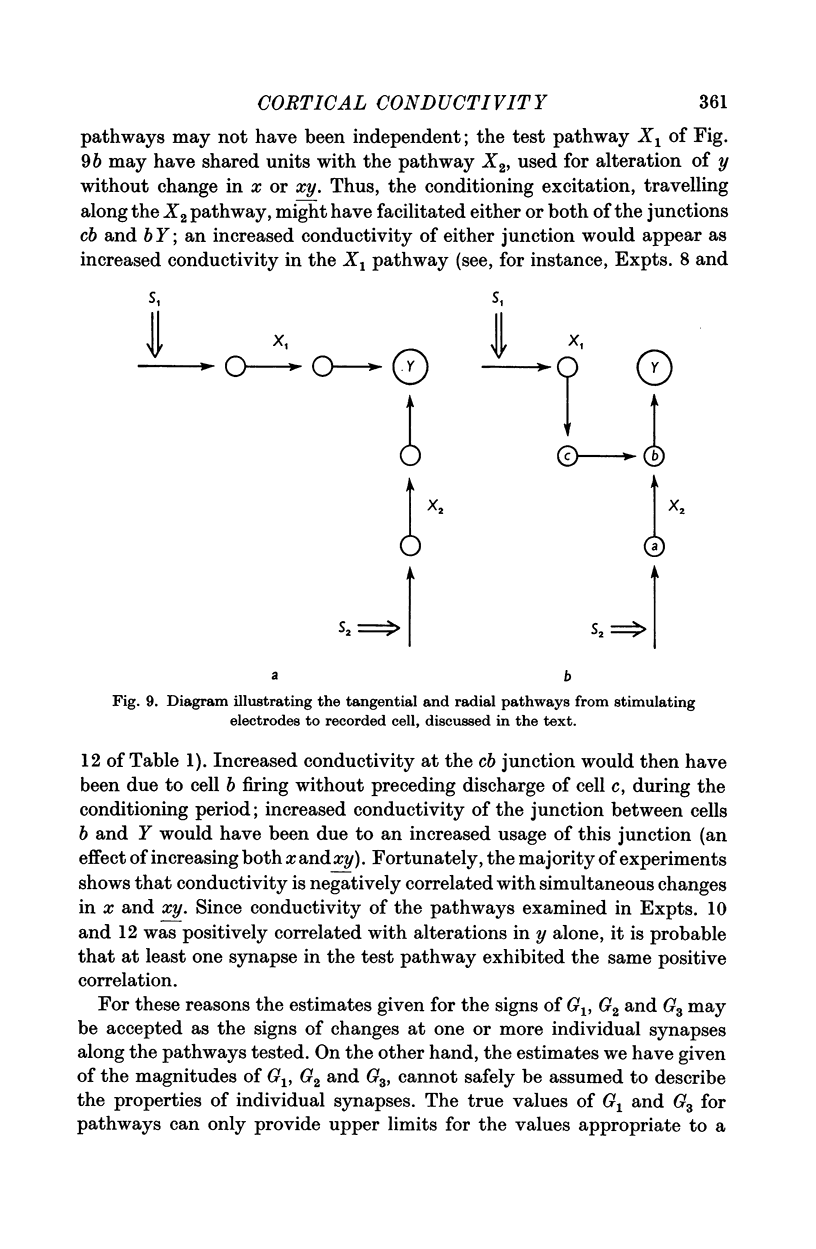



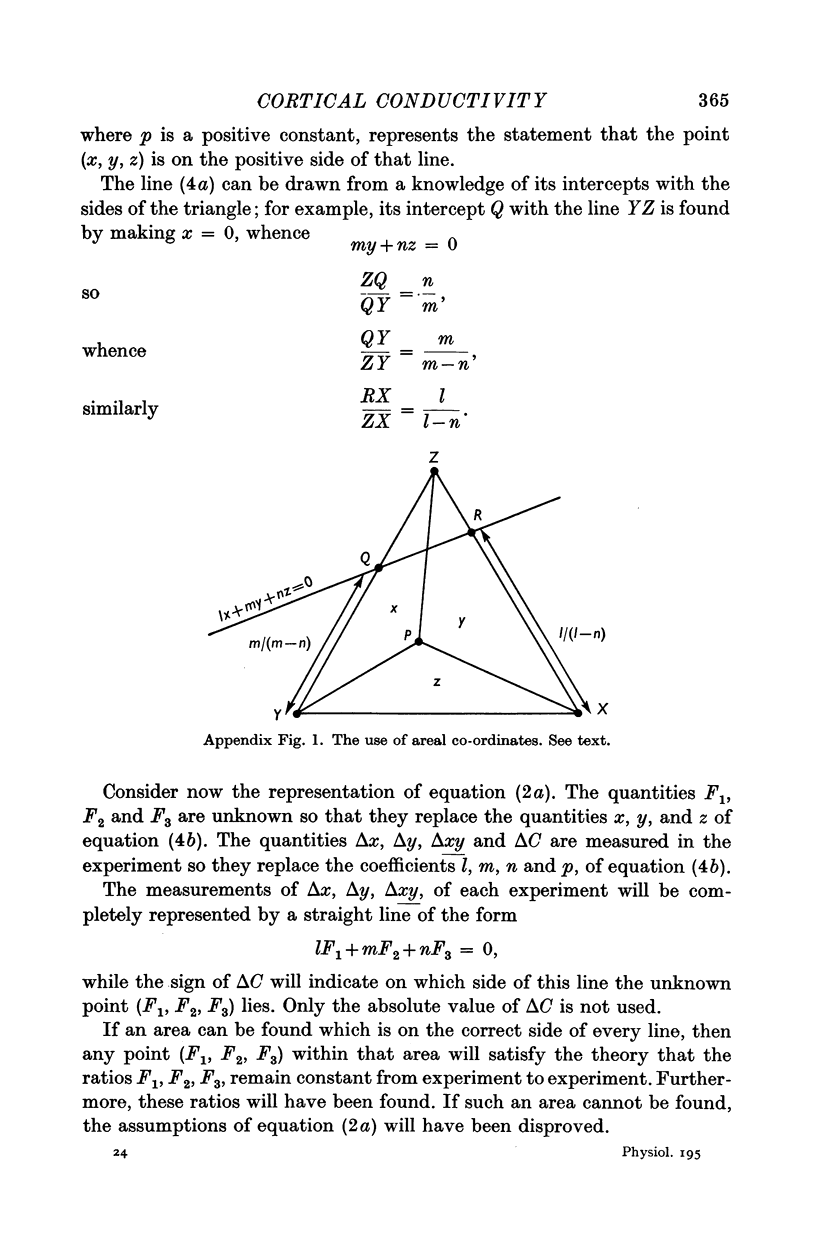


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BINDMAN L. J., LIPPOLD O. C., REDFEARN J. W. THE ACTION OF BRIEF POLARIZING CURRENTS ON THE CEREBRAL CORTEX OF THE RAT (1) DURING CURRENT FLOW AND (2) IN THE PRODUCTION OF LONG-LASTING AFTER-EFFECTS. J Physiol. 1964 Aug;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURES J., BURESOVA O. A study on the metabolic nature and physiological manifestations of the spreading EEG depression of Leao. Physiol Bohemoslov. 1956;5(Suppl):4–6. [PubMed] [Google Scholar]
- BURNS B. D., GRAFSTEIN B. The function and structure of some neurones in the cat's cerebral cortex. J Physiol. 1952 Nov;118(3):412–433. doi: 10.1113/jphysiol.1952.sp004805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B. D., HERON W., PRITCHARD R. Physiological excitation of visual cortex in cat's unanesthetized isolated forebrain. J Neurophysiol. 1962 Mar;25:165–181. doi: 10.1152/jn.1962.25.2.165. [DOI] [PubMed] [Google Scholar]
- BURNS B. D., SMITH G. K. Transmission of information in the unanaesthetized cat's isolated forebrain. J Physiol. 1962 Nov;164:238–251. doi: 10.1113/jphysiol.1962.sp007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B. D. Some properties of isolated cerebral cortex in the unanesthetized cat. J Physiol. 1951 Jan;112(1-2):156–175. doi: 10.1113/jphysiol.1951.sp004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B. D. The production of after-bursts in isolated unanesthetized cerebral cortex. J Physiol. 1954 Sep 28;125(3):427–446. doi: 10.1113/jphysiol.1954.sp005170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B. D. Use of extracellular microelectrodes. Methods Med Res. 1961;9:354–380. [PubMed] [Google Scholar]
- Brown G. L., von Euler U. S. The after effects of a tetanus on mammalian muscle. J Physiol. 1938 Jun 14;93(1):39–60. doi: 10.1113/jphysiol.1938.sp003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., McINTYRE A. K. The effects of disuse and of activity on mammalian spinal reflexes. J Physiol. 1953 Sep;121(3):492–516. doi: 10.1113/jphysiol.1953.sp004961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- MILNER P. M. The cell assembly: Mark II. Psychol Rev. 1957 Jul;64(4):242–252. doi: 10.1037/h0042287. [DOI] [PubMed] [Google Scholar]
- ROBSON J. G., BURNS B. D., WELT P. J. The effect of inhaling dilute nitrous oxide upon recent memory and time estimation. Can Anaesth Soc J. 1960 Oct;7:399–410. doi: 10.1007/BF03021298. [DOI] [PubMed] [Google Scholar]
- SHARPLESS S. K. REORGANIZATION OF FUNCTION IN THE NERVOUS SYSTEM--USE AND DISUSE. Annu Rev Physiol. 1964;26:357–388. doi: 10.1146/annurev.ph.26.030164.002041. [DOI] [PubMed] [Google Scholar]
- SMITH D. R., SMITH G. K. A STATISTICAL ANALYSIS OF THE CONTINUAL ACTIVITY OF SINGLE CORTICAL NEURONES IN THE CAT UNANAESTHETIZED ISOLATED FOREBRAIN. Biophys J. 1965 Jan;5:47–74. doi: 10.1016/s0006-3495(65)86702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]




