Summary
The neck and shoulder region of vertebrates has undergone a complex evolutionary history. In order to identify its underlying mechanisms we map the destinations of embryonic neural crest and mesodermal stem cells using novel Cre-recombinase mediated transgenesis. The single-cell resolution of this genetic labelling reveals cryptic cell boundaries traversing seemingly homogeneous skeleton of neck and shoulders. Within this complex assembly of bones and muscles we discern a precise code of connectivity that mesenchymal stem cells of neural crest and mesodermal origin both obey as they form muscle scaffolds. Neural crest anchors the head onto the anterior lining of the shoulder girdle, while a Hox gene controlled mesoderm links trunk muscles to the posterior neck and shoulder skeleton. The skeleton that we identify as neural crest is specifically affected in human Klippel-Feil syndrome, Sprengel’s deformity and Arnold-Chiari I/II malformation, providing first insights into their likely aetiology. We identify genes involved in the cellular modularity of neck and shoulder skeleton and propose a new methodology for determining skeletal homologies that is based on muscle attachments. This has allowed us to trace the whereabouts of the cleithrum, the major shoulder bone of extinct land vertebrate ancestors which appears to survive as the scapular spine in living mammals.
The vertebrate neck has undergone a dramatic evolutionary transformation from an immobile bony bridge between head and shoulder in early vertebrates with paired fins1 to a mobile system of muscle scaffolds inter-connecting head and shoulders in early jaw-bearing fish such as placoderms2. These scaffolds have retained a remarkably conserved structure and function in jaw opening and head mobility ever since3. The fundamental changes in the skeleton of neck and shoulders reflect evolving embryonic differentiation processes of mesenchymal stem cells: from bone to muscle connective tissues and cartilage. These have defied mechanistic analysis as the detection of fate changes among homologous cell populations requires experimental long-term lineage labeling which was so far only possible in the chick4, a species with a highly modified neck architecture5. Gills and the majority of the head skeleton are derived from the embryonic cranial neural crest4, while the limb skeleton is derived from trunk mesoderm6. Neural crest and mesoderm do not provide obvious landmarks for their respective boundaries in the intervening neck transition zone: cranial neural crest is not segmentally deployed in this post-otic region (behind rhombomere 5)7 and limb lateral plate mesoderm does not appear to pattern the shoulder girdle proper8. With post-otic neural crest (PONC) and paraxial (somitic) mesoderm as candidate components, the neck between the ear (otic) capsule of the head and the trunk forelimbs has remained an uncharted embryonic territory.
Bone formation versus muscle scaffolds
Neural crest and mesodermal cells appear to differ in the way they form bones: neural crest forms dermal and endochondral bones in the head whereas mesoderm forms endochondral skeleton in the trunk. To date no evidence for mesoderm-derived dermal bones has been produced. The shoulder girdle and neck in between head and limbs contains dermal as well as endochondral bones. All previous investigations into the evolution of this region have therefore assumed this dermal-endochondral distinction to be a safe indicator for bone origins and homologies: Accordingly, all dermal bones in the post-otic region are considered to be exclusively neural crest-derived while all endochondral bones are mesodermal 9,10. The validity of this widely held ‘ossification model’ has remained untested in the neck of any living vertebrate. Indeed, in apparent contradiction to it, a current view holds the posterior boundary of neural crest-derived skeleton to be the parietal (or frontal) bone of the skull 11,12 : no neural crest-derived skeleton behind the ear capsule has as yet been identified.
Comparative neck anatomy in living jawed vertebrates challenges the likelihood of the prevailing ‘ossification model 1’: We note that the pattern of neck muscles (red in Fig.1) is far more conserved than the ossification modes of the shoulder bones to which these muscles are attached (Fig. 1, pale grey regions are dermal, dark grey ones are endochondral bones). This poses a serious problem for muscle homologies: In all (cranial and trunk) regions of the vertebrate body so far examined the embryonic cellular origin of muscle connective tissues and their respective skeletal attachment regions is identical 13,14. This would imply that if attachment regions change in their cellular origins (and ossification type), their coordinated muscle connective tissues also change in their composition. This would force us to reject homology of all neck musculature among jawed vertebrates, although it displays a highly similar and complex connectivity pattern (red in Fig.1)5. A similar problem is posed by the composition of the clavicular bone itself (box 3, Fig. 1). It is dermal in fish and amphibians and of mixed dermal+endochondral composition in mammals15,16. If the ‘ossification model’ holds, we would have to refute homology of a bone that has changed its histogenesis but not its position inside the head-neck assembly over more than 400 million years. In this light a competing ’muscle scaffold model 2‘ can be considered, according to which bones ‘morph’ around a highly constrained muscle attachment scaffold13. Cell population boundaries remain stable (and are the structural basis for conserved muscle scaffolds) but differentiation of PONC and mesodermal cells into bone, cartilage or muscle connective tissues varies due to changes in signaling pathways. This implies that cell population boundaries are cryptic, they do not coincide with bone sutures but with muscle attachment sites.
Fig. 1.
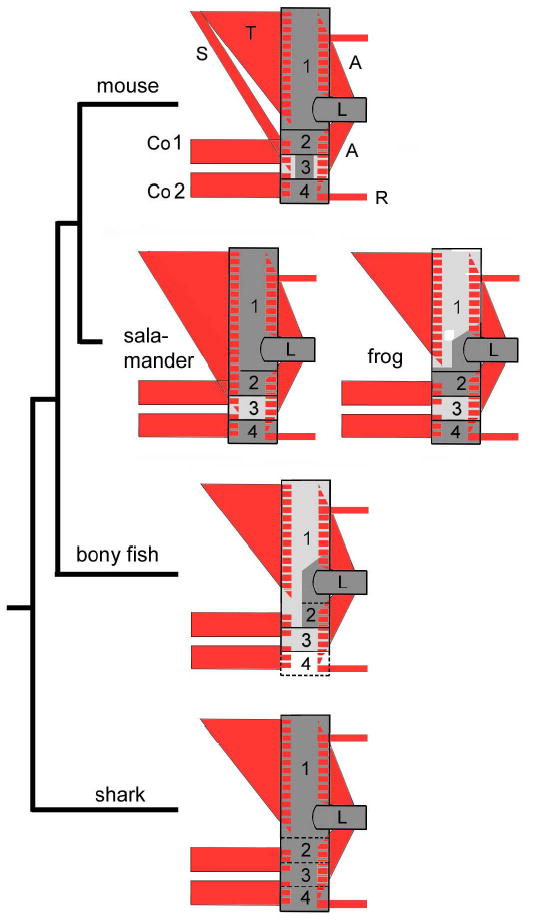
Highly conserved neck muscle scaffolds (red) attached (hatched areas) on a shoulder skeleton (boxes 1–4) displaying variable dermal (light grey,d) and endochondral (dark grey, e) ossification type. Attachment regions (hatching) of the gnathostome trapezius muscle (T), 5,49, are e in sharks, salamanders5 and all amniotes but d in fish and frogs. S, sterno-cleido-mastoid; Co1,2, coraco-branchialis, -hyoideus; A, limb muscles; R, trunk muscles. Shoulder skeleton : 1. dorsal cleithrum (d) in bony fish (Polyodon5 or Amia 35), frog (Rana 36) and scapular region (e) in salamander 5, mouse and living amniotes. 2. acromio-coracoid (e) 3. clavicular region (d/d+e), in sharks5 bone is absent and its space taken by part of the scapulo-coracoid (stippled) 4. sternal region, comprising the sternum (e) or connective tissue (bony fish); L, limb skeleton.
By utilizing a (recombinase-mediated) genetic lineage labelling strategy in transgenic mice we can now discriminate between these two models. We map neck neural crest and mesoderm with single-cell resolution onto muscular (connective tissue) attachment points and skeletal structures of a given (dermal versus endochondral) ossification type. The two models make mutually exclusive predictions for shoulder girdle origins: If model 1 is correct the anterior scapular spine would be mesodermal as it is endochondral (left part of box1 in Fig. 1). If model 2 is correct, the same skeletal region (box1, Fig. 1) would be neural crest as it serves as the attachment region for the trapezius muscle (T in Fig1) with expected neural crest connective tissues.
Here we reveal a cryptic neural crest-mesoderm boundary inside the neck and shoulder girdle skeleton, which ignores traditional skeletal landmarks or (endochondral vs. dermal) ossification types and thus invalidates the traditional ‘ossification model’. Instead, cellular distributions of neural crest and mesoderm precisely correspond to muscle attachment scaffolds onto the shoulder girdle, corroborating the nonintuitive ‘scaffold model ’. This finding illuminates the aetiology of various hitherto poorly understood congenital diseases in humans that are co-extensive with neural crest derived shoulder structures. By using the ‘scaffold model’ as a new arbiter for bone homologies, paleontology can date fate changes of common precursor populations in fossils. This reveals an unexpected evolutionary directionality in underlying fate decisions of mesenchymal stem cells that originate from mesoderm and neural crest.
Cryptic neural crest in neck and shoulders
The key problem we wish to address is the full distribution of skeletal post-otic neural crest (PONC). By using Wnt-1 17 and Sox-10-Cre-recombinase mediated fate mapping we ask three questions: 1. Can we find evidence for post-otic neural crest (PONC) to form endochondral bones? This determines whether either the ‘ossification’ or the ‘scaffold model’ are applicable to the neck region. 2. Is the entire dermal skeleton behind the otic capsule neural crest derived, or is some of it mesodermal? This will test the validity of the ‘ossification model’ in the only species that is currently accessible to high-resolution lineage mapping: the mouse. 3. Does the distribution of neural crest and mesoderm correlate with muscle attachment points or with ossification types in the neck and shoulder skeleton? This will distinguish the explanatory value of the ‘ossification model’ from that of the ‘scaffold model’ as each model makes non-overlapping predictions about anterior shoulder girdle origins.
Neural crest proves to have an unexpectedly pervasive role in the mouse neck region, forming bone, cartilage and muscle connective tissue within two domains. First, an external, essentially tubular domain dominated by pharyngeal arch muscles that extends from the head to the entire ancestral shoulder girdle and incorporates its anterior part (Fig.3). Secondly, a ventral internal domain comprising internal pharynx and larynx constrictors, tongue muscles, thyroid, cricoid and arytenoid cartilages and their respective muscle attachments at the oesophageal entry (Fig.4,6). Ensheathed between these two tubular domains lies a mesodermal domain centered on the somite-derived vertebral column, that reaches forward to the occipital region of the head.
Fig.3.
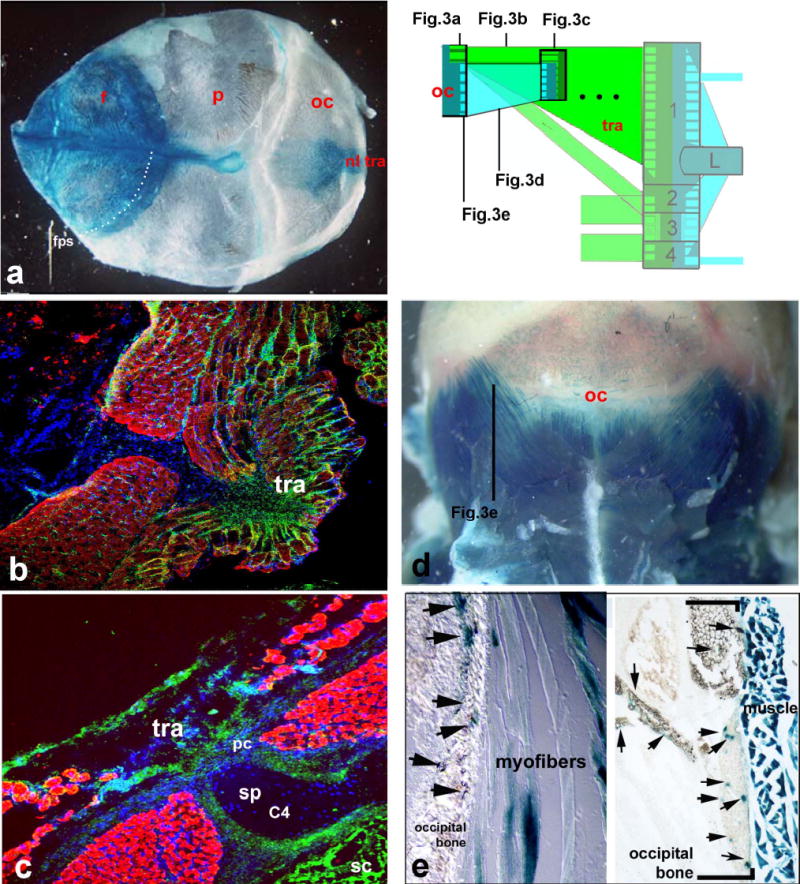
Neural crest and somitic mesodermal origins of the neck. Green areas in schematic are neural crest-derived connective tissues, blue areas are mesodermal (somitic) connective tissues and muscle fibres. Neural crest-derived (left column a,b,c) (nuchal ligament nl)attachment regions of the trapezius (tra) onto the occipital skull (oc in a) and perichondral (pc) sheaths around spinous processes (sp) of cervical vertebrae (C4) are LacZ+ in Wnt-1- (a) and GFP+ (green) in Sox10-transgenics (b,c). As in all ensuing figures nuclear DAPI stain is blue, red is myosin heavy chain IR of muscle fibres, green cells are GFP+ neural crest cells. The fronto-parietal suture (stippled line in a, fps) does not correspond to any cellular boundary of LacZ+ neural crest (cf. supplementary method S 1). The mesodermal connectivity system is shown in the right column. HoxD4-LacZ+ mesodermal occipital bone (oc in schematic, d, arrows in e) is connected to neck vertebrae (box in schematic, dotted lines represent other vertebrae omitted) directly via striated myofibres (in e) of the same genetic (HoxD4-LacZ+) axial identity.
Fig.4.
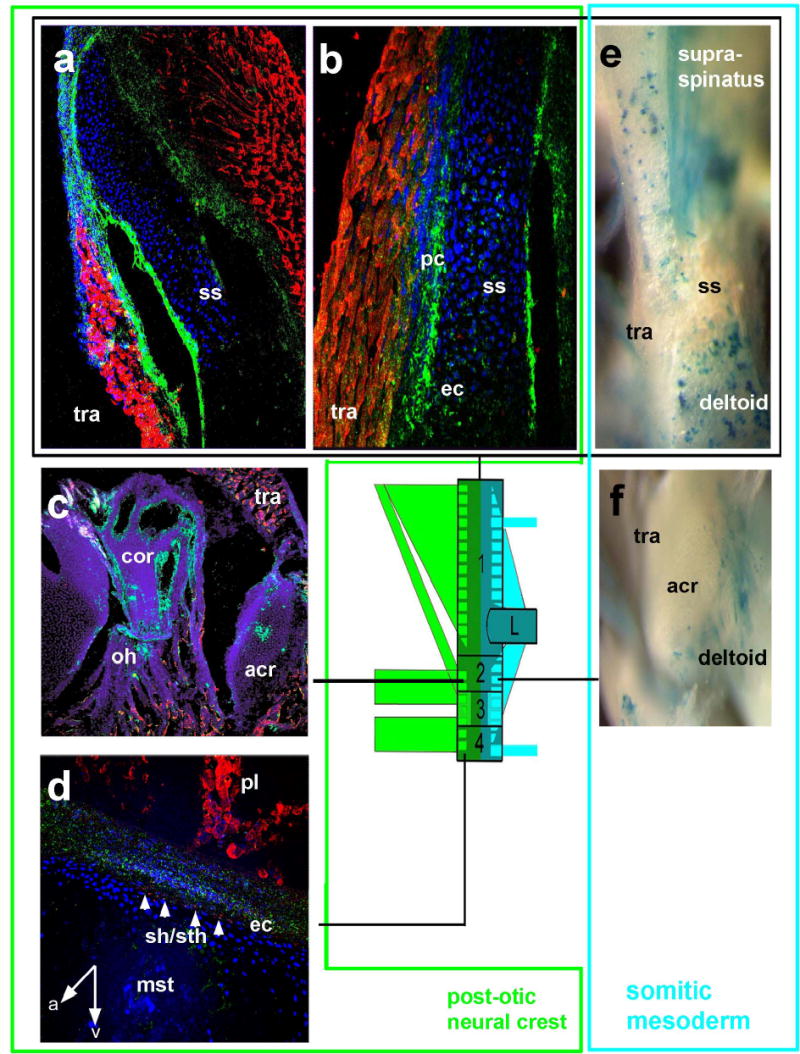
Dual neural crest and mesodermal origins of the endochondral shoulder girdle. Green box PONC GFP+ green (a,b) components, turquoise box mesodermal LacZ+ blue (e) components of the scapular spine (box 1, a,b,e), the acromio-coracoid (box 2, c,f,) and sternum (box4,d). Inside the scapular spine (ss, a,b) GFP+ neural crest cells are found to form endochondral skeleton (ec) and perichondrium (pc) exactly at the places where the trapezius (tra) carrying crest connective tissue is attached. Conversely, blue mesodermal muscle fibres are attached to punctuate areas of the posterior scapular spine (e) and acromion (f). Note that areas of trapezius attachment in mice with mesodermal labeling are white, i.e. unlabelled, showing that neural crest and mesodermal muscle attachment systems do not mix with each other. coracobranchial muscle fibres (Sh/sth in d) are inserted by the endochondral PONC component (white arrows) onto the mesodermal sternum (mst). A anterior, v ventral, pl pleural muscles.
Fig.6.
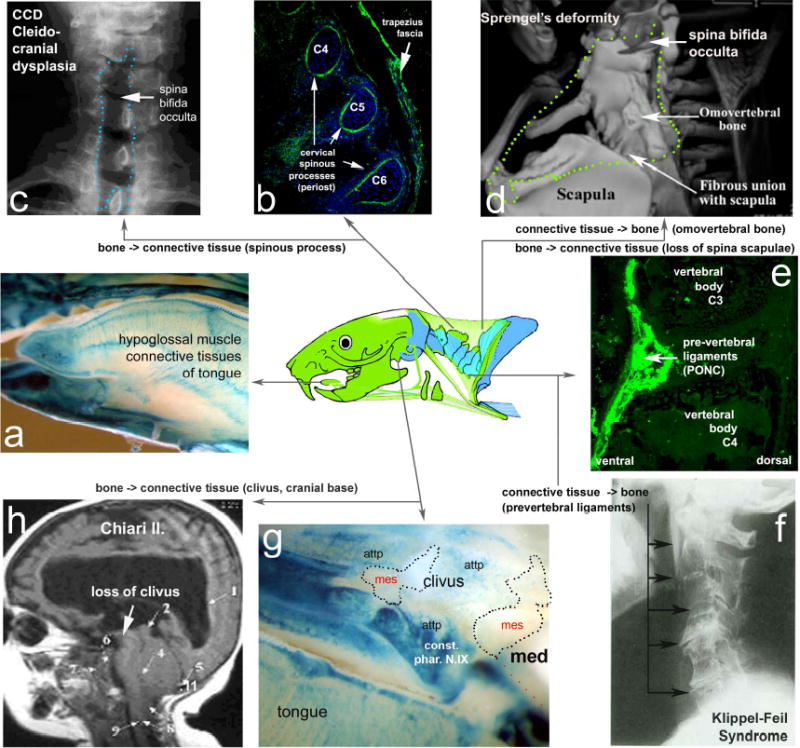
Pathological flexibility of post-otic neural crest differentiation. Changes in fate specification from bone (periosteum) into connective tissue (left half of figure) can explain localized cervical defects in cleidocranial dysplasia (b,c) and Chiari I.+II syndrome (h). In the latter, the PONC-derived clivus (blue in g) of the otherwise mesodermal (mes) cranial base which serves as the attachment point (attp) for pharynx constrictor muscles (constr. Phar.N.X) in front of the medulla (med) fails to form and is replaced by fragile connective tissue (CT). Conversely, PONC-derived CT of pharynx constrictor muscles (e) that are connected to cervical vertebrae can ectopically become bone (right half of figure), leading to neck immobility in the Klippel-Feil syndrome (KFS, in f) or ectopic, ‘omovertebral’ bones inside trapezius territory (stippled line in d) in patients with Sprengel’s deformity, a frequent facet of KFS19,20. Note also the concomitant loss of the PONC derived (but not mesodermal) scapular spine in these patients (d). All tongue muscle CT are entirely neural crest derived (blue areas in a), explaining enlarged tongues (in patients with trisomies) as neurocristopathic.
The largest component of the external crest domain is the trapezius muscle and its attachment regions (tra in Fig. 3a–c). This is a branchial muscle, innervated by the accessory nerve X/XI with a position that has remained remarkably conserved in all jawed vertebrates 5. The cranial neural crest connectivity code revealed by our previous chick-quail work 13 led us to predict that the connective tissue of the trapezius and all its postotic attachments should be formed from PONC - even if they are endochondral. This indeed proves to be the case. At the anterodorsal end, a patch of LacZ+/GFP+ PONC endoskeleton forms the occipital protuberance inside otherwise mesodermal (occipital) territory (i.e. the trapezius attachment point in the skull, Fig. 3a). The labelling extends to the attached nuchal ligament (nl, Fig.3a), the trapezius muscle connective tissues (Fig.3b), as well as their respective endoskeletal attachment region on the entire anterior margin and inside the scapular spine (Fig.4,a,b), the coracoid, acromion (Fig.4c, box 2 in Fig. 1) as well as the periosteal muscle attachment caps on spinous processes of all cervical vertebrae (pc in Fig.3c,6b). Thus, the posterior neural crest boundary is found inside the shoulder girdle endoskeleton where it forms an anterior attachment region of branchial muscles. PONC thus generates extensive areas of endochondral ossification in the shoulder girdle and cervical vertebral column, contradicting the traditional notion that these regions are wholly mesodermal4,11. However, the crest contributions are morphologically cryptic: their only visible anatomical landmarks are the branchial muscle attachments. Tracing the posterior PONC boundary more ventrally we investigate the sternocleidomastoid muscle (N. XI, scm in Fig.5a,b,c,e). This originates on the (postotic) mastoid process of the skull (m in Fig.5b) and attaches onto the endoskeletal anterior sternum (st in Fig. 5a,c,e) as well as along the anterior margin of the dermal clavicle (cl in Fig.5a, d). We find green LacZ+/GFP+ PONC cells inside all of these attachment sites. Our genetic labelling provides first detailed insights into cellular architecture and origins of the clavicle (black box, Fig.5c,d,f,g). The clavicle bone (black box in Fig.5) forms from an anterior (buccal) dermal (Fig. 5d) as well as a posterior dermal ossification centre (Fig.5g), which later fuse and surround a cartilaginous core in mammals (Fig. 5c,g) 15,16. The anterior dermal ossification is purely PONC derived. (green box, Fig. 5d). More medially, attachment regions of branchial muscles (sternomastoid, cleidomastoid, fascial sling of omohyoid ohs) and most of the cartilaginous core of the clavicle are also neural crest derived (Fig. 5c). Thus, as inside the scapula, PONC inside the clavicle gives rise to endochondral bone.
Fig.5.
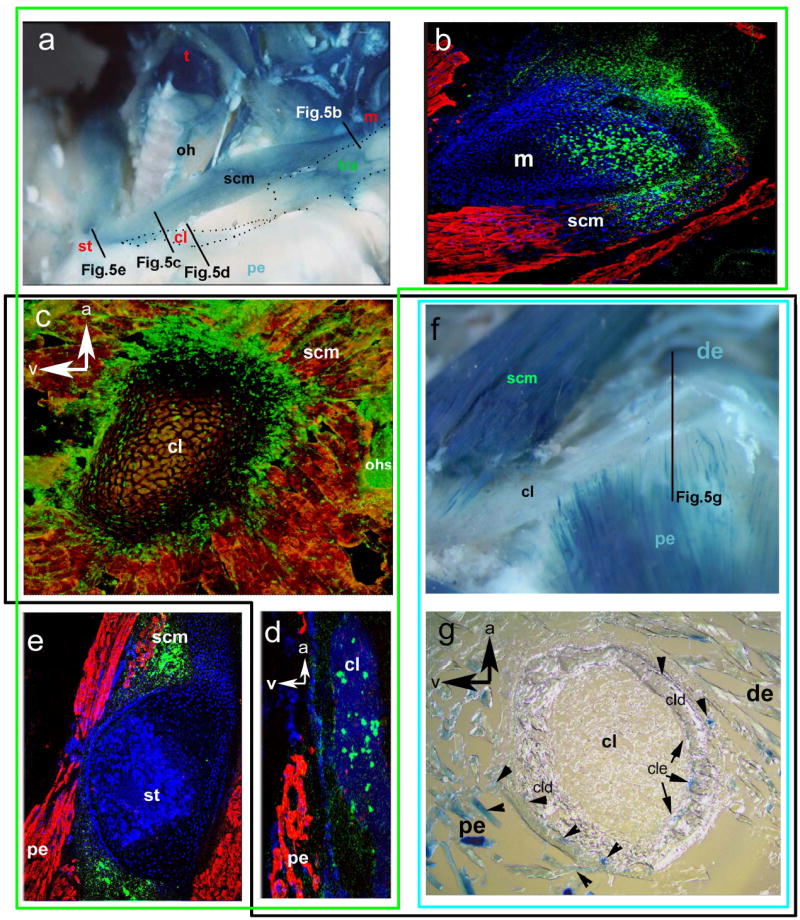
The dual architecture of the clavicle: cell population boundaries coincide with muscle attachments and not with ossification modes. Black box surrounds data of the clavicle. a–e PONC-derived parts, f–g mesodermal parts. PONC connective tissue of the sternocleidomastoid (scm) attaches onto the mastoid process (m) of the postotic skull (b) and reaches down to the clavicle (cl) which is endochondral (in c) and dermal (in d) anteriorly and onto an endoskeletal PONC patch of the sternum (st in e). Contiguous DAPI staining of bone matrix in d shows that no other unlabelled (i.e. mesodermal) cells are present inside the anterior clavicle. Complementary to this, LacZ+ mesodermal myocytes (f,g) of the pectoral (pe) and deltoid (de) muscles are exclusively attached onto the posterior dermal (cld!) and endochondral (cle) clavicle margin (g),. The anterior clavicle is unlabelled in the transgenic with mesodermal labeling (white in f): LacZ+ mesodermal cells are confined to mesodermal attachment points.
The ventral shoulder girdle carries a series of muscles that connect it to ventral branchial elements (Box4, Co1, Co2 in Fig. 1, 7): M.omohyoideus (connecting the anterior scapula next to the coracoid, and the internal clavicle, with the hyoid; oh, Fig.4c,5a,c), M. sternohyoideus (connecting the manubrium sterni and clavicle to the hyoid; sth, Figs.2b,4d) and M. sternothyroideus (sth, Fig. 4d connecting the manubrium sterni with the thyroid cartilage). These are homologs of the coracobranchial muscles (Co1,2) present in all jawed vertebrates and effect rapid jaw opening and retraction of the branchial skeleton 3,5. As these are in origin branchial muscles (innervated by cranial nerves 5) we hypothesized that their connective tissue would be crest derived and this is indeed the case. Swallowing in all jawed vertebrates requires internal pharynx and larynx constrictors which constitute the internal tubular crest domain outlined above. These are connected to the mesodermal cranial base (the so-called pharyngeal tubercle) anterior to the foramen magnum via the pharyngobasilar fascia, as well as to the ventral neck vertebrae via the pharyngeal raphe (Fig.2b, 6e,g). Their branchial innervation (by N.IX, X.) suggests their attachments regions to be PONC-derived too in all vertebrates. Despite being at odds with the commonly held notion that ‘chordal’ cranial base is entirely mesodermal 4,11, our PONC-labelled mice show constrictor muscle attachment points of neural crest origin focally inserted into the otherwise mesodermal endochondral cranial base (Fig. 6g). More posteriorly, thyroid, cricoid and arytenoid cartilages and their respective muscle attachments at the oesophageal entry are also neural crest-derived with tracheal cartilages demarcating the anterior mesoderm boundary (data not shown). More surprisingly, the entire intrinsic tongue musculature that is attached to crest-derived branchial skeleton has crest-derived connective tissue, despite being innervated by N.XII and cervical spinal nerves (Fig.6a). This demonstrates that motor-innervation alone cannot serve as a reliable indicator of embryonic muscle connective tissue origins, but skeleton-muscular connectivity can. These genetic PONC-labelling experiments demonstrate that post-otic neural crest in mouse behaves in the same way as the pre-otic crest studied in our previous experiments 13. Crest cells form not only the connective tissue of the muscle but all its attachment points, irrespective of how these attachment points ossify: endochondrally or dermally or – as inside the tongue (Fig.6a)- they do not ossify. This unveils a pervasive but anatomically cryptic ‘muscle scaffold system’ that is sharply defined at the single cell level.
Fig.7.
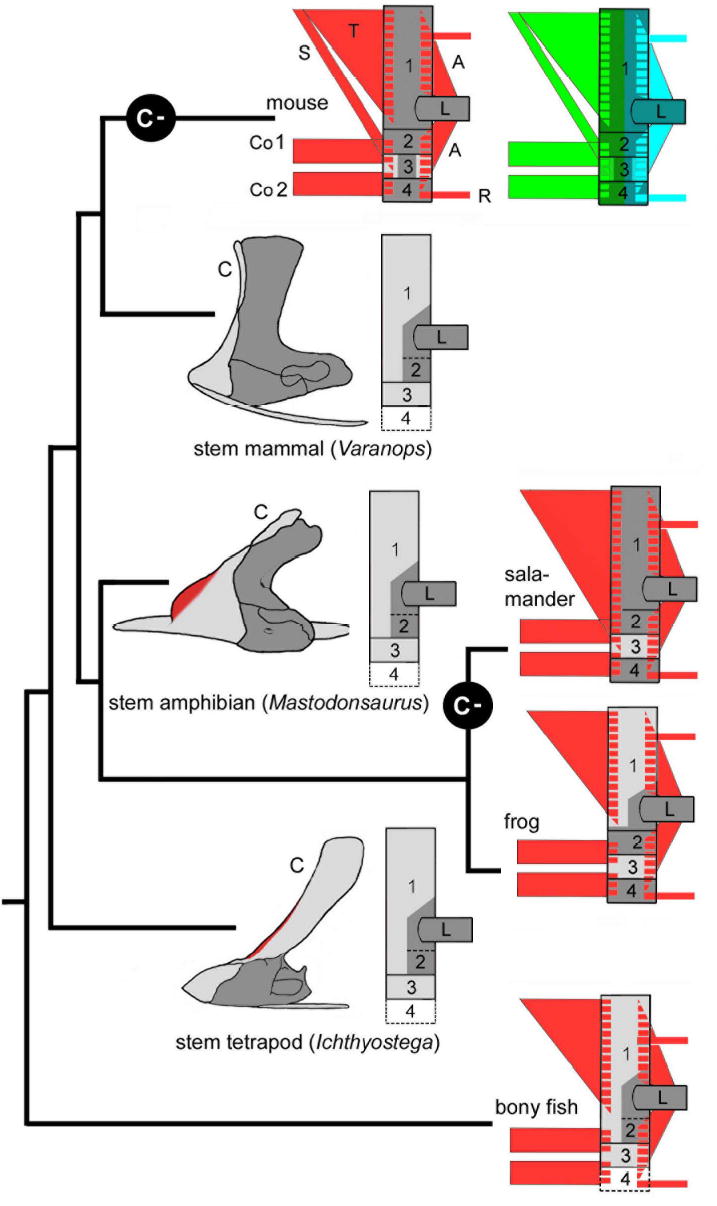
Muscle scaffolds in fossils: the cleithrum (box 1) goes into hiding (C-) several times independently in evolution and ‘morphs’ into scapular spines. In all species with a cleithrum - be they fossil mammalian (Varanops ref38), amphibian (Mastodonsaurus 37) or tetrapod (Ichthyostega 35, P.E.A. pers. obs.) stem group representatives or extant frogs36 - it is identical in position to the scapular spine of extant tetrapods with respect to the dual trapezius (T) and mesodermal muscle connectivity and its dorsoventral position. Red zones on fossil forms indicate observed trapezius (T) complex attachment regions.
Fig.2.
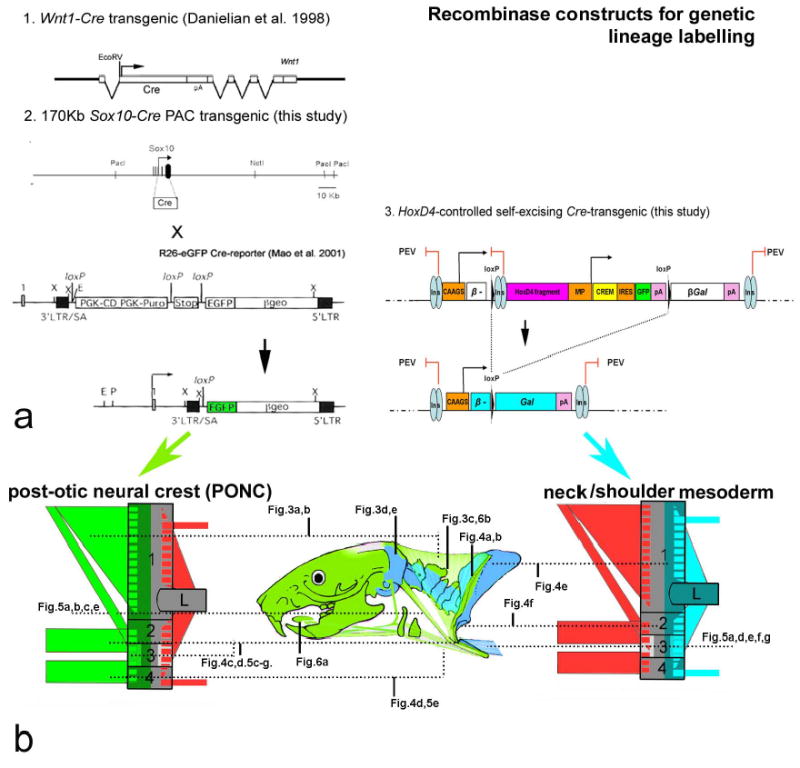
Genetic lineage labeling of post-otic neural crest and somitic mesoderm in the neck. a. transgenics carrying 1.Wnt—Cre-17, 2.Sox10Cre- constructs were crossed into R26-RLacZ 45 and –GFP 46 reporters in order to permanently label all pre- and post-migratory post-otic/neck neural crest (green). 3. A self-excising Cre-recombinase leads to re-constitution of a β-Gal reading frame in the complementary population, the somitic (HoxD4+) mesoderm of somite 5 and posterior (blue). See supplementary method S1 for technical details. b. Anatomical guide through mouse neck and shoulder skeleton, reference to schematics and data figs.
Rules of engagement in the mesodermal neck
In addition to branchial muscles at its anterior margin, the shoulder girdle in all jawed vertebrates serves as attachment for mesodermal trunk and limb muscles (with spinal motor neuron innervation and connective tissues derived from mesoderm) at its posterior margin5. Mesodermal trunk muscles also attach to the occipital head (Fig.2b)11,14. While the mesodermal (somitic) origins of vertebrae and occiput have long been established, the rules of connectivity between muscles and bony elements have remained unclear. This prompted us to test whether skeletal attachment sites of muscles with mesodermal connective tissues are also mesodermal and of the same (Hox gene) axial identity. As axial Hox gene expression boundaries are sometimes not coinciding with somitic boundaries18- a genetic approach is required (see online supplementary methods S1).
Analysis of HoxD4-CREM-LacZ transgenics shows that that posterior margin of the scapular spine (right part of Box1 in Fig. 1,7, Fig.4e) as well as the entire scapular blade is of somitic (LacZ+) origin at places where mesodermal muscles (supraspinatus, deltoid, Fig. 4e) are attached. Similar to the scapular spine, the more ventral coracoid and acromion (Box2 in Figs. 1,7, Fig4c,f) are also of dual neural crest-mesoderm origin with attachment regions corresponding to muscle connective tissue origins. More ventrally, the clavicle reveals an as yet unrecognized differentiative potential of somitic mesoderm (Box3 in Fig. 1,7, Fig.5 g). According to the commonly held ‘ossification model’ all dermal clavicular parts (i.e. the anterior as well as posterior one) are expected to be neural crest derived9,10. We find this to be an incorrect prediction for the clavicle. The posterior margin of the clavicle in HoxD4-LacZ transgenics is LacZ+ at all dermal (cld in Fig.5g) as well as endochondral attachment sites (cle in Fig.5g) of LacZ+ i.e. mesodermal pectoral and deltoid muscle fibres (pe and de in Fig. 5f,g).
This demonstrates not only that the clavicle itself is a neural crest-mesodermal interface, but also that postcranial mesoderm gives rise to dermal skeletal elements. It was well-known that the posterior dermal clavicle ossifies independently from the anterior dermal ossification centre, but its separate (mesodermal) origin was unknown15,16. This is the first experimental precedent for interpreting other trunk dermal armour plates among fossil and extant vertebrates as mesodermal. Based on our findings it is conceivable that the posterior dermal clavicle is the last remnant of a more widespread body armour of mesodermal origin. More ventrally, the sternum (Box4 in Fig. 1,7) is of mesodermal origin at its core and posterior margin, in line with the connectivities described above (data not shown), while its anterior margin (manubrium sterni, mst in Fig.4d,5a) harbours crest derived endochondral attachment points for coracobranchial musculature (Co2, arrows in Fig.4d).
In the occipital head region (Fig.3d,e), examination of HoxD4 transgenics demonstrates that blue (HoxD4LacZ+) muscle fibres are focally and directly attached onto HoxD4+ skeletal regions (arrows in Fig. 3e). This shows for the first time by genetic means that somitic mesoderm strictly obeys a (Hox-mediated) genetic connectivity code directly comparable to that of the neural crest. Interestingly, although the trapezius and sterno-cleidomastoid muscle also receives all its muscle fibres from (HoxD4+) somitic mesoderm (Fig.3d, Fig.5f blue scm) these fibres are connected to skeletal elements only via PONC-derived connective tissues (Fig.4b,5c). In contrast, (HoxD4+)mesodermal muscle fibres (of occipital, pectoral or deltoid muscles) directly connect to mesodermal skeletal structures – without the mediation through mesodermal connective tissues (Fig.3e). Post-otic somitic mesoderm is similarly capable of forming dermal and endoskeletal bone as well as connective tissue, a fact that invalidates the universal use of the ‘ossification model’ in comparative morphology or paleontology. Instead, we find that the muscular ‘scaffold model’ more appropriately describes the dual composition and complex connectivity pattern of the neck and shoulder girdle.
Neural crest and human neck pathology
The flexibility of shoulder ossification types inside a highly constrained (trapezius) muscle scaffold as observed in comparative neck anatomy (Fig. 1) might reflect an innate flexibility of PONC cells to respond to osteogenic stimuli which, in turn, might render them prone to pathological malformation. The dual origin of neck and shoulder structures would also make it likely to find modular i.e. PONC- versus mesoderm-specific pathological phenotypes. We therefore searched for human congenital syndromes with a tissue distribution that precisely corresponds to the PONC-population territory but displays pathological differentiation of PONC cells only: crest connective tissues would adopt new cartilaginous or osseous fates and vice versa (Fig.6). The complex distribution of PONC-derived structures and the strict muscular connectivity rules yield safe criteria for discriminating patterning from differentiation defects. Interestingly, several hitherto poorly understood human syndromes precisely match the profile of a PONC syndrome and permit first insights into their common cellular aetiology. Klippel-Feil-Disease (Fig.6f)19, Sprengel’s deformity (Fig.6d)20, CCD (cleidocranial dysplasia) (Fig.6c)21, Arnold-Chiari I/II malformation (Fig.6h)22 and Cri-du-Chat 23 syndrome are all characterized by co-occurrence of pharyngeal/laryngeal, (sub-)occipital, cervical and shoulder dysmorphologies and swallowing problems. They also share a spina bifida occulta (Fig.6d) which is confined to the cervical region normally occupied by the trapezius. In Sprengel’s deformity a large fibrous, sometimes endochondral so-called omo-vertebral bone replaces all dorsal neural crest derived endochondral elements of occipital region, cervical spinous processes, spina scapulae and trapezius 20 inside the PONC trapezius territory (Fig.6d). On this basis we identify Sprengel’s deformity, which is one of the phenotypic facets of Klippel-Feil syndrome, as primarily affecting PONC fate choices and not cervical segmentation as is currently thought19. Moreover, the cervical hypomobility of Klippel-Feil patients can also be understood as caused by defects in PONC fate choices: ectopic ossifications of PONC (trapezius) connective tissues around the (somitic mesodermal) neck vertebrae and an ectopic ossification of the PONC pre-vertebral ligaments of pharyngeal muscles (Fig.6e,f). Similarly, loss or dysplasia of PONC-derived basicranial (clivus) bone attachments for the internal pharynx and larynx constrictors (Fig.6g, attp, constr.phar.NIX) and ensuing widening of the foramen magnum is the primary mechanical cause of the Arnold-Chiari I+II malformation, a serious human congenital malformation associated with swallowing problems and sudden infant (cot) death syndrome (SIDS)(Fig.6h) 22. In this case PONC re-specification from (endochondral) attachment bone to connective tissue is the likely cause of basicranial instability and early death. Detailed aetiologies of these congenital syndromes will be discussed elsewhere (TM, GK, ms. in preparation). Given the cellular identification of these defects and corresponding phenotypes of transcription factor mutants in mice [Supplementary Fig. S2] the underlying genetic causes can now be investigated in a more focused manner: impaired transcription factor networks acting inside PONC cell populations during development.
Discussion: Scaffold model, homologies and mechanisms
We have identified the neck and shoulder region as the interface of the neural crest and mesodermal cell populations. We show that boundaries of embryonic cell populations precisely correspond to muscle attachment regions but not to ossification modes. The evolutionary conservation of muscle patterns (Fig. 1) is therefore likely to be a reflection of conserved cell population boundaries. The latter appear to be far more stable than the signaling pathways that determine their (dermal-endochondral) ossification as attachment points (Fig. 1). An alternative hypothesis would have to find multiple independent developmental explanations for such highly constrained muscle patterns (Fig. 1). Verification of cell boundary stability and the validity of the ‘scaffold model’ will have to await further genetic fate mapping in a wider phylogenetic range of species when this becomes possible. However, our present high-resolution data set for the mouse allows us to refute the widely held competing ‘ossification model’9,10. Dermal versus endochondral ossification modes are not safe criteria for identifying cellular origins and homologies of neck and shoulder structures. The rather counter-intuitive ‘scaffold model’ perceives muscle connectivities as the basic units (as they precisely correspond to cell populations) but the bones everyone can see as mere epi-phenomena and subjects of change. This prompts a new heuristic strategy for experimentally establishing neck homologies on the basis of attachment criteria details of which can be found in supplementary methods S3.
The connectivity patterns which we observe with single cell resolution in the mesodermal occipital and shoulder girdle are stricter than anticipated 14,24. Muscles are directly connected onto skeleton of the same axial HoxD4+ gene identity– without mediation through connective tissue (dots in Fig.4,e,f, Fig.3e). Our findings corroborate the emerging notion 25, 26 that in vertebrates, as previously found in Drosophila 27, myoblast (muscle) precursors still harbour positional identity (inherited from their somitic mesenchymal stem cell precursors) and are not as naïve as commonly perceived. Neural crest and mesoderm independently acquire Hox gene expression during evolution 28. Hox genes have been proposed to be responsible for population contiguity in neural crest 13,29,30. We note that mouse mutants of Pbx1 31, Pax132,, Pax 9 33, interactors or downstream targets of autonomously acting Hox genes, display modular neck/shoulder defects of PONC but not mesodermal bones and replicate human syndromes mentioned above ( supplementary Fig. S2, data not shown). This supports the notion that (cell type-specific) modularity and connectivity is Hox gene controlled. Complementary to these, Emx2 mouse mutants lack the mesodermal scapular blade but not PONC shoulder derivatives34. While HoxD4+ somites also provide muscle cells to the trapezius and other branchial neck muscles, these myoblasts appear to be subjugated to (neural crest derived) muscle connective tissue: they do not attach directly onto skeletal regions as mesodermal muscles do. It will be an interesting challenge to disentangle the molecular causes for such patterning dominance of neural crest over mesoderm in areas of spatial overlap, where neural crest and somitic Hox codes ‘collide’.
Fossil fates: chasing the cleithrum’s ghost [43, preferred or] Chasing the ghost of the cleithrum [34]
The conservation of the neck muscle scaffold among jawed vertebrates and its precise correspondence to cell population boundaries provides refined (single cell) criteria for tracing skeletal fate changes of a more fundamental nature. This permits us to determine the whereabouts of elements such as the elusive cleithrum, the central most shoulder bone of all bony fish (osteichthyan) ancestors which is absent in all extant land living vertebrates (tetrapods)35 except frogs36. The cleithrum is uniquely defined by its position and connectivity. In extant bony fish and frogs it serves as the sole attachment region for the (trapezius/cucullaris) muscles anteriorly5,36 and for fin/limb/trunk muscles at its posterior margin (Fig.7)35,36. Several historical hypotheses have been proposed to explain its absence among most living tetrapods. 1. the cleithrum was lost among common tetrapod (stem group) ancestors and re-acquired only in frogs, or. 2. it was independently lost in the lineages leading to living salamanders, diapsids, turtles and mammals. These two hypotheses have divergent implications for macro-evolutionary trends in neck skeletogenesis. Comparative anatomy of extant species cannot distinguish between these two hypotheses (Fig. 1,7 compare salamander and mammal). Paleontology, on the other hand, has unearthed a rich data set of fossil stem group shoulder morphologies that can resolve the timing and polarity of these changes. Using these attachment criteria as a guide we re-examine representative examples of stem tetrapods (Ichthyostega), stem amphibians (Mastodonsaurus)37, stem amniotes (Seymouria and Diadectes, not shown) and stem group mammals (Varanops) 38 ( Fig.7). All stem group tetrapods discovered to date possess a cleithrum (C in Fig. 7) covering the entire antero-dorsal margin of the shoulder girdle. Indeed, trapezius/cucullaris muscle scars can be found on the cleithra of Jacubsonia 39, Pederpes (Clack & Finney 2005, pers. comm.)40 and Ichthyostega (PEA, pers. obs.) (red in Fig.7) which demonstrates a cleithrum muscle connectivity pattern primitively retained from the fish condition. We also find a cleithrum in stem reptiles such as Araeoscelis 41 and stem turtles Proganochelys42 and Kayentachelys 43. Thus, the cleithrum has been lost at least 4 times independently - –in salamanders, mammals, turtles and diapsids (C- in Fig. 7, data not shown). Meanwhile, the muscle connectivities embracing the cleithrum have remained unchanged from the fish condition: exactly like the ancestral cleithrum, the mammalian scapular spine is positioned between the conserved trapezius and limb/trunk muscle attachment systems as a common abutment of the two. Based on identical (cell population-mediated) connectivity and position, our genetic labeling proposes to identify the endochondral scapular spine as the ‘cell population ghost’ of the cleithrum. It will be interesting to illuminate the molecular causes for this uni-directional trend to dismantle the dermal shoulder girdle, replace it by endochondral skeleton or lose it altogether which appears to continue in mammals and amphibian and also extends to other bones such as the clavicle16, 36. In the framework of the highly constrained neck muscle scaffolds we find no evidence for histogenetic reversals, i.e. that endochondral bones of ancestors turned into dermal bones of descendants during the course of evolution. We speculate that a common as yet unknown genomic cis-regulatory architecture governing neck ossifications in tetrapod ancestors might have pre-disposed different descending tetrapod lineages to similar parallel trends.
The present study provides a first identification of the embryonic cell populations involved in neck patterning: postotic neural crest and somitic mesoderm. These mesenchymal stem cell populations are subject to considerable muscle patterning constraints while they retain (pathological and evolutionary) flexibility in their osteogenic differentiation. The molecular basis of such constraints and flexibilities and their integration in single cells remains to be discovered. Ultimately, this ‘Protean’ flexibility of mesenchymal stem cells to ’morph’ into cartilage, bone and connective tissue will have to be explained in the language of evolving gene-regulatory circuitry. This genetic circuitry will have to be placed into future reconstructions of phylogenetic trees as it was causative for the diverse neck morphologies that we observe. We anticipate that traces of other major evolutionary transformations and novelties will become detectable on a single cell level once comparative genetic lineage-mapping becomes possible.
Methods
We generated two independent transgenic mouse lines in which all postotic neural crest (PONC) are permanently labelled by means of recombinase-activated marker cassettes. Wnt1 is expressed in all premigratory PONC cell precursors 17 and Sox10 is expressed strongly in the entire post-migratory PONC population during early embryonic development and not at all in mesoderm 44. We therefore labeled pre-migratory PONC with a Wnt-1-Cre transgene 17 and post-migratory PONC by a Sox 10-Cre BAC transgene, introduced into founders by pronuclear injection (Fig.2a, 1. and 2.). Transgenic founders were crossed to Cre-conditional R26R-LacZ and -GFP reporter lines 45,46. This allowed us to visualize for the first time the entire LacZ+ and GFP+ postotic neural crest (PONC) population of the head/neck/shoulder region with single cell resolution (Fig.2b). Complementary to these lines, we generated a third transgenic line in which all post-occipital paraxial (neck and trunk) mesoderm was permanently labeled, carrying a novel HoxD4-CREM-LacZ construct (Fig.2,a 3.). Landmark studies on the correspondence between Hox gene expression and axial somitic identity had predicted Hox 4 paralogues to define the non-occipital/occipital boundary 18,47. These genetic and embryological studies 24 had shown that the occipital plane in amniotes runs exactly through somite 5 which expresses HoxD4. HoxD4 is not expressed in neural crest and its mesodermal expression is regulated by a well-characterized genomic fragment 48. We cloned this fragment into a novel lineage labeling construct for HoxD4+ somitic mesenchymal stem cells and all of their progeny from somite 5 backwards (Fig.2b). Briefly, LacZ marker activation is made conditional upon a HoxD4-enhancer controlled self-excision of Cre-recombinase. This new strategy obviates traditional problems of differential position-effect variegation (PEV in Fig.2a3.) of transgenic Cre-drivers and reporters. It also avoids deleterious effects of high Cre-recombinase levels in tissues50. Please refer to supplementary Methods S1 for technical details on the constructs, controls and analysis.
Supplementary Material
Acknowledgments
We are indebted to A. Lumsden for help with a complex manuscript. P. Soriano and S. Orkin kindly provided Cre-reporters. A. West, G. Felsenfeld and J. Green gave advice on insulators and plasmids. This work was funded by BBSRC (GK, PEA), the Wellcome Trust (GK, WDR), the UK-MRC (WDR), the Swedish Research Council (PEA), NIH (APM) and WIBR-UCL (GK). GK and TM were long-term postdoctoral fellows of HFSPO. GK gratefully acknowledges Salvador Moncada for support in establishing a new lab.
Footnotes
Supplementary information accompanies the paper on www.nature.com/nature.
Authors’ contribution T.M. and P.E.A are equally contributing first-authors of this work.
Competing interests statement The authors declare that they have no competing financial interests.
References
- 1.Janvier, P. Early Vertebrates (Oxford Science Publications, 1996).
- 2.Johanson Z. Placoderm branchial and hypobranchial muscles and origins in jawed vertebrates. J Vert Pal. 2003;23:735–749. [Google Scholar]
- 3.Motta, P.J. & Wilga, C.D. Advances in the study of feeding behaviours, mechanisms, and mechanics of sharks. in Environmental Biology of Fishes. 60, 131–156 (Kluwer Academic Publishers, Netherlands, 2001).
- 4.LeDouarin N. & Kalcheim, C. The Neural Crest (Cambridge University Press, 2nd Edition 1999
- 5.Edgeworth, F. H. The cranial muscles of vertebrates (Cambridge University Press, 1935)
- 6.Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Nature. 1997 Aug 14;388(6643):639–48. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- 7.Lumsden A, Sprawson N, Graham A. Segmental origin and migration of neural crest cells in the hindbrain region of the chick embryo. Development. 1991;113:1281–1291. doi: 10.1242/dev.113.4.1281. [DOI] [PubMed] [Google Scholar]
- 8.Saunders JWJ. The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. J Exp Zool. 1948;108:363–403. doi: 10.1002/jez.1401080304. [DOI] [PubMed] [Google Scholar]
- 9.Smith MM, Hall BK. Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol Rev. 1990;65:277–373. doi: 10.1111/j.1469-185x.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith MM & Hall, B.K. A developmental model for evolution of the vertebrate exoskeleton and teeth: The role of cranial and trunk neural crest. In: M. K. Hecht, R. J. MacIntyre and M. T. Clegg, Editors, Evolutionary Biology, Volume 27, Plenum Press, pp. 387–448 New York (1993)
- 11.Couly GF, Coltey PM, LeDouarin NM. The triple origin of skull in higher vertebrates: a study inquail-chick chimeras. Development. 1993;114:1–15. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in themammalian skull vault. Dev Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- 13.Koentges G, Lumsden AGS. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- 14.Huang R, et al. Contribution of single somites to the skeleton and muscles of the occipital and cervicalregions in avian embryos. Anat Embryol. 2000;202:375–383. doi: 10.1007/s004290000131. [DOI] [PubMed] [Google Scholar]
- 15.Huang LF, et al. Mouse clavicular development: analysis of wild-type and cleidocranial dysplasia mutant mice. Dev Dyn. 1997;210:33–40. doi: 10.1002/(SICI)1097-0177(199709)210:1<33::AID-AJA4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Hall BK. Development of the clavicles in birds and mammals. J Exp Zool. 2001;289:153–161. doi: 10.1002/1097-010x(20010215)289:3<153::aid-jez1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 17.Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of geneactivity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- 18.Burke AC, Nelson CE, Morgan BA, Tabin C. Hox genes and the evolution of vertebrate axialmorphology, Development 121, 333–346 (1995) doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- 19.Clarke RA, Catalan G, Diwan AD, Kearsley JH. Heterogeneity in Klippel-Feil syndrome: a newclassification. Pediatr Radiol. 1998 Dec;28(12):967–74. doi: 10.1007/s002470050511. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz AE. Congenital elevation of the scapula – Sprengel’s deformity. Am J Orthop Surg. 1908;6:260–311. [Google Scholar]
- 21.Otto F, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 22.Greenfield’s Neuropathology, 7th edition, D.I. Graham & Lantos, P.L. (Oxford University Press 2002)
- 23.Kjaer I, Niebuhr E. Studies of the cranial base in 23 patients with cri-du-chat syndrome suggest a cranial developmental field involved in the condition. Am J Med Genet. 1999 Jan 1;82(1):6–14. doi: 10.1002/(sici)1096-8628(19990101)82:1<6::aid-ajmg2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 24.Huang R, Zhi Q, Patel K, Wilting J, Christ B. Dual origin and segmental organisation of the avian scapula. Development. 2000;127:3789–3794. doi: 10.1242/dev.127.17.3789. [DOI] [PubMed] [Google Scholar]
- 25.Alvares LE, et al. Intrinsic, Hox-dependant cues determine the fate of skeletal muscle precursors. Devel Cell. 2003;5:379–390. doi: 10.1016/s1534-5807(03)00263-6. [DOI] [PubMed] [Google Scholar]
- 26.Schweitzer R, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001 Oct;128(19):3855–66. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 27.Baylies MK, et al. Myogenesis: a view from Drosophila. Cell. 1998;93:921–7. doi: 10.1016/s0092-8674(00)81198-8. [DOI] [PubMed] [Google Scholar]
- 28.Takio Y, et al. Evolutionary Biology: lamprey Hox genes and the evolution of jaws. Nature. 2004;429:262. doi: 10.1038/nature02616. [DOI] [PubMed] [Google Scholar]
- 29.Barrow JR, Capecchi MR. Compensatory defects associated with mutations in Hoxa1 restore normal palatogenesis to Hoxa2 mutants. Development. 1999;126:5011–26. doi: 10.1242/dev.126.22.5011. [DOI] [PubMed] [Google Scholar]
- 30.Smith A, et al. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr Biol. 1997 Aug 1;7(8):561–70. doi: 10.1016/s0960-9822(06)00255-7. [DOI] [PubMed] [Google Scholar]
- 31.Selleri L, et al. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development. 2001 Sep;128(18):3543–57. doi: 10.1242/dev.128.18.3543. [DOI] [PubMed] [Google Scholar]
- 32.Dietrich S, Gruss P. undulated phenotypes suggest a role of Pax-1 for the development of vertebral andextravertebral structures. Dev Biol. 1995 Feb;167(2):529–48. doi: 10.1006/dbio.1995.1047. [DOI] [PubMed] [Google Scholar]
- 33.Peters H, et al. Pax1 and Pax9 synergistically regulate vertebral column development. Development. 1999 Dec;126(23):5399–408. doi: 10.1242/dev.126.23.5399. [DOI] [PubMed] [Google Scholar]
- 34.Prols F, et al. The role of Emx2 during scapula formation. Dev Biol. 2004 Nov 15;275(2):315–24. doi: 10.1016/j.ydbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Jarvik, E. Basic Structure and Evolution of Vertebrates, vol. 1 (Academic Press, London, 1980)
- 36.Shearman RM. Growth of the pectoral girdle of the Leopard Frog Rana pipiens (Anura:Ranidae) J Morph. 2005;264:94–104. doi: 10.1002/jmor.10322. [DOI] [PubMed] [Google Scholar]
- 37.Schoch RR. Comparative osteology of Mastodonsaurus giganteus (Jaeger, 1828) from the Middle Triassic (Lettenkeuper: Longobardian) of Germany (Baden-Württemberg, Bayern, Thüringen) Stuttgarter Beiträge zur Naturkunde, Serie B. 1999;278:1–175. [Google Scholar]
- 38.Sumida, S. S. in Amniote Origins (eds. Sumida, S. S. & Martin, K. L. M.) 353–398 (Academic, San Diego,1997).
- 39.Lebedev, O. A. in The Second Gross Symposium “Advances in Palaeoichthyology” (ed. Luksevics, E.) 79–98 (Acta Universitatis Latviensis 679, 2005)
- 40.Clack JA, Finney SM. Pederpes finneyae, an articulated tetrapod from the Tournaisian of WesternScotland. J Syst Palaeont. 2005;2:311–346. [Google Scholar]
- 41.Reisz RR, Berman DS, Scott D. The anatomy and relationships of the Lower Permian reptile Araeoscelis. J Vert Paleontol. 1984;4:57–67. [Google Scholar]
- 42.Jaekel O. Die Wirbeltierfunde aus dem Keuper von Halberstadt. Paläont Zeitschrift. 1915–16;2:88–214. [Google Scholar]
- 43.Joyce W. The presence of cleithra in the primitive turtle Kayentachelys aprix. J Vert Paleontol Suppl. 2003;23(3):66A. [Google Scholar]
- 44.Ferguson CA, Graham A. Redefining the head-trunk interface for the neural crest. Dev Biol. 2004;269:70–80. doi: 10.1016/j.ydbio.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 46.Mao X, et al. Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood. 2001;97:324–326. doi: 10.1182/blood.v97.1.324. [DOI] [PubMed] [Google Scholar]
- 47.Condie BG, Capecchi MR. Mice with targeted disruptions in the paralogous genes Hoxa-3 and Hoxd-3 reveal synergistic interactions. Nature. 1994;370:304–307. doi: 10.1038/370304a0. [DOI] [PubMed] [Google Scholar]
- 48.Zhang F, et al. Elements both 5′ and 3′ to the murine Hoxd4 gene establish anterior borders of expression in mesoderm and neuroectoderm. Mech Dev. 1997;67:49–58. doi: 10.1016/s0925-4773(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 49.Winterbottom R. A descriptive synonymy of the striated muscles of the teleostei. Proc Acad Nat Scil, Philadelphia. 1974;125:225–317. [Google Scholar]
- 50.Loonstra A, et al. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci U S A. 2001 Jul 31;98(16):9209–14. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


