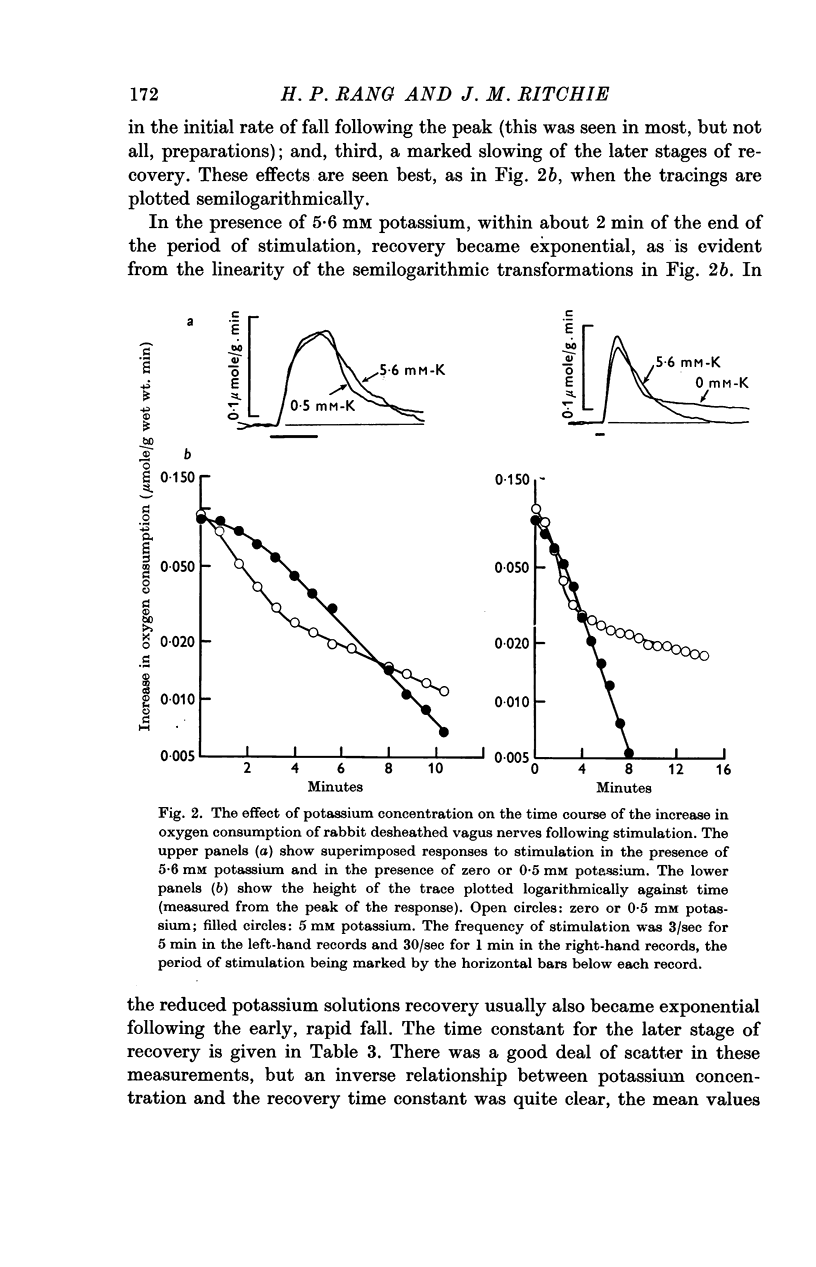
Abstract
1. A study has been made of the effect of potassium and other cations on the oxygen consumption of rabbit desheathed vagus nerves at rest and during activity.
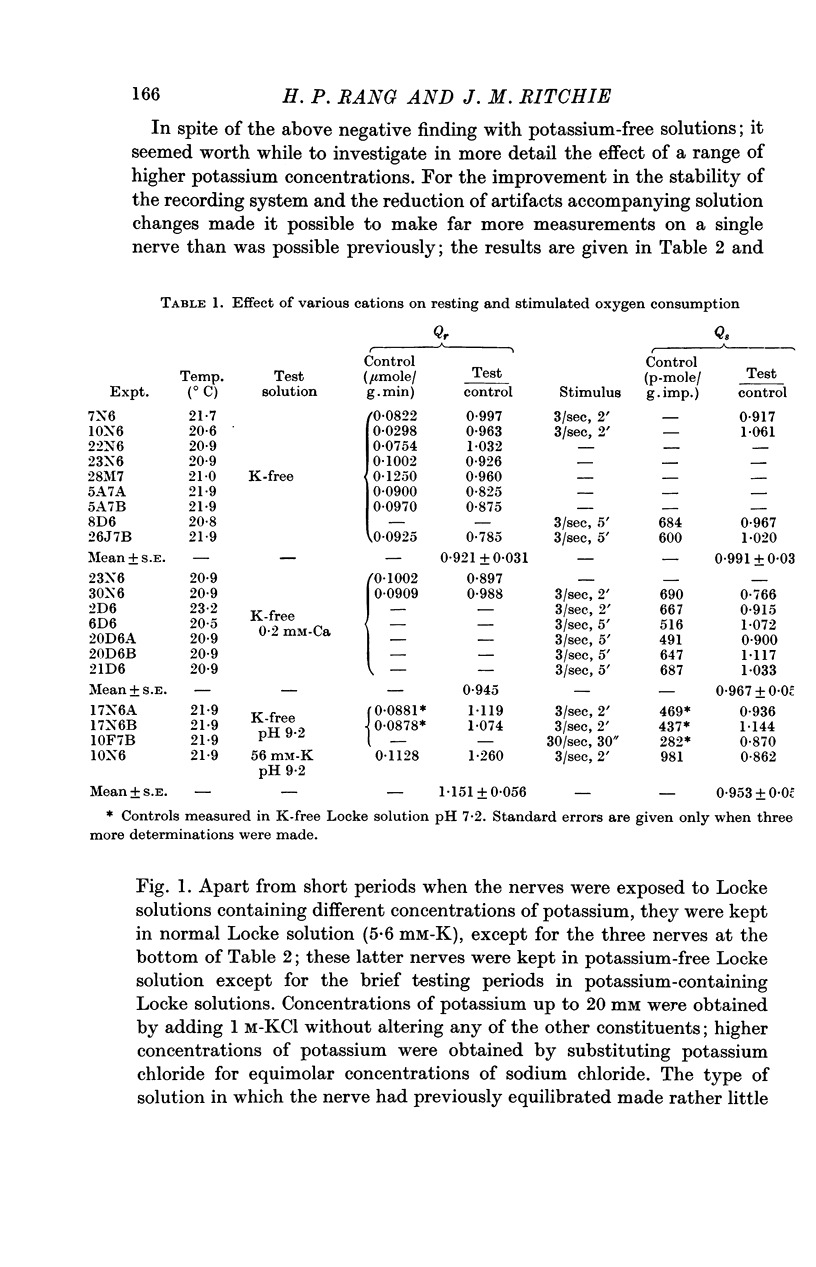
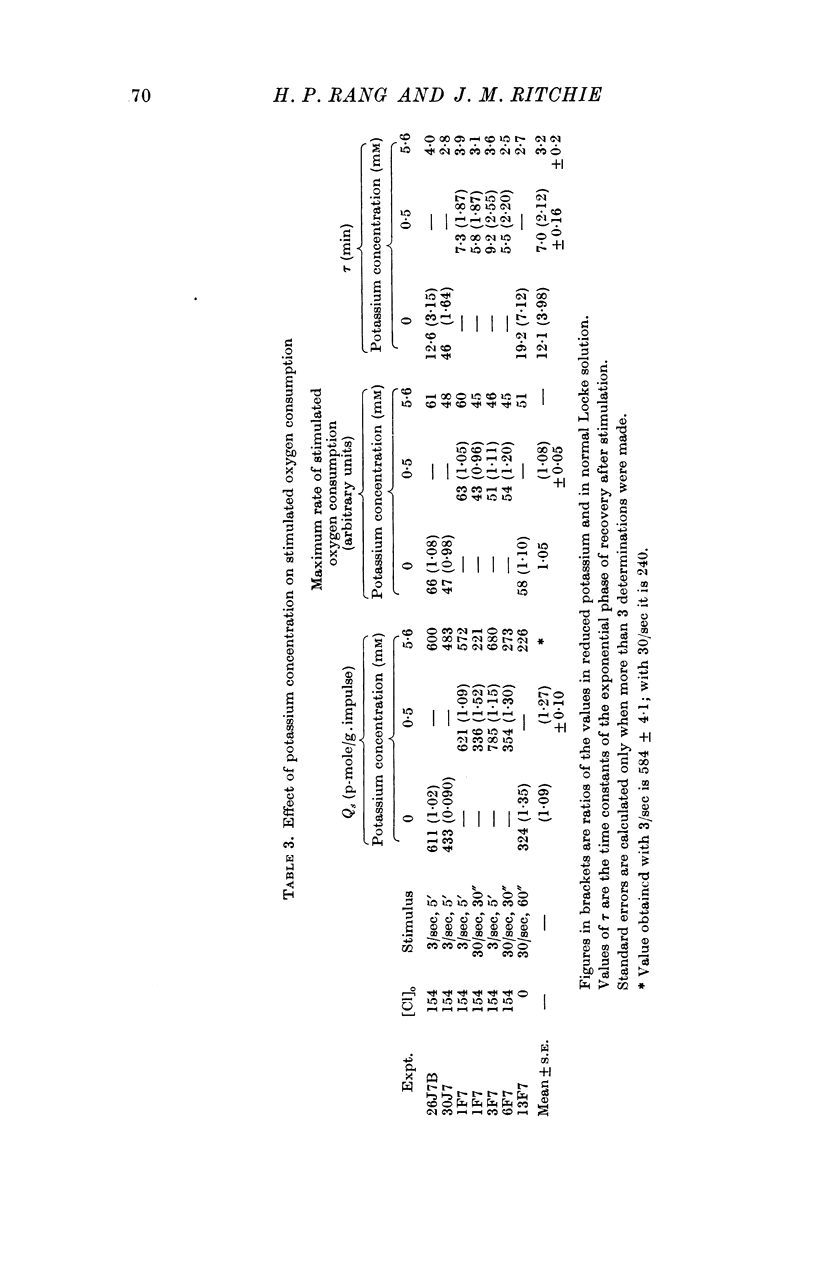
2. In normal Locke solution (containing 5·6 mM-K) the resting oxygen consumption, Qr, was 0·0903 μmole/g wet wt. min. The extra oxygen consumed as a result of stimulation, Qs, at 3 stimuli/sec was about 600 p-mole/g.impulse; at 30 stimuli/sec it was less, being 240 p-mole/g.impulse.
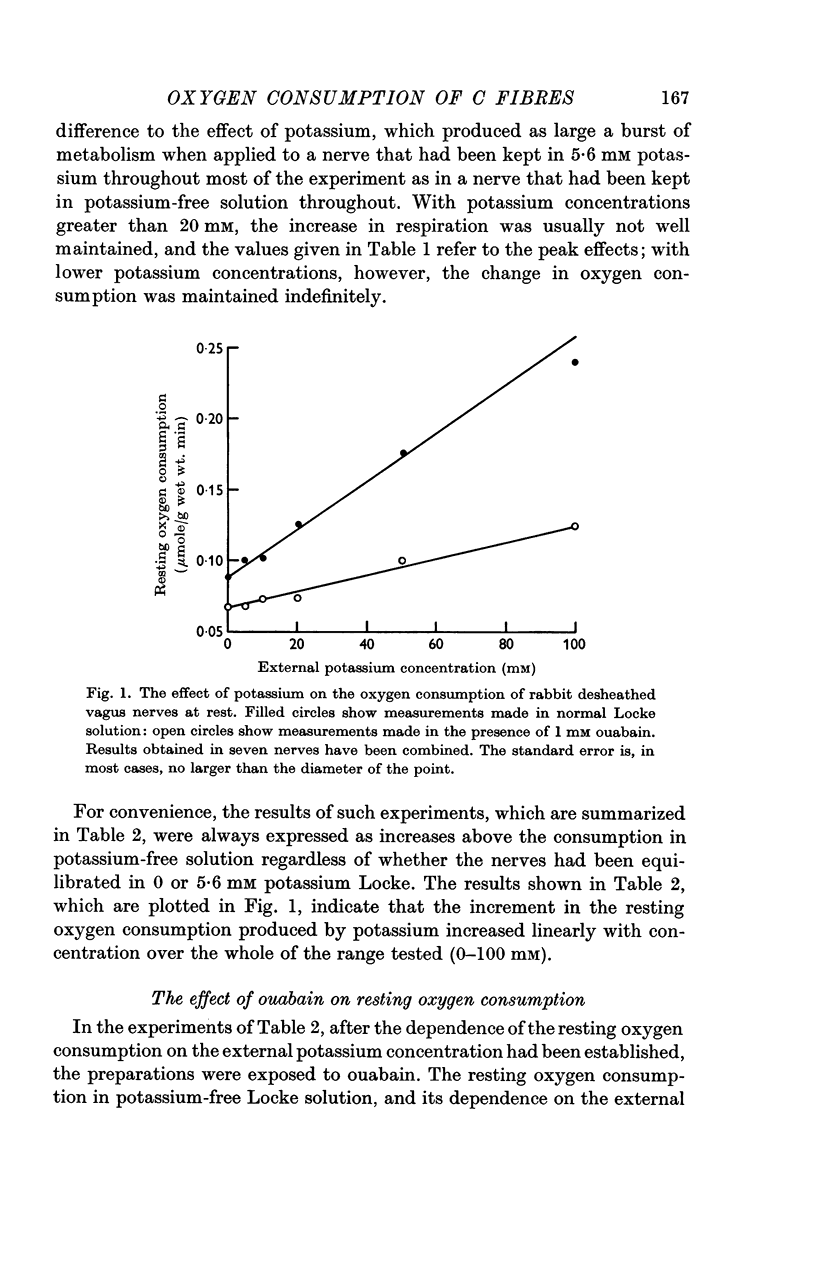
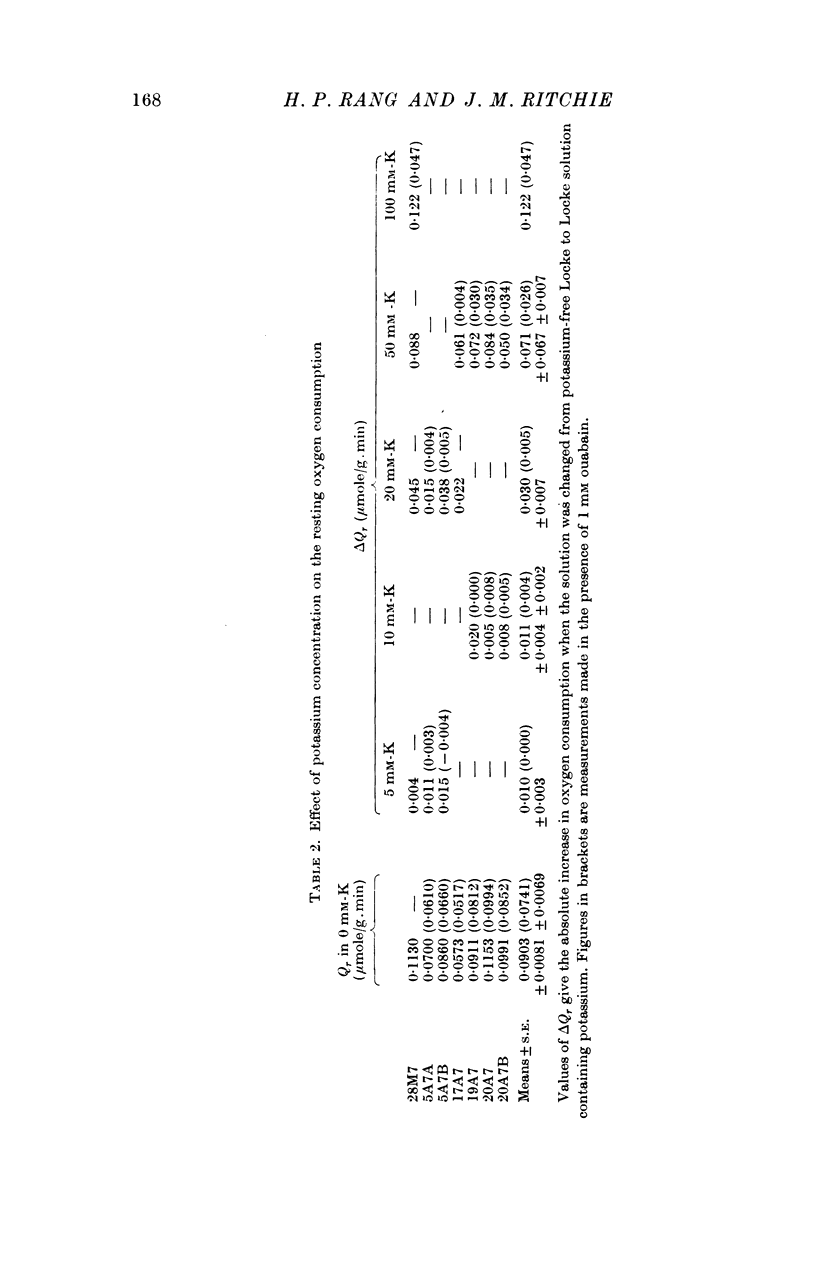
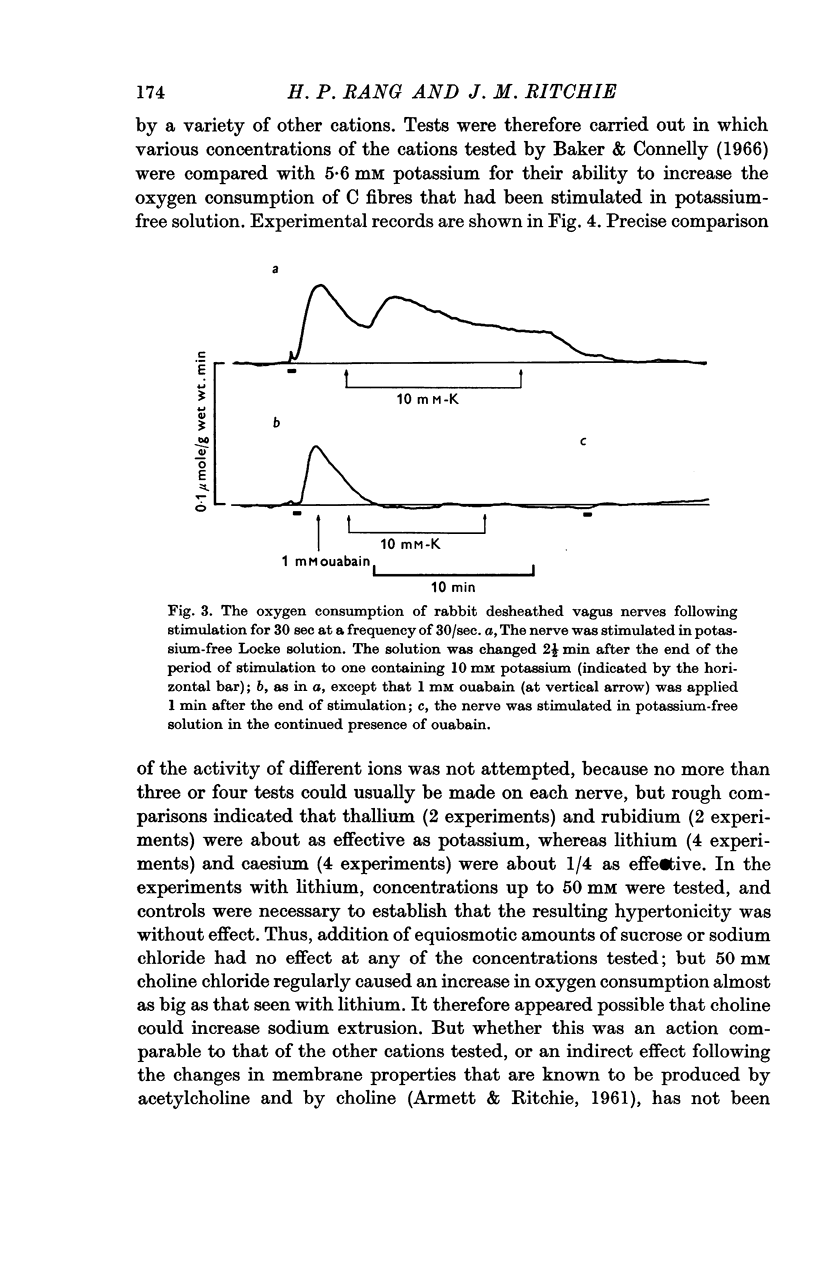
3. Only a fraction of Qr (18% at 5·6 mM-K) was sensitive to ouabain (1 mM). The ouabain-sensitive component, however, increased as the external potassium concentration was increased, in the range 0-100 mM. Qs was virtually abolished by ouabain.
4. Reduction of the external potassium concentration from 5·6 mM to zero reduced Qr (by 10%) and increased Qs, but the changes were scarcely significant statistically.
5. The conclusions were drawn: that Qs reflected the pumping of cations to restore the ionic imbalance following activity, particularly reflecting the extrusion of sodium ions from the fibre; that this pumping was normally absolutely dependent on the presence of potassium externally and that no pumping could occur in its absence; and that Qs was not reduced to zero in ostensibly potassium-free solutions because enough potassium was released into the periaxonal space during activity to maintain pumping.
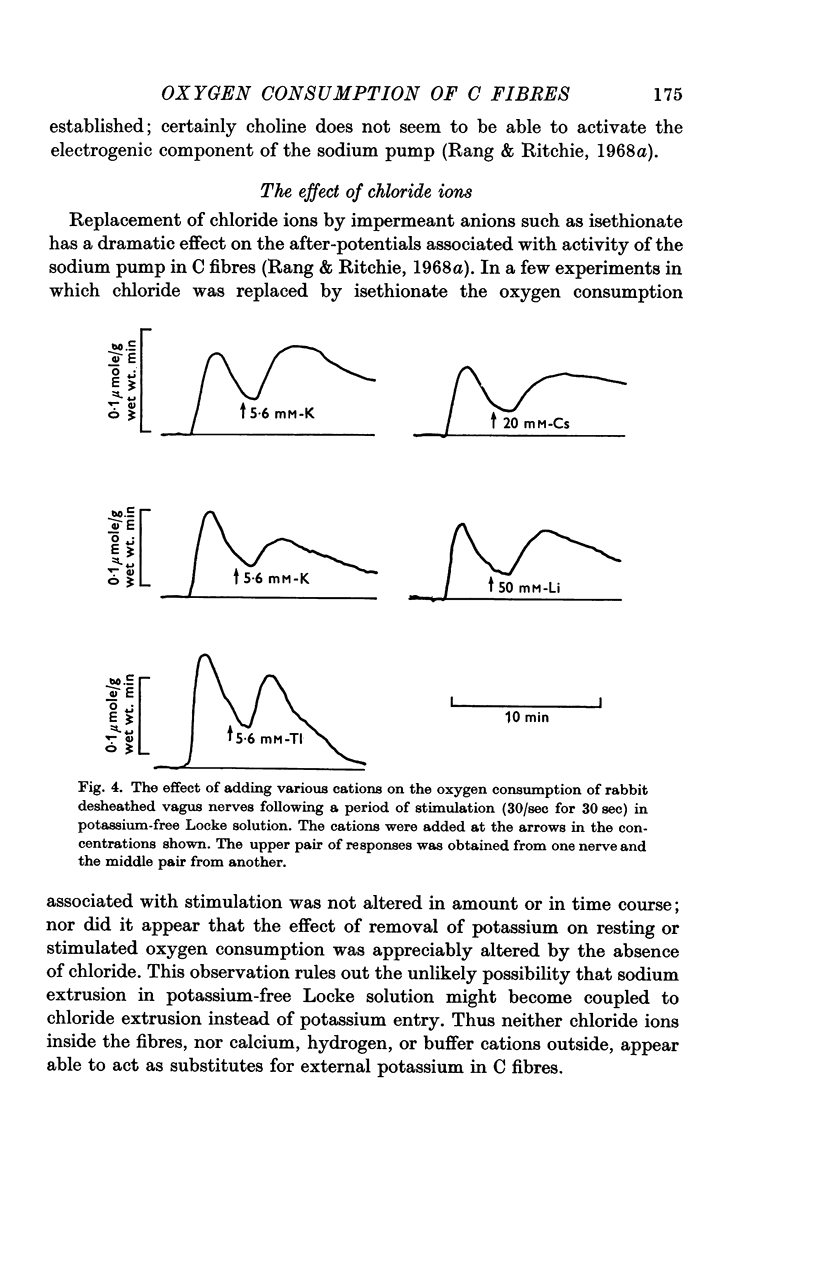
6. Thallium, rubidium, caesium and lithium could replace potassium and allowed pumping to occur.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABBOTT B. C., HILL A. V., HOWARTH J. V. The positive and negative heat production associated with a nerve impulse. Proc R Soc Lond B Biol Sci. 1958 Feb 18;148(931):149–187. doi: 10.1098/rspb.1958.0012. [DOI] [PubMed] [Google Scholar]
- ARMETT C. J., RITCHIE J. M. The action of acetylcholine and some related substances on conduction in mammalian non-myelinated nerve fibres. J Physiol. 1961 Feb;155:372–384. doi: 10.1113/jphysiol.1961.sp006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINK F., Jr, BRONK D. W., CARLSON F. D., CONNELLY C. M. The oxygen uptake of active axons. Cold Spring Harb Symp Quant Biol. 1952;17:53–67. doi: 10.1101/sqb.1952.017.01.008. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Connelly C. M. Some properties of the external activation site of the sodium pump in crab nerve. J Physiol. 1966 Jul;185(2):270–297. doi: 10.1113/jphysiol.1966.sp007987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. Phosphorus metabolism of intact crab nerve and its relation to the active transport of ions. J Physiol. 1965 Sep;180(2):383–423. doi: 10.1113/jphysiol.1965.sp007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F. The sodium pump. Endeavour. 1966 Sep;25(96):166–172. doi: 10.1016/0160-9327(66)90124-4. [DOI] [PubMed] [Google Scholar]
- Bronk D. W. The initial and recovery heat production of vertebrate nerve. J Physiol. 1931 Feb 25;71(2):136–144. doi: 10.1113/jphysiol.1931.sp002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugnard L. The heat production of cat's nerve. J Physiol. 1936 Jan 15;86(1):29–36. doi: 10.1113/jphysiol.1936.sp003340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENGARD P., STRAUB R. W. Metabolic studies on the hyperpolarization following activity in mammalian non-myelinated nerve fibres. J Physiol. 1962 May;161:414–423. doi: 10.1113/jphysiol.1962.sp006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The stoicheiometry of the sodium pump. J Physiol. 1967 Sep;192(1):217–235. doi: 10.1113/jphysiol.1967.sp008297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWICZ P., GERBER C. J. EFFECTS OF EXTERNAL POTASSIUM AND STROPHANTHIDIN ON SODIUM FLUXES IN FROG STRIATED MUSCLE. J Gen Physiol. 1965 Jan;48:489–514. doi: 10.1085/jgp.48.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth J. V., Keynes R. D., Ritchie J. M. The origin of the initial heat associated with a single impulse in mammalian non-myelinated nerve fibres. J Physiol. 1968 Feb;194(3):745–793. doi: 10.1113/jphysiol.1968.sp008434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., SWAN R. C. The effect of external sodium concentration on the sodium fluxes in frog skeletal muscle. J Physiol. 1959 Oct;147:591–625. doi: 10.1113/jphysiol.1959.sp006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITCHIE J. M., STRAUB R. W. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol. 1957 Apr 3;136(1):80–97. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. On the electrogenic sodium pump in mammalian non-myelinated nerve fibres and its activation by various external cations. J Physiol. 1968 May;196(1):183–221. doi: 10.1113/jphysiol.1968.sp008502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang H. P., Ritchie J. M. The ionic content of mammalian non-myelinated nerve fibres and its alteration as a result of electrical activity. J Physiol. 1968 May;196(1):223–236. doi: 10.1113/jphysiol.1968.sp008503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M. The oxygen consumption of mammalian non-myelinated nerve fibres at rest and during activity. J Physiol. 1967 Feb;188(3):309–329. doi: 10.1113/jphysiol.1967.sp008141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN A. K., POST R. L. STOICHIOMETRY AND LOCALIZATION OF ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT IN THE ERYTHROCYTE. J Biol Chem. 1964 Jan;239:345–352. [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- Van Der Kloot W. G. Potassium-stimulated respiration and intra-cellular calcium releae in from skeletal muscle. J Physiol. 1967 Jul;191(1):141–165. doi: 10.1113/jphysiol.1967.sp008242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. The connexion between active cation transport and metabolism in erythrocytes. Biochem J. 1965 Oct;97(1):214–227. doi: 10.1042/bj0970214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. Vectorial aspects of adenosine-triphosphatase activity in erythrocyte membranes. Biochem J. 1964 Nov;93(2):337–348. doi: 10.1042/bj0930337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZERAHN K. Oxygen consumption and active sodium transport in the isolated and short-circuited frog skin. Acta Physiol Scand. 1956 May 31;36(4):300–318. doi: 10.1111/j.1748-1716.1956.tb01327.x. [DOI] [PubMed] [Google Scholar]


