Abstract
1. The content and distribution of sodium in the epithelium of the frog skin (Leptodactylus ocellatus L.) have been studied.
2. The inulin space, the 22Na exchange, and the amounts of water and sodium were measured in samples of connective tissue. The results indicate that the necessary assumptions generally made to calculate the sodium and water contents of the epithelial cells as the difference between the total content in the tissue and the amounts contained in the inulin space are not valid in the frog skin.
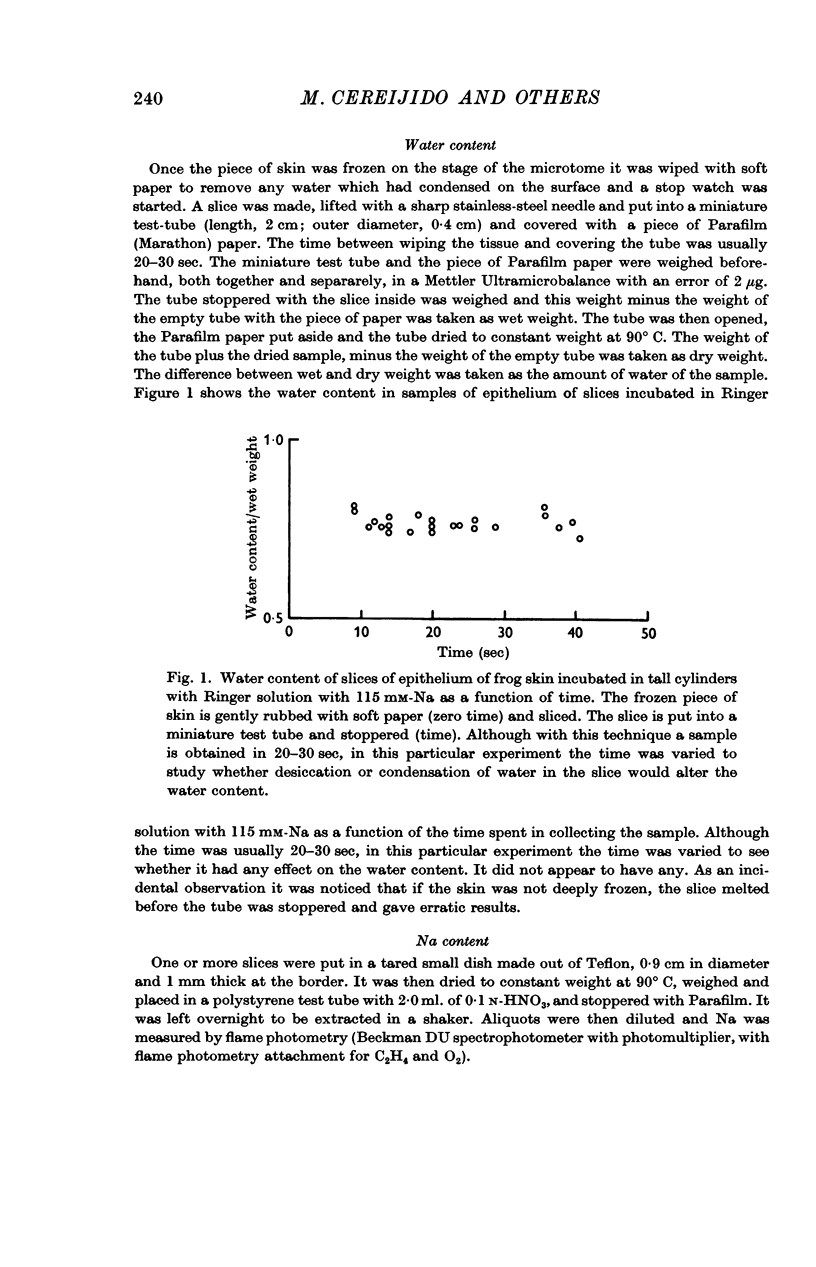
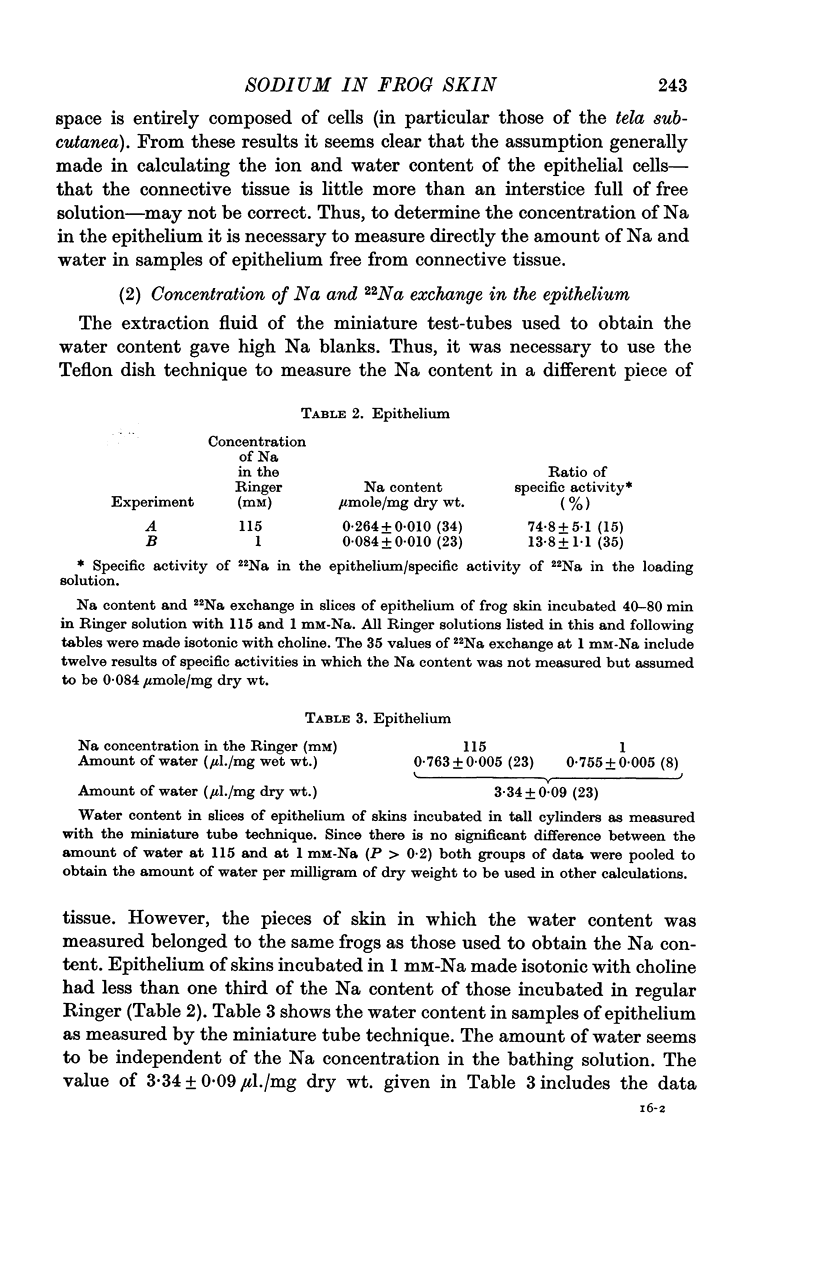
3. The mean concentration of sodium in the epithelium has been obtained from direct measurements of sodium and water in samples of epithelium. To measure the water content of the epithelium a new technique has been developed. When the skin is bathed with Ringer solution containing 115 mM-Na on both sides, the mean concentration of sodium in the epithelium is 79 mM. When the concentration of sodium in the Ringer is 1 mM the mean concentration in the epithelium is 25 mM. When the skin is bathed with Ringer with 1 mM-Na on the outside and 115 mM-Na on the inside—a situation which resembles the natural condition in the skin—the mean concentration of sodium in the epithelium is 52 mM.
4. The compartmentalization of Na was studied by comparing the sodium content and the degree of exchange with 22Na in the bathing solutions. In these experiments the skins were exposed to Ringer solutions with different concentrations of sodium, and 22Na on one or both sides.
5. The results indicate that the epithelium has a compartment of sodium which is not exchangeable in 40-80 min and whose size is not appreciably changed by a threefold change in the Na content in the epithelium and a hundredfold change in the concentration of the bathing solution.
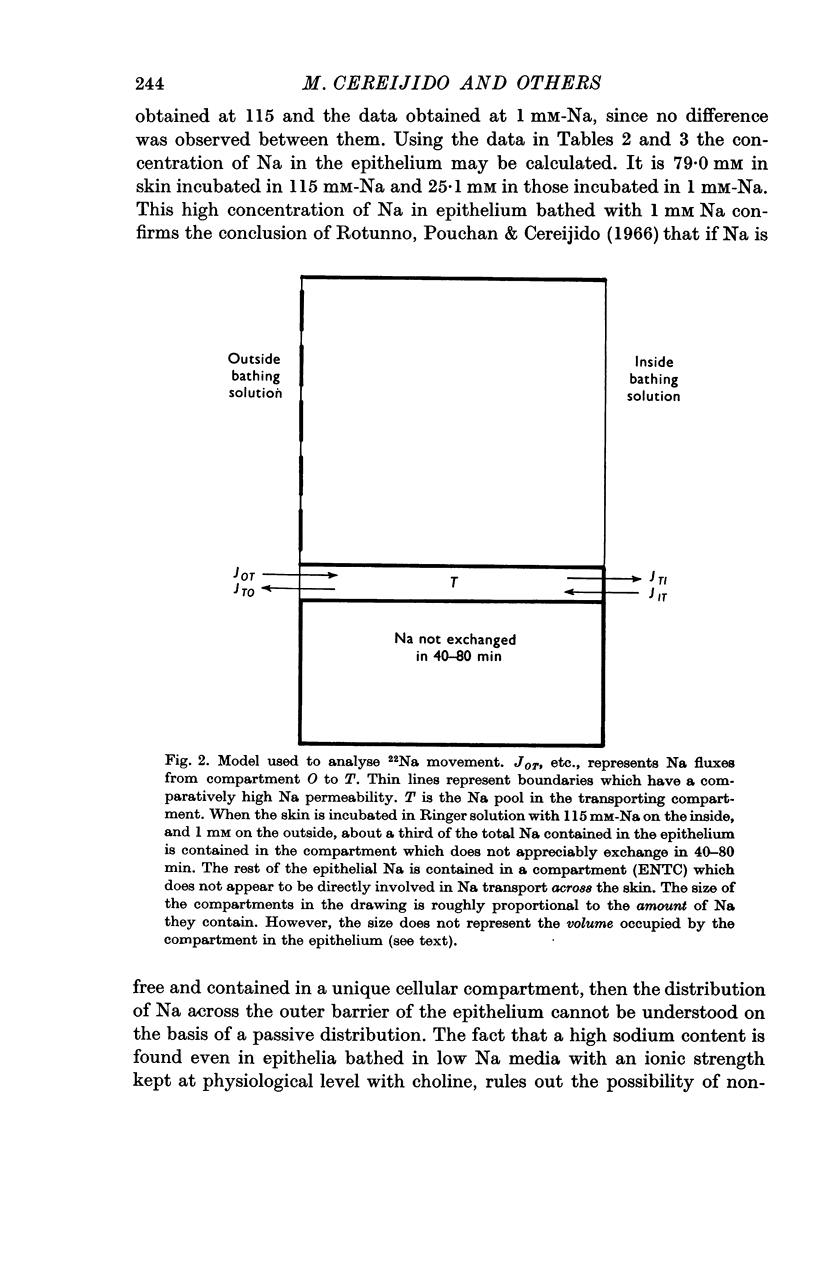
6. Sodium exchangeable in 40-80 min seems to be contained in two different compartments: (a) a large one that contains fixed sodium is mainly connected to the inside, and does not appear to participate directly in sodium transport across the frog skin; (b) a small one, that is bounded on the inside by a Na-impermeable barrier, and that seems to comprise the sodium involved in active transport. When the skin is bathed with Ringer solutions with 115 mM-Na on the inside and 1 mM-Na on the outside, the transporting compartment contains some 13% of the total sodium in the epithelium.
7. The results are interpreted on the basis of a model recently proposed by Cereijido & Rotunno (1968). The major feature of this model is that the sodium transporting compartment is confined to the plasma membrane of the epithelial cells.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CEREIJIDO M., CURRAN P. F. INTRACELLULAR ELECTRICAL POTENTIALS IN FROG SKIN. J Gen Physiol. 1965 Mar;48:543–557. doi: 10.1085/jgp.48.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CEREIJIDO M., HERRERA F. C., FLANIGAN W. J., CURRAN P. F. THE INFLUENCE OF NA CONCENTRATION ON NA TRANSPORT ACROSS FROG SKIN. J Gen Physiol. 1964 May;47:879–893. doi: 10.1085/jgp.47.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., HERRERA F. C., FLANIGAN W. J. The effect of Ca and antidiuretic hormone on Na transport across frog skin. II. Sites and mechanisms of action. J Gen Physiol. 1963 May;46:1011–1027. doi: 10.1085/jgp.46.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Rotunno C. A. Transport and distribution of sodium across frog skin. J Physiol. 1967 Jun;190(3):481–497. doi: 10.1113/jphysiol.1967.sp008223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope F. W. NMR evidence for complexing of Na+ in muscle, kidney, and brain, and by actomyosin. The relation of cellular complexing of Na+ to water structure and to transport kinetics. J Gen Physiol. 1967 May;50(5):1353–1375. doi: 10.1085/jgp.50.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran P. F., Cereijido M. K fluxes in frog skin. J Gen Physiol. 1965 Jul;48(6):1011–1033. doi: 10.1085/jgp.48.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAZIER H. S., DEMPSEY E. F., LEAF A. Movement of sodium across the mucosal surface of the isolated toad bladder and its modification by vasopressin. J Gen Physiol. 1962 Jan;45:529–543. doi: 10.1085/jgp.45.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. Cell junctions in amphibian skin. J Cell Biol. 1965 Jul;26(1):263–291. doi: 10.1083/jcb.26.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSHIKO T., USSING H. H. The kinetics of Na24 flux across amphibian skin and bladder. Acta Physiol Scand. 1960 May 25;49:74–81. doi: 10.1111/j.1748-1716.1960.tb01931.x. [DOI] [PubMed] [Google Scholar]
- HUF E. G., DOSS N. S., WILLS J. P. Effects of metabolic inhibitors and drugs on ion transport and oxygen consumption in isolated frog skin. J Gen Physiol. 1957 Nov 20;41(2):397–417. doi: 10.1085/jgp.41.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura A., Takeda H., Sasaki N. The accumulation of sodium and calcium in a specific layer of frog skin. J Cell Physiol. 1965 Oct;66(2):221–225. doi: 10.1002/jcp.1030660208. [DOI] [PubMed] [Google Scholar]
- KOEFOED-JOHNSEN V., USSING H. H. The nature of the frog skin potential. Acta Physiol Scand. 1958 Jun 2;42(3-4):298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- LEVINSKY N. G., SAWYER W. H. Relation of metabolism of frog skin to cellular integrity and electrolyte transfer. J Gen Physiol. 1953 May;36(5):607–615. doi: 10.1085/jgp.36.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACROBBIE E. A., USSING H. H. Osmotic behaviour of the epithelial cells of frog skin. Acta Physiol Scand. 1961 Nov-Dec;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- Rotunno C. A., Kowalewski V., Cereijido M. Nuclear spin resonance evidence for complexing of sodium in frog skin. Biochim Biophys Acta. 1967 Feb 1;135(1):170–173. doi: 10.1016/0005-2736(67)90022-3. [DOI] [PubMed] [Google Scholar]
- Routunno C. A., Pouchan M. I., Cereijido M. Location of the mechanism of active transport of sodium across the frog skin. Nature. 1966 May 7;210(5036):597–599. doi: 10.1038/210597a0. [DOI] [PubMed] [Google Scholar]
- SHAW F. H., SIMON S. E. The nature of the sodium and potassium balance in nerve and muscle cells. Aust J Exp Biol Med Sci. 1955 Apr;33(2):153–177. doi: 10.1038/icb.1955.17. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]


