Abstract
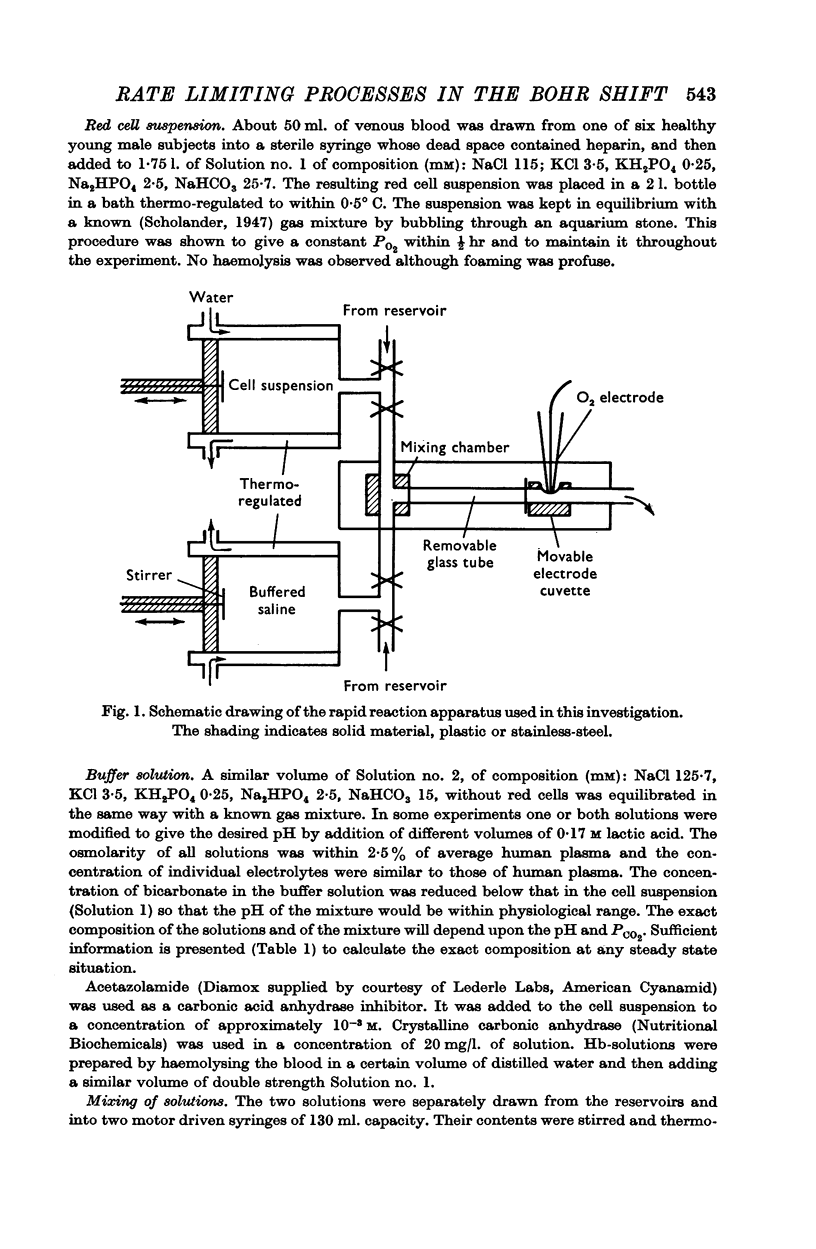
1. The rates of the Bohr shift of human red cells and some of its constituent reactions have been studied with a modified Hartridge—Roughton rapid reaction apparatus using an oxygen electrode to measure the progress of the reaction.
2. The rate of the Bohr shift was compatible with the hypothesis that the transfer of H+ across the membrane by means of CO2 exchange and reaction with buffers is generally the rate-limiting step.
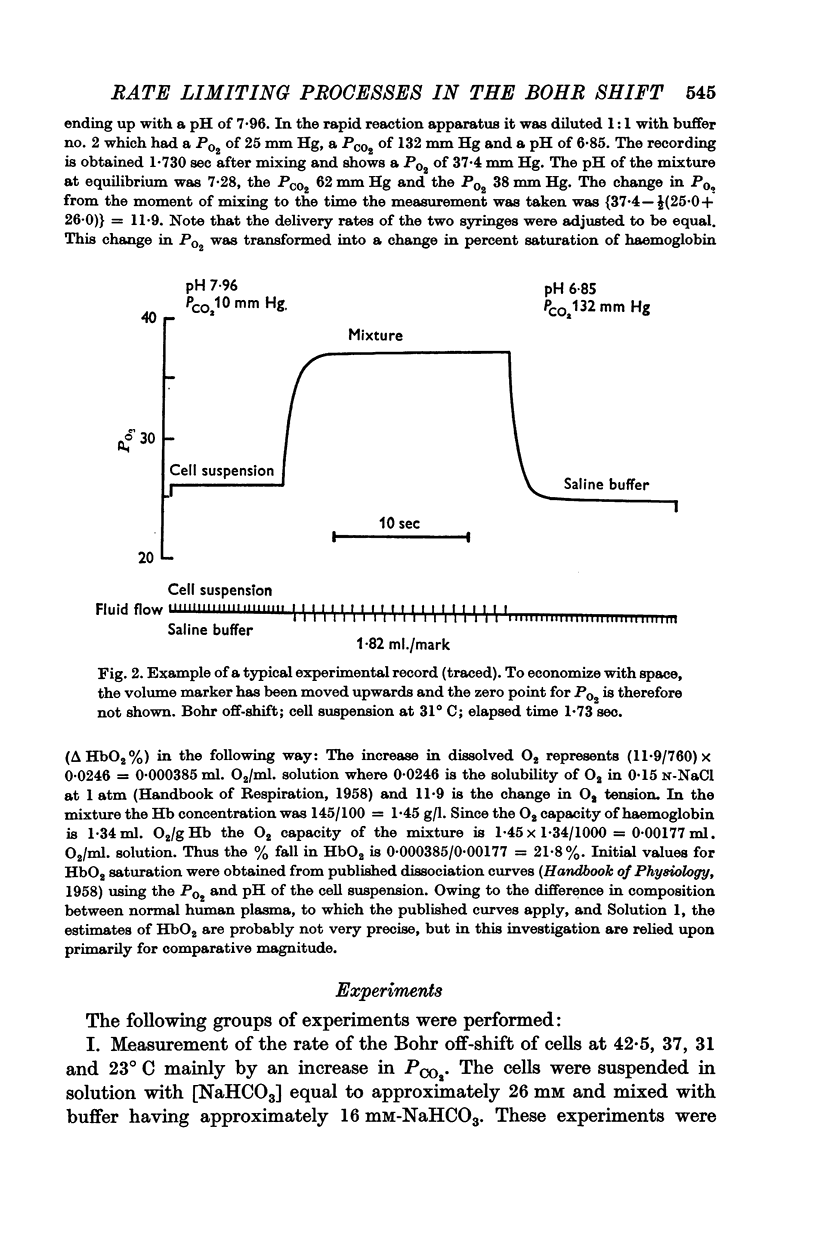
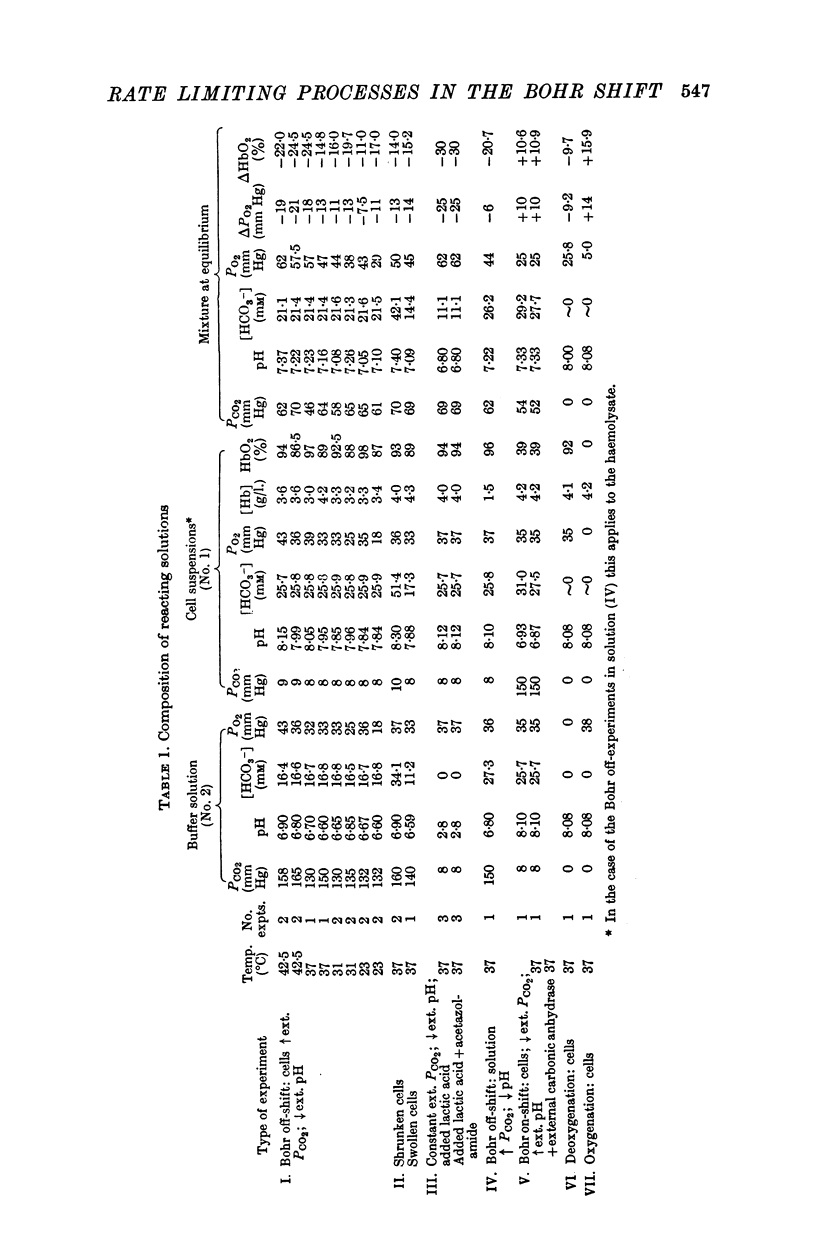
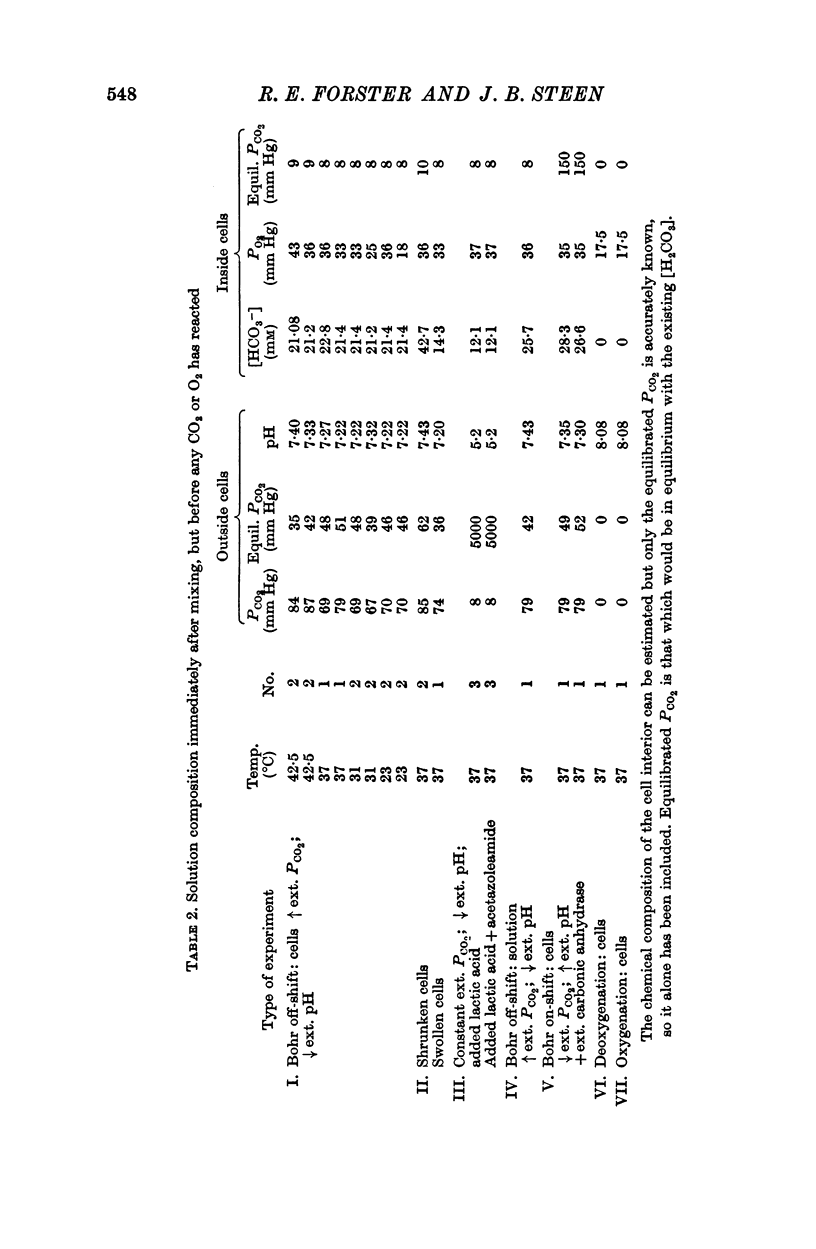
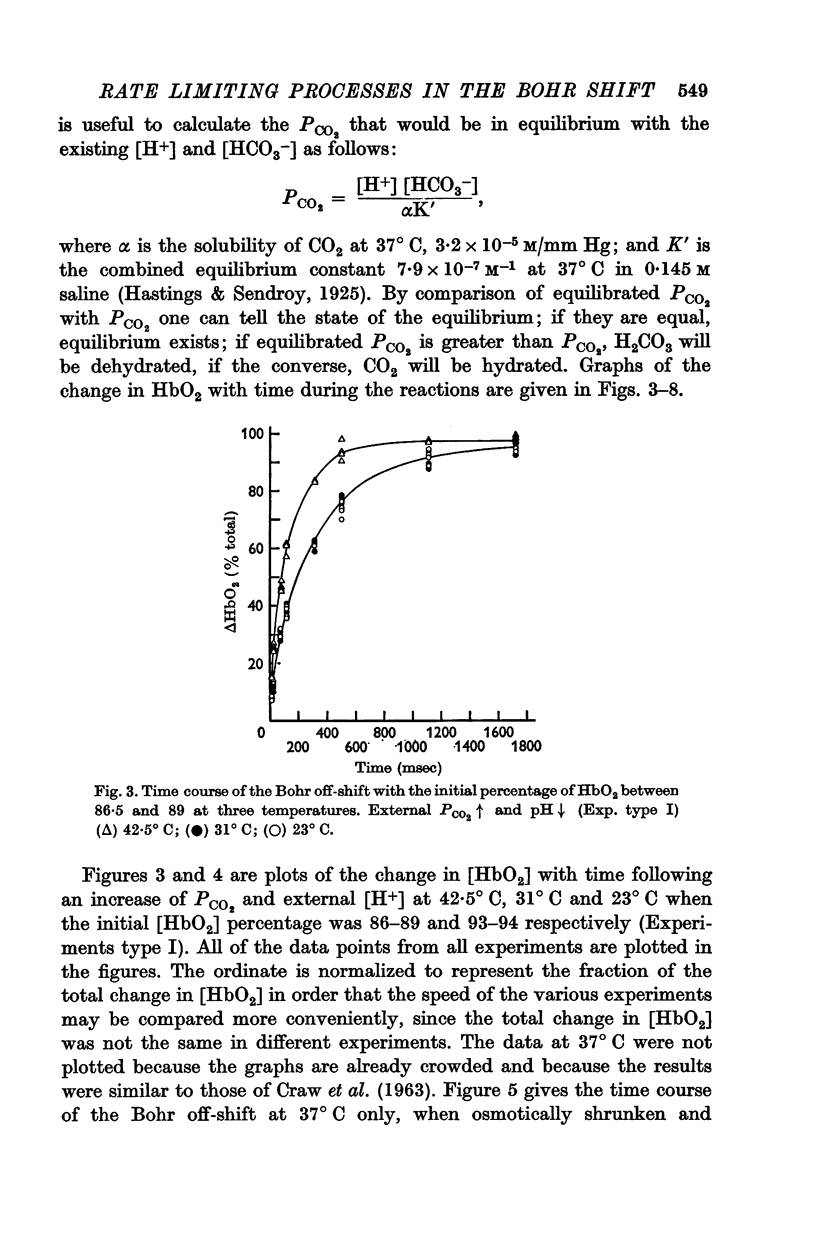
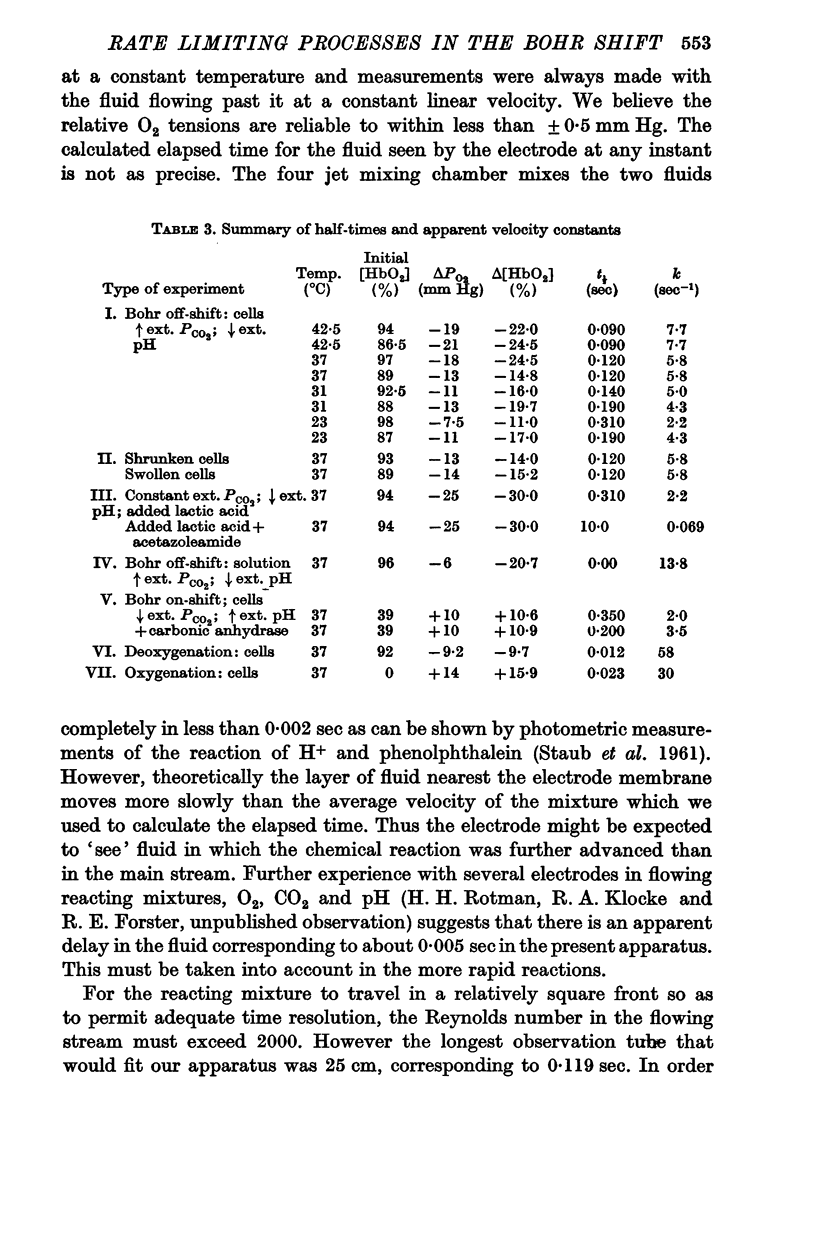
(a) When the Bohr off-reaction was produced by a marked increase in PCO2 around the cells, the half-time at 37° C was 0·12 sec. In this case CO2 was available initially to diffuse into the cells, the process being predominantly limited by the rate of intracellular CO2 hydration.
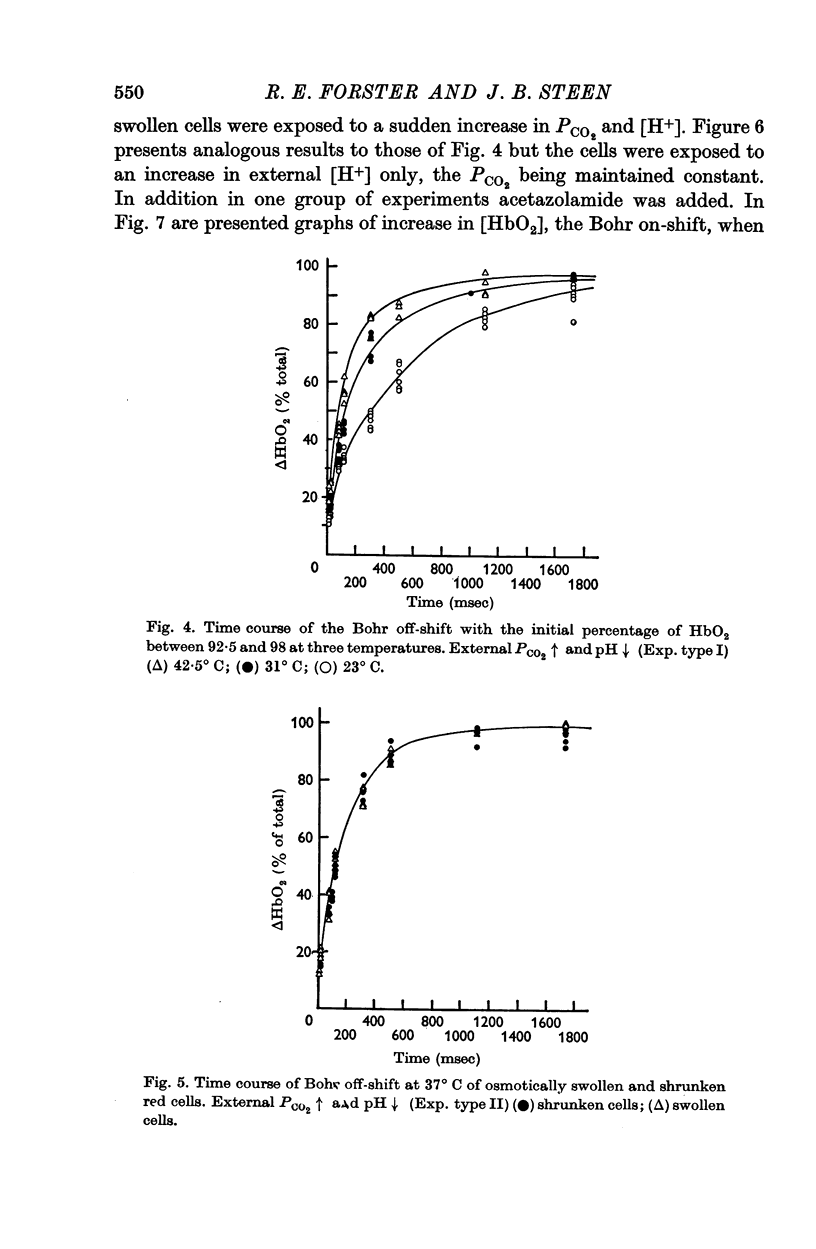
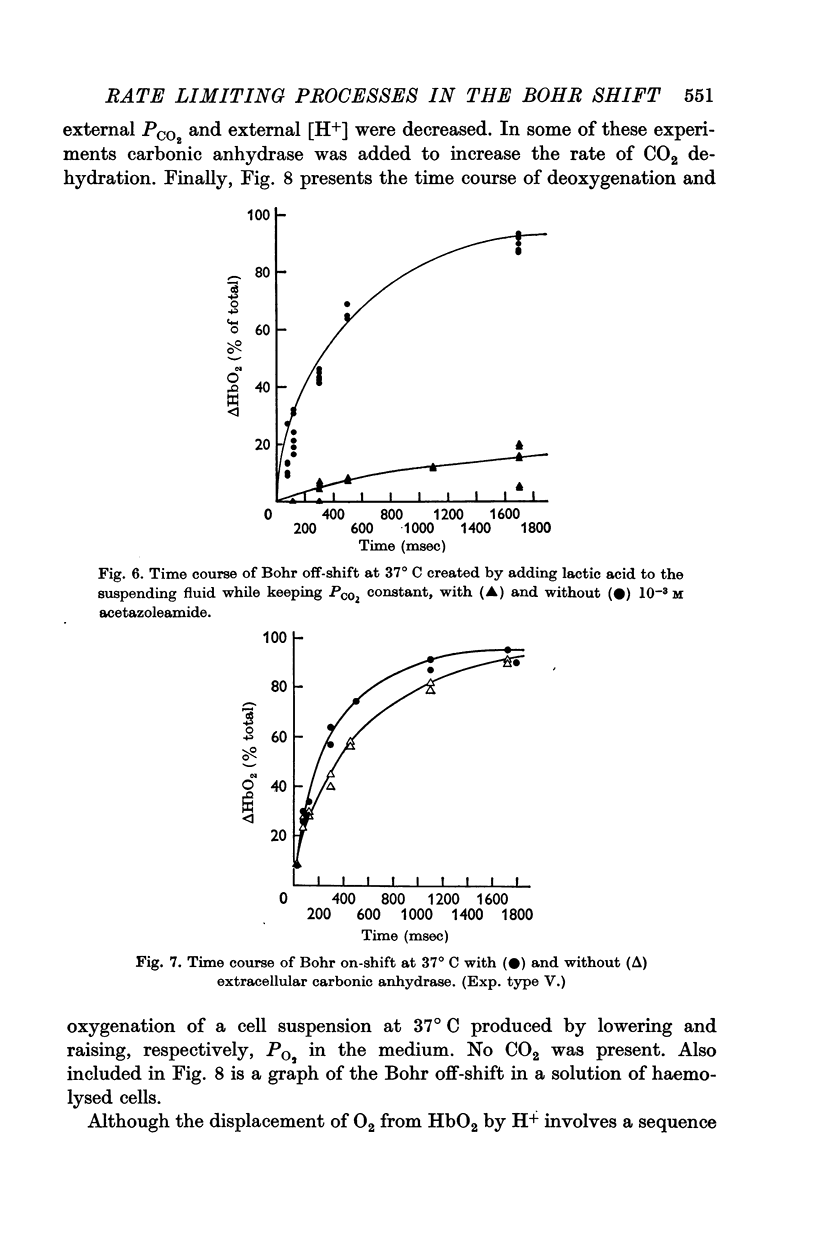
(b) When the Bohr off-shift was produced by an increase of [H+] outside the cell, PCO2 being low and equal within and outside the cells, the half time became 0·31 sec. In this case, even at the start, the H2CO3 formed by the almost instantaneous neutralization reaction of H+ and HCO3- had to dehydrate to form CO2 and this in turn had to diffuse into and react within the red cell before the [HbO2] could change. When a carbonic anhydrase inhibitor was added to slow the CO2 reaction inside the cell, the half-time rose to 10 sec.
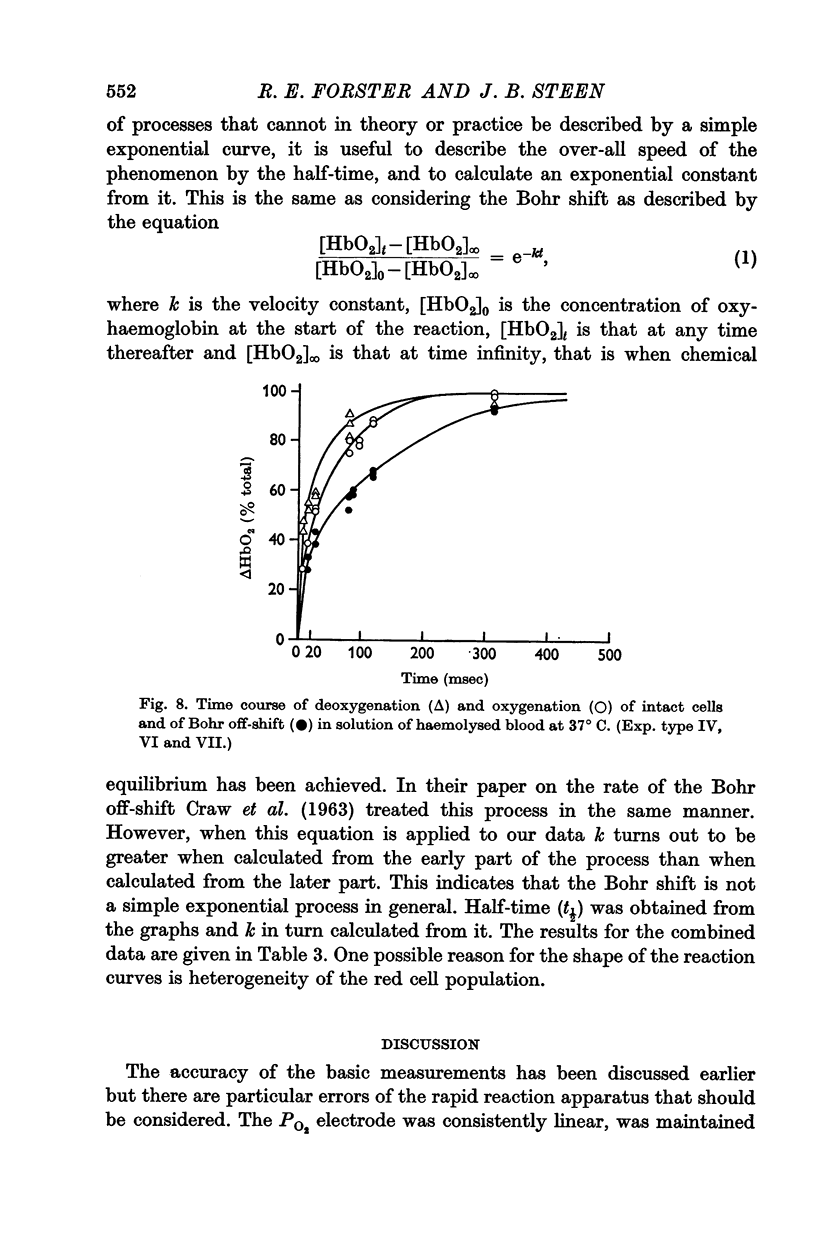
(c) The Bohr off-shift in a haemolysed cell suspension produced by an increase in PCO2 appeared to be limited by the rate at which the CO2 could hydrate to form H+.
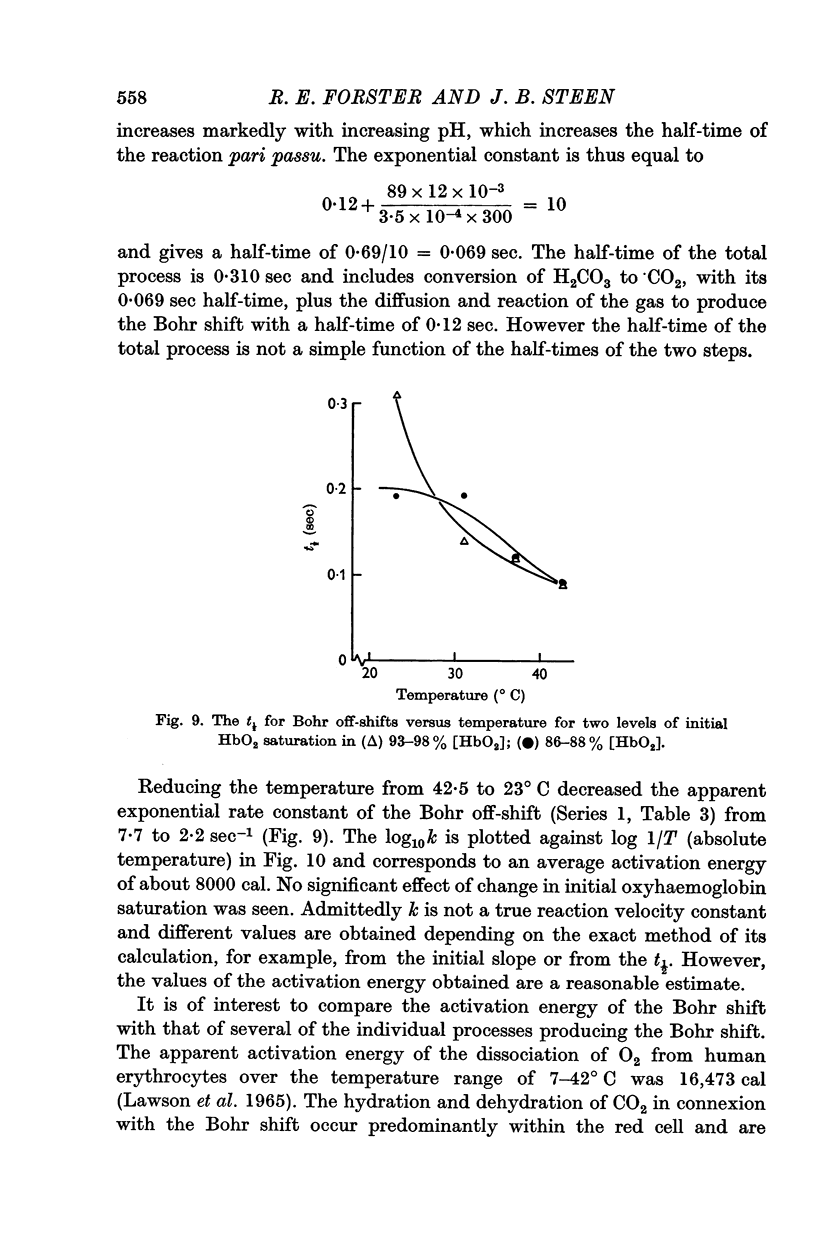
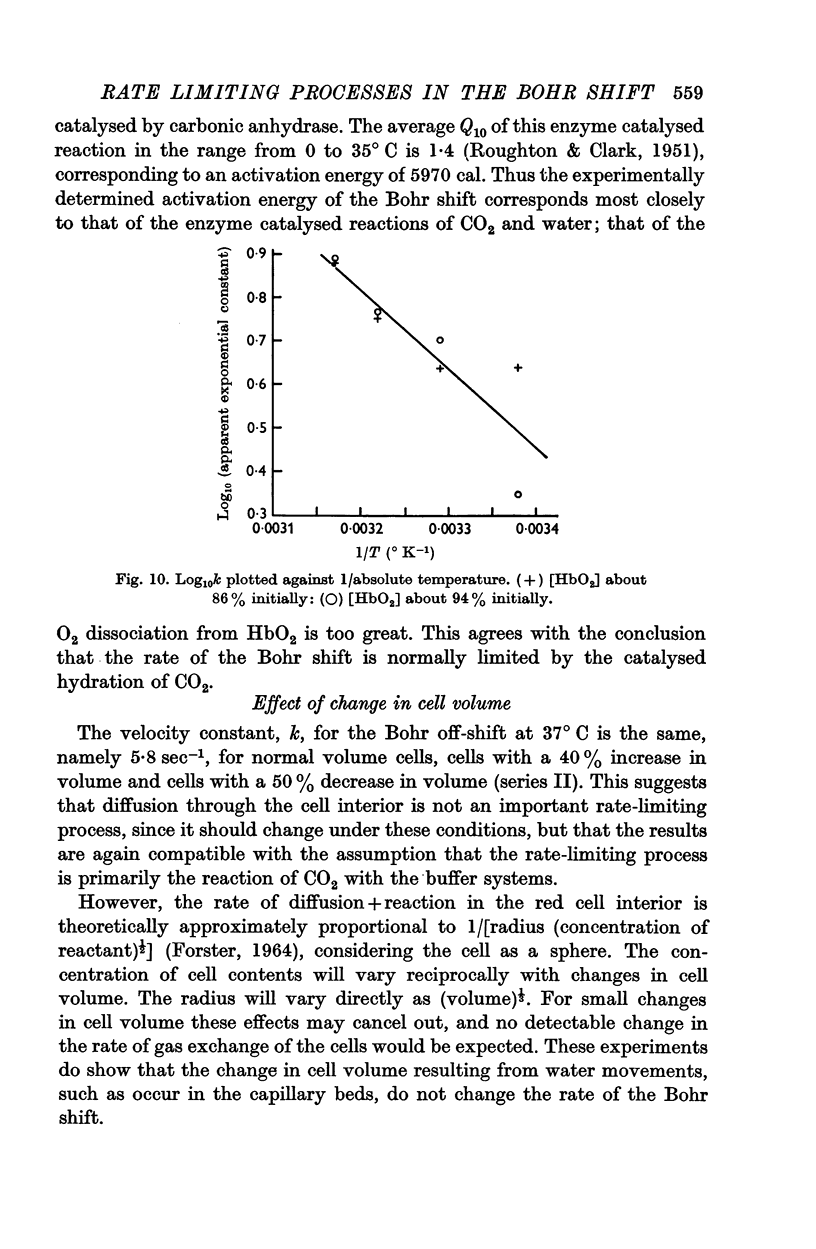
3. The Bohr off-shift has an average Q10 of 2·5 between 42·5 and 28° C with an activation energy of 8000 cal.
4. The pronounced importance of the CO2-bicarbonate system for rapid intracellular pH changes is discussed in connexion with some physiological situations.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Booth V. H. Carbonic anhydrase activity inside corpuscles. Enzyme-substrate accessibility factors. J Physiol. 1938 Jul 14;93(2):117–128. doi: 10.1113/jphysiol.1938.sp003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAW M. R., CONSTANTINE H. P., MORELLO J. A., FORSTER R. E. Rate of the Bohr shift in human red cell suspensions. J Appl Physiol. 1963 Mar;18:317–324. doi: 10.1152/jappl.1963.18.2.317. [DOI] [PubMed] [Google Scholar]
- GIEBEL O., PASSOW H. [The permeability of erythrocyte membranes for organic anions. On the problem of diffusion through the pores]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;271:378–388. [PubMed] [Google Scholar]
- KERNOHAN J. C., FORREST W. W., ROUGHTON F. J. The activity of concentrated solutions of carbonic anhydrase. Biochim Biophys Acta. 1963 Jan 8;67:31–41. doi: 10.1016/0006-3002(63)91794-3. [DOI] [PubMed] [Google Scholar]
- Lawson W. H., Jr, Holland R. A., Forster R. E. Effect of temperature on deoxygenation rate of human red cells. J Appl Physiol. 1965 Sep;20(5):912–918. doi: 10.1152/jappl.1965.20.5.912. [DOI] [PubMed] [Google Scholar]
- NAKAMURA T., STAUB N. C. SYNERGISM IN THE KINETIC REACTIONS OF O2 AND CO2 WITH HUMAN RED BLOOD CELLS. J Physiol. 1964 Sep;173:161–177. doi: 10.1113/jphysiol.1964.sp007449. [DOI] [PMC free article] [PubMed] [Google Scholar]


