Abstract
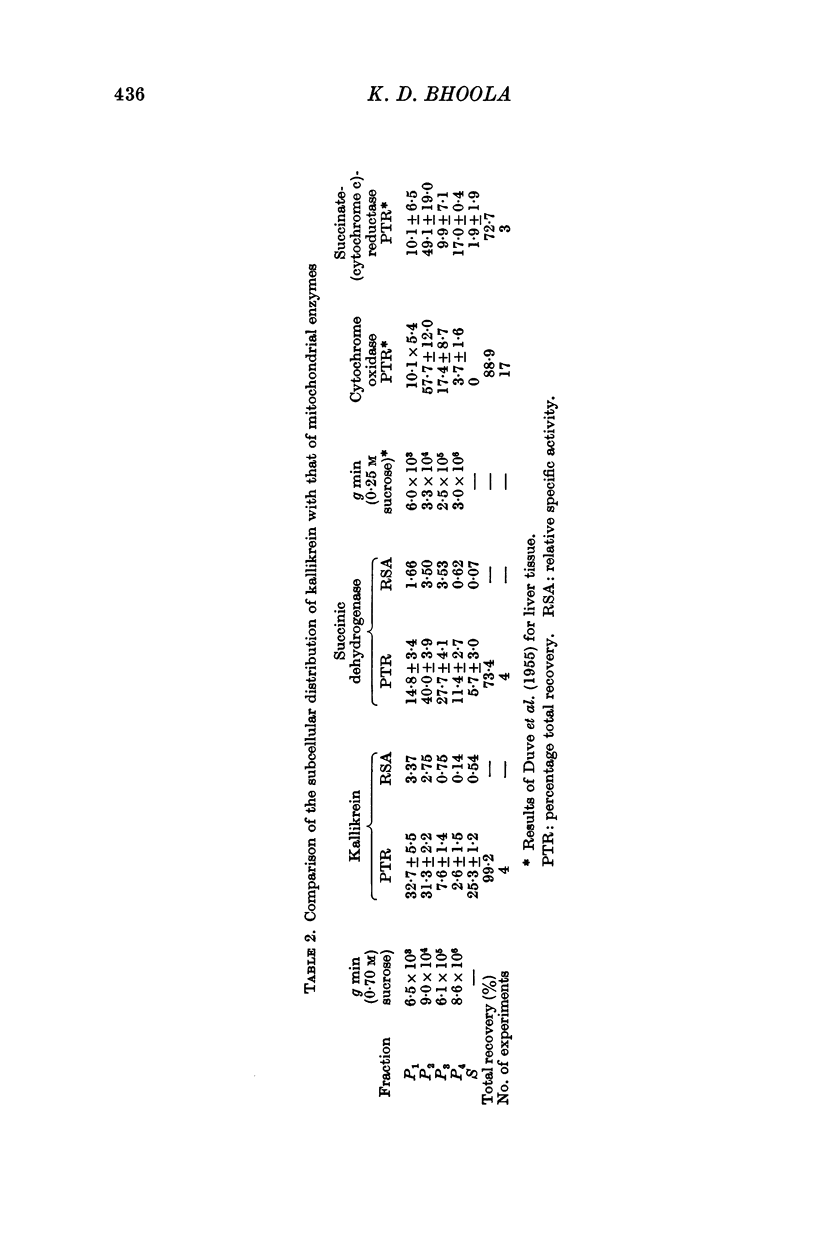
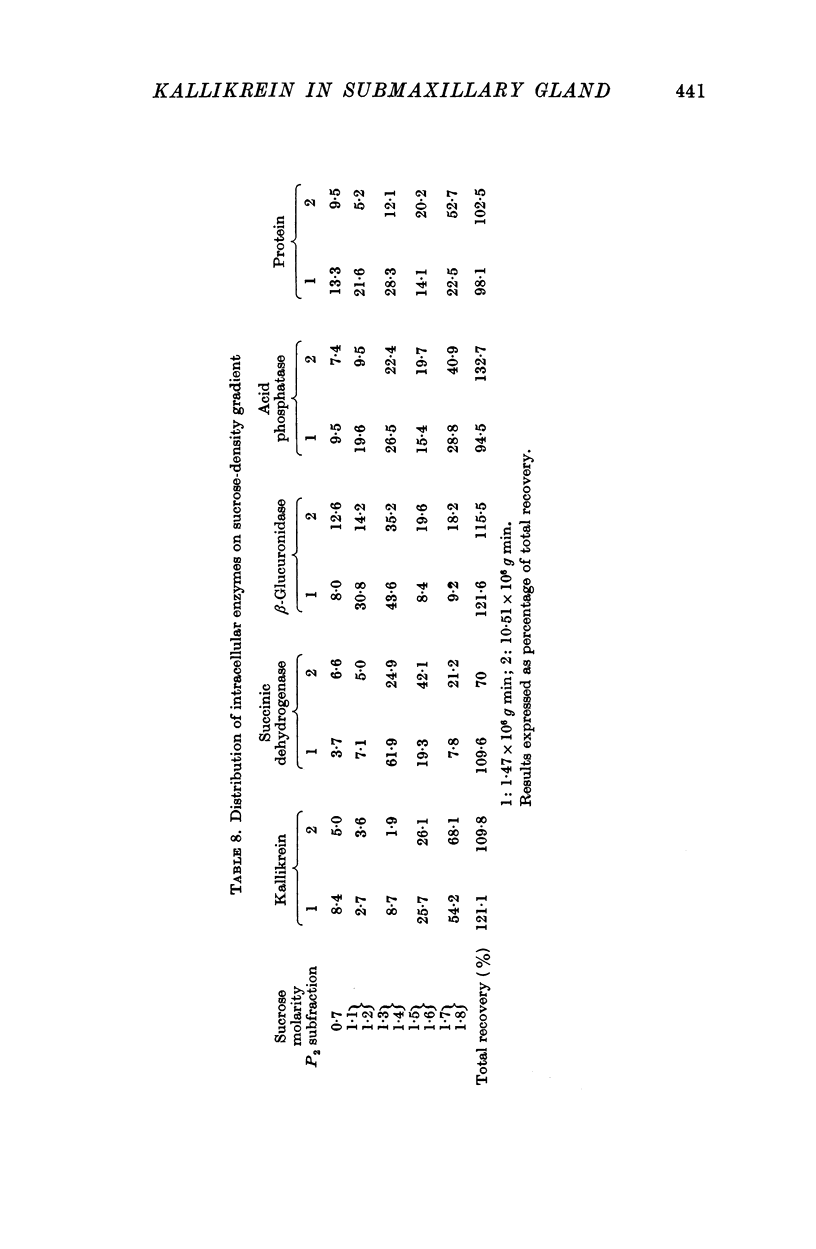
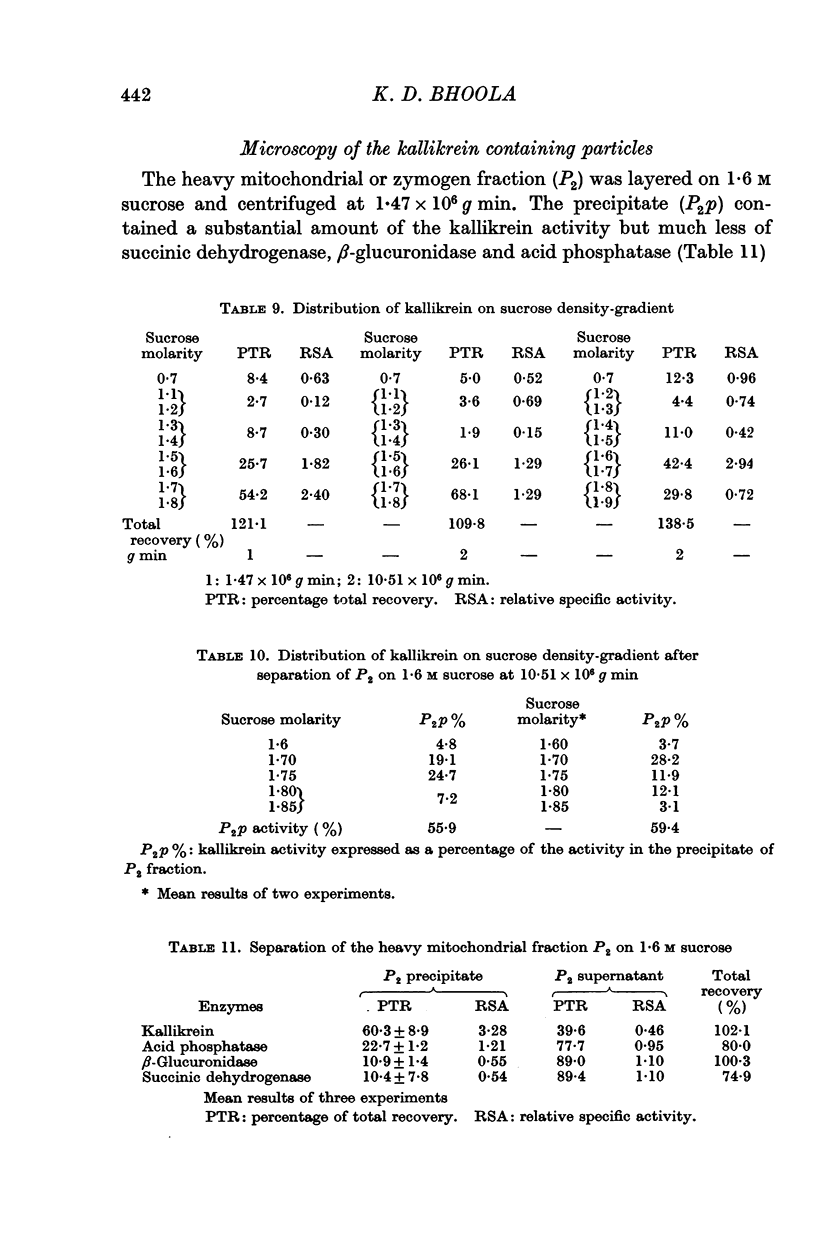
1. Differential centrifugation and sucrose density-gradient data indicate that kallikrein in the submaxillary gland is associated with particles which sediment with the nuclear and heavy mitochondrial fractions and equilibrate with 1·7-1·8 M sucrose.
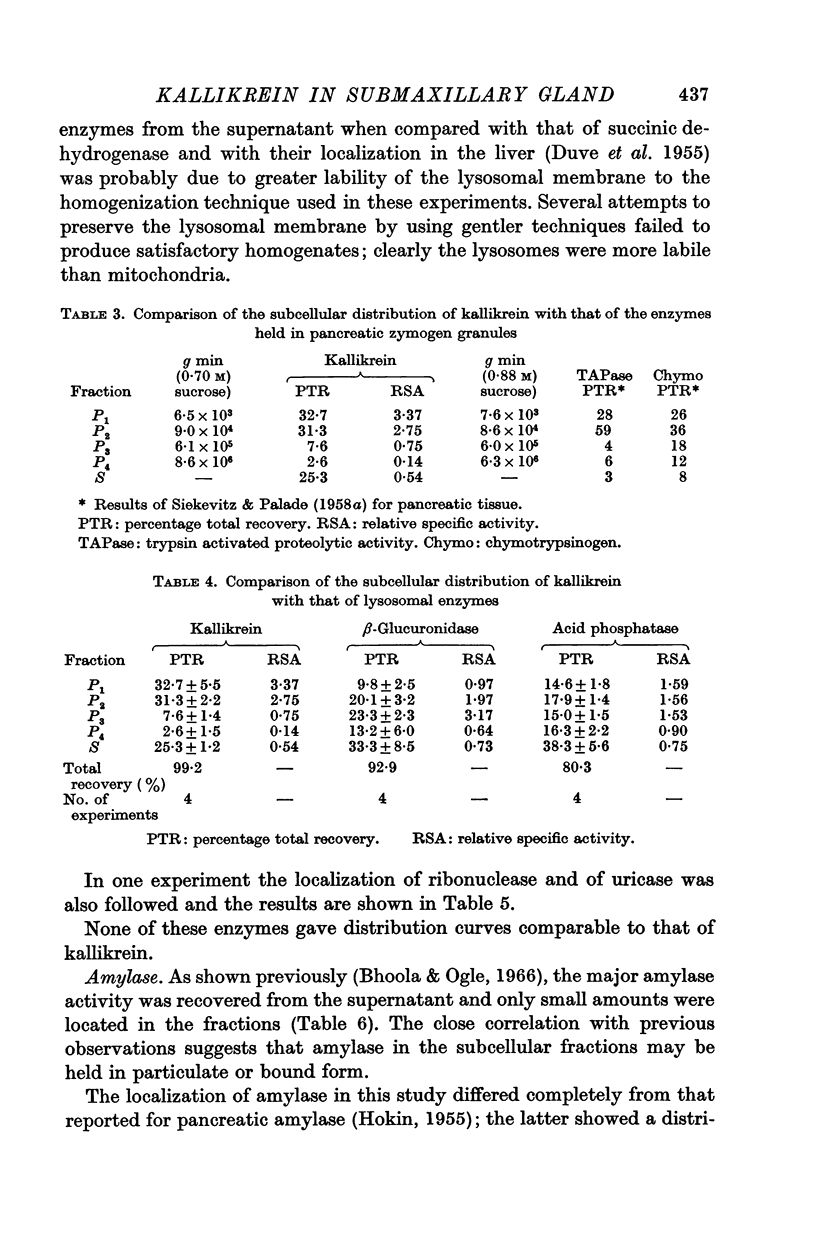
2. The sedimentation properties of the kallikrein containing particles differ from those of mitochondria and lysosomes, but resemble those of the pancreatic zymogen granules.
3. Histological observations indicate that kallikrein is associated with basophilic particles which stain positively with Periodic Acid-Schiff reagent.
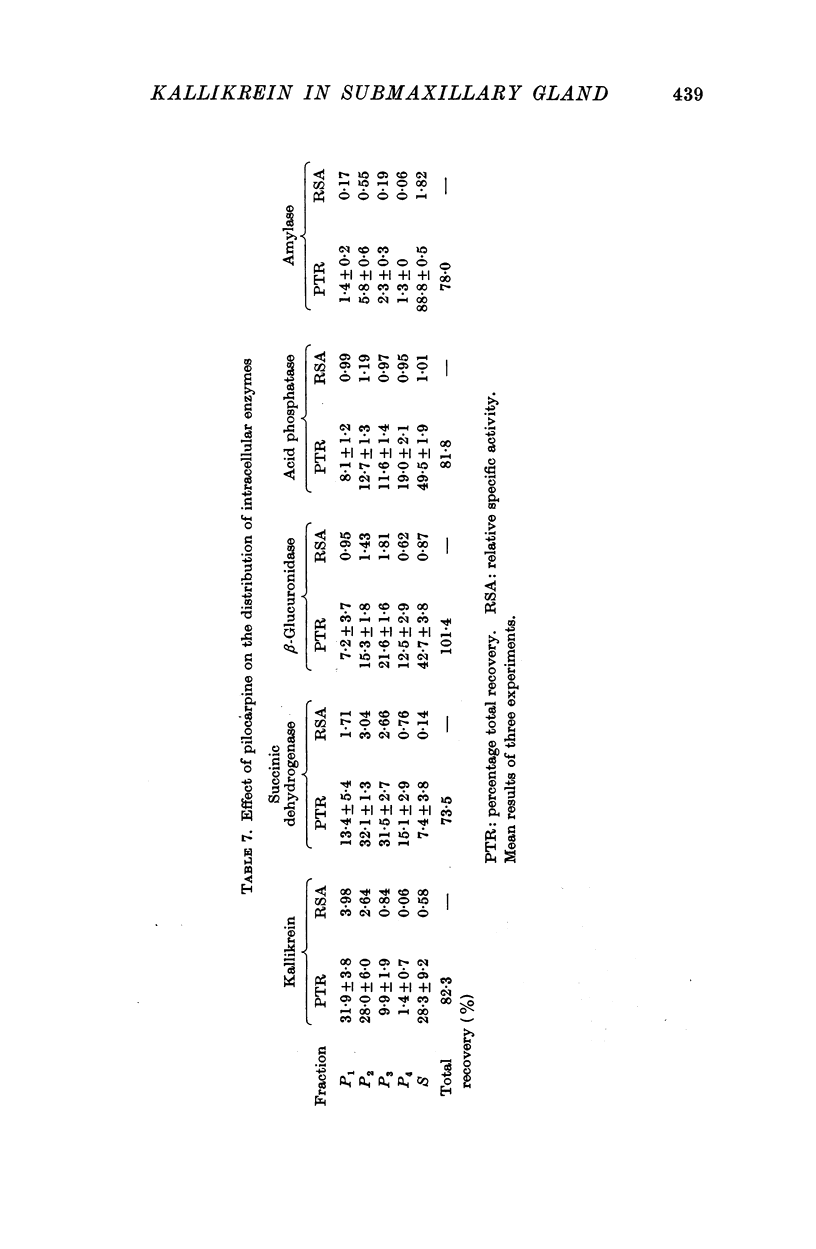
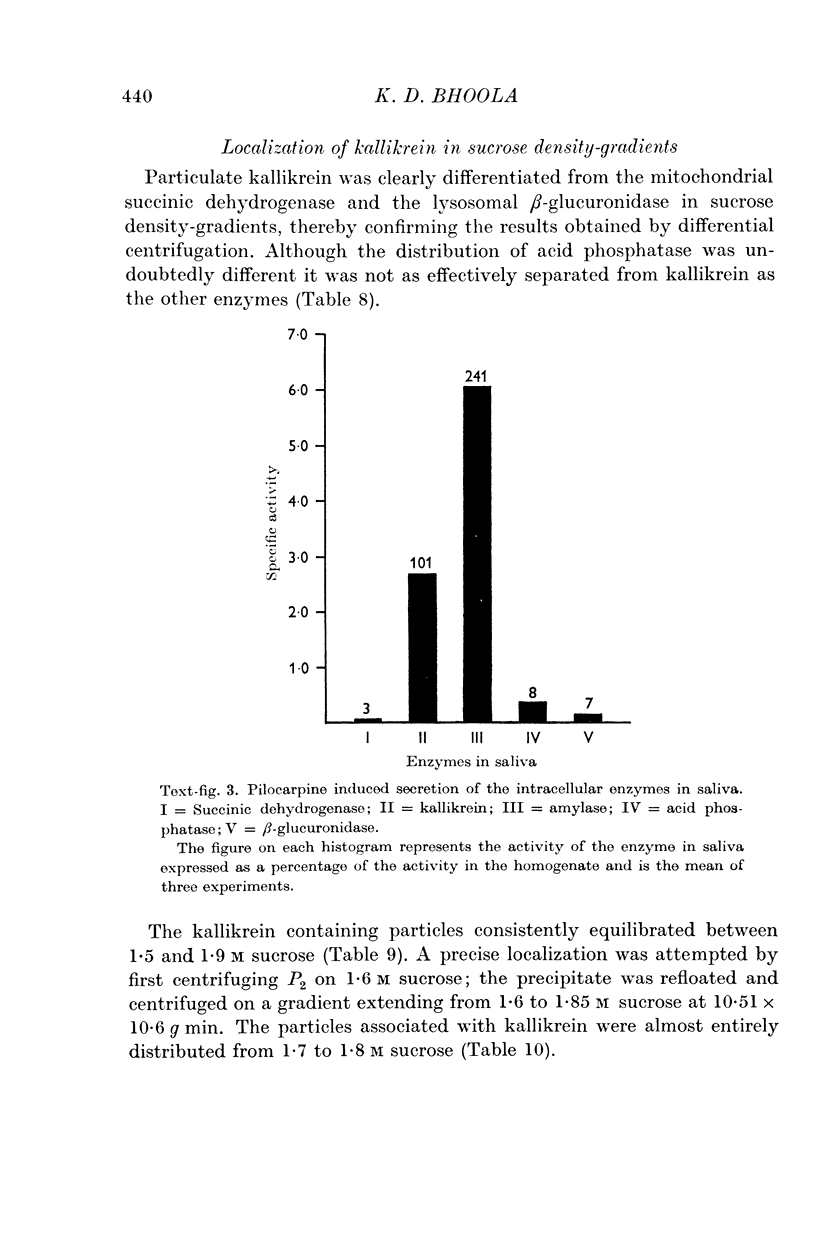
4. Pilocarpine causes a preferential secretion of kallikrein and amylase into saliva.
5. The results would support an exocrine role for submaxillary kallikrein.
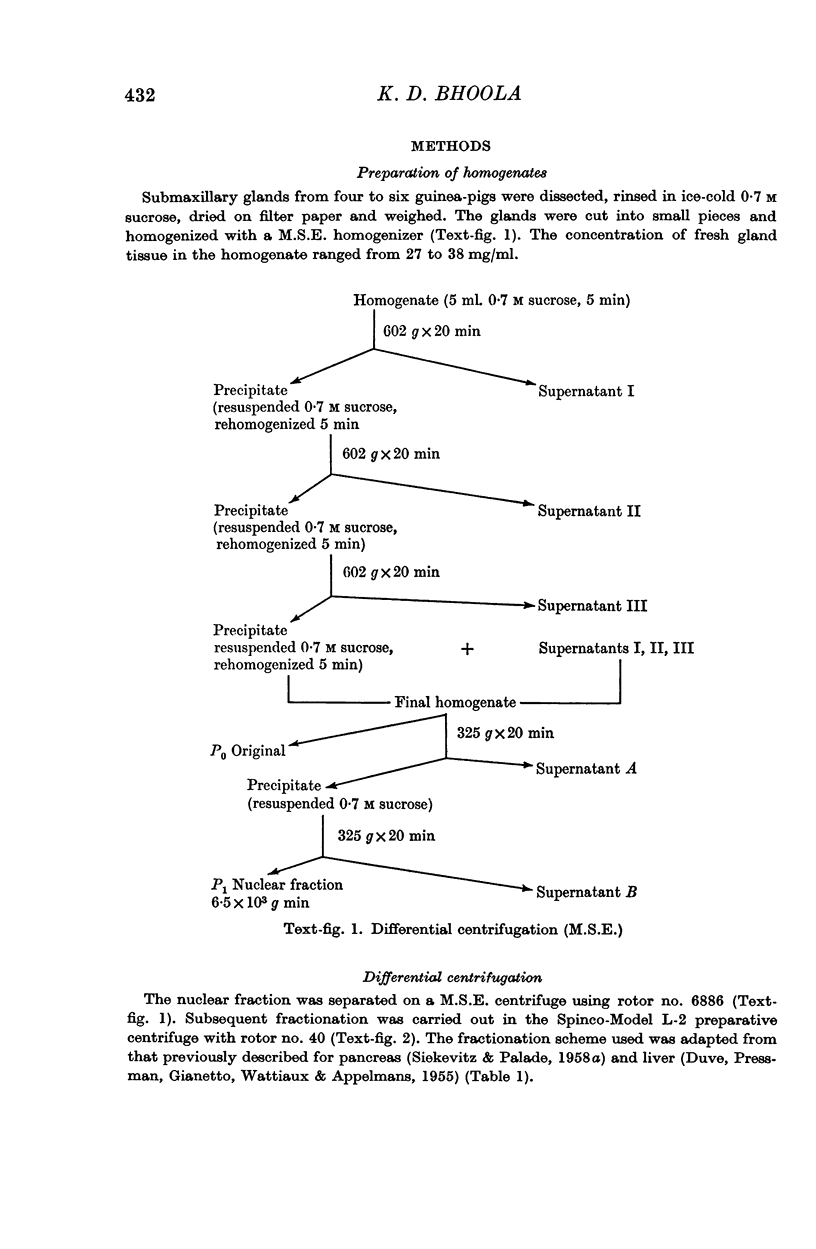
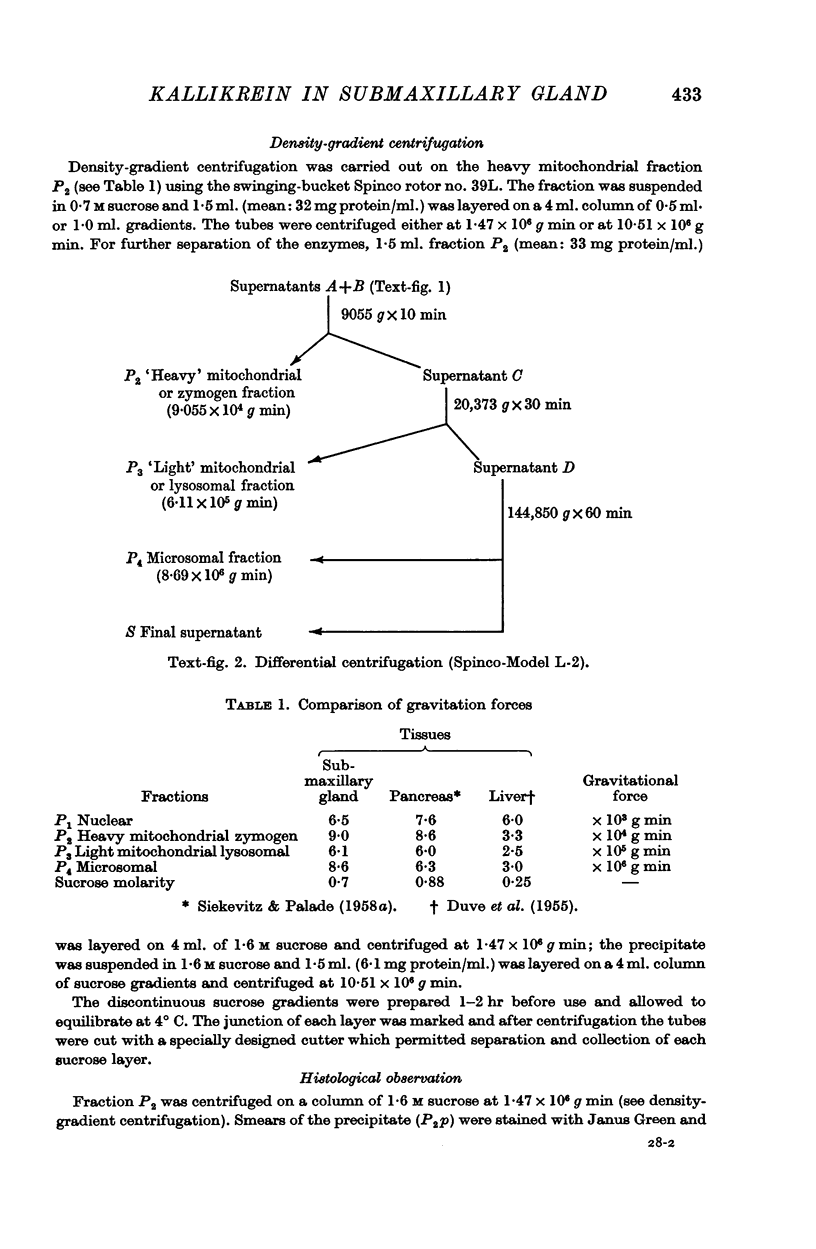
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTHET J., DE DUVE C. Tissue fractionation studies. I. The existence of a mitochondria-linked, enzymically inactive form of acid phosphatase in rat-liver tissue. Biochem J. 1951 Dec;50(2):174–181. doi: 10.1042/bj0500174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Jacques P., Baudhuin P., Sellinger O. Z., Berthet J., De Duve C. Tissue fractionation studies. 18. Resolution of mitochondrial fractions from rat liver into three distinct populations of cytoplasmic particles by means of density equilibration in various gradients. Biochem J. 1964 Jul;92(1):184–205. doi: 10.1042/bj0920184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhoola K. D., Ogle C. W. The subcellular localization of kallikrein, amylase and acetylcholine in the submaxillary gland of the guinea-pig. J Physiol. 1966 Jun;184(3):663–672. doi: 10.1113/jphysiol.1966.sp007939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLDSTOCK D. J., MATHIAS A. P., SCHACHTER M. A comparative study of kinin, kallidin, and bradykinin. Br J Pharmacol Chemother. 1957 Jun;12(2):149–158. doi: 10.1111/j.1476-5381.1957.tb00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERR L. M. H., LEVVY G. A. The preparation and properties of glucuronidase, the fractionation of buffered water homogenates. Biochem J. 1951 Feb;48(2):209–216. doi: 10.1042/bj0480209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENT B., NORBERG B. Phosphatase assay in serum with p-nitrophenyl phosphate as substrate. Scalpel (Brux) 1960;12:154–157. doi: 10.3109/00365516009062417. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. I. Isolation and enzymatic activities of cell fractions. J Biophys Biochem Cytol. 1958 Mar 25;4(2):203–218. doi: 10.1083/jcb.4.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. II. Functional variations in the enzymatic activity of microsomes. J Biophys Biochem Cytol. 1958 May 25;4(3):309–318. doi: 10.1083/jcb.4.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITTAKER V. P. The isolation and characterization of acetylcholine-containing particles from brain. Biochem J. 1959 Aug;72:694–706. doi: 10.1042/bj0720694. [DOI] [PMC free article] [PubMed] [Google Scholar]



