Abstract
The genes encoding cholera toxin (CT), ctxAB, are coregulated with those for other Vibrio cholerae virulence factors by a cascade of transcriptional activators, including ToxR, TcpP, and ToxT. Additional regulators that modulate expression of ctxAB during infection were recently identified in a genetic selection. A transposon insertion in vieS, the sensor kinase of the VieSAB three-component signal transduction system, resulted in failure to induce expression of a ctxA-recombinase fusion during murine infection. To determine which components of the VieSAB system are essential for CT regulation, ctxAB transcript levels were assessed by RNase protection assay in various vieSAB in-frame deletion mutants after growth in vitro under virulence gene inducing conditions. A threefold reduction in ctxAB transcript levels was observed for the ΔvieSAB strain; consistent with this, the ΔvieSAB strain produced twofold less CT protein than the wild type, and this defect was complementable in trans. These results suggest that the VieSAB three-component system is required for full activation of the ctxAB operon during in vitro growth as well as during infection. The VieSAB system may regulate ctxAB expression indirectly by affecting production of ToxT, because decreased toxT transcript levels were observed in the ΔvieSAB strain.
The gram-negative bacterium Vibrio cholerae requires both colonization of the small intestinal epithelium, mediated by the toxin-coregulated pilus (TCP), and production of cholera toxin (CT), an ADP-ribosylating toxin, to cause the severe diarrheal disease cholera. CT and TCP are regulated by a cascade of transcription factors: these include, but are not limited to, ToxR and TcpP, which are both membrane-localized proteins of the OmpR transcriptional activator family that sense environmental signals and cooperatively induce expression of ToxT, an AraC family transcriptional regulator (5, 15, 24). Both ToxT and ToxR activate transcription of the ctxAB operon, which encodes the A and B subunits of CT (3, 29). ToxT also controls expression of the major pilin subunit TcpA (5), but induction of transcription of tcpA differs from that of ctxA during an infection with respect to both timing and dependence on colonization. Specifically, tcpA is induced prior to ctxA, and ctxA induction is dependent upon successful colonization, because it does not occur in a tcpA mutant background (16). These observations suggest that regulation of ctxA is complex and may require the action of additional regulatory factors.
In vivo positive regulators of ctxA have previously been identified by transposon mutagenesis coupled with recombination-based in vivo expression technology (RIVET) (18). RIVET uses a transcriptional fusion between the promoter of interest and tnpR, which encodes a resolvase, and a res-tet-res cassette located elsewhere on the chromosome. When the promoter is active, TnpR is produced and acts at the res sites to excise the tetracycline resistance (Tcr) marker. Transposon insertion mutants that fail to induce expression of a ctxA::tnpR fusion during infection of the infant mouse remain tetracycline resistant and were selected on this basis. Among the mutants identified by this method was a transposon insertion in vieS, which encodes the sensor kinase of the three-component vieSAB signal transduction system (18).
One family of bacterial signal transduction systems consists of two components: a membrane-localized sensor histidine kinase and a DNA-binding response regulator. The kinase activity of the sensor is stimulated in response to a particular environmental signal, and phospho-transfer to the response regulator alters its affinity for DNA (25). The VieSAB system differs from conventional two-component signal transduction systems, because it encodes two putative response regulators, VieA and VieB. The vieSAB genes are encoded adjacent to each other and in the same transcriptional orientation on the V. cholerae large chromosome, but genetic evidence suggests they are differentially expressed. Both vieS and vieA are expressed during in vitro growth, but vieS expression is constitutive, while vieA expression is VieA dependent (17). Induction of vieB, in contrast, occurs only during infection and requires successful colonization mediated by TCP (16; Sang Ho Lee and Andrew Camilli, unpublished data). Because different response regulators are expressed during in vivo and in vitro growth, the VieSAB system has the potential to differentially regulate ctxA expression under these two conditions. VieA appears to be a typical response regulator, because it contains a helix-turn-helix domain for DNA binding in addition to the phosphoreceiver domain. VieB, however, contains a phosphoreceiver domain, but lacks any recognizable DNA binding motif.
Because only one other member of the two-component signal transduction family, the response regulator VarA, has been implicated in V. cholerae virulence gene regulation (28) and the cognate sensor kinase for this regulator has not been identified, study of the VieSAB three-component system may provide insight into the inducing signals that activate virulence factor expression as well as contribute to understanding the differential regulation of ctxA and tcpA during infection. In this report, we demonstrate that the VieSAB system is required for full CT expression both in vitro and in vivo and suggest that regulation may be indirect through modulated ToxT expression.
MATERIALS AND METHODS
Growth conditions.
Bacteria were grown in Luria-Bertani (LB) broth with aeration at 37°C and maintained at −80°C in LB broth containing 30% glycerol. To induce ctxA expression, bacteria were grown in AKI broth (13). For counterselection of the sacB-containing pCVD442 plasmid, bacteria were grown at 30°C on LB agar lacking NaCl and supplemented with 10% sucrose. Antibiotics were used at the following concentrations unless otherwise noted: streptomycin, 100 μg/ml; ampicillin, 50 μg/ml; tetracycline, 3 μg/ml; kanamycin, 50 μg/ml.
Plasmid and strain construction.
All strains and plasmids used in this study are listed in Table 1. All oligonucleotide primers used are listed in Table 2. Plasmid pSL482, which encodes a transcriptional fusion between ctxA and a ribosome-binding site mutant of tnpR, tnpR168 (16), was constructed by inserting a ctxA′ fragment (bp −516 to +53) into pIVET5 immediately upstream of tnpR168. The ctxA′ fragment was amplified in a PCR from AC-V66 genomic DNA by using Taq polymerase and primers CtxF1 and CtxR1, digested with XbaI and BglII and ligated into similarly digested pIVET5::tnpR168.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5αλpir | F− Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 λ::pir | 10, 14 |
| Sm10λpir | thi recA thr leu tonA lacY supE RP4-2-Tc::Mu λ::pir | Laboratory strain |
| AC-E479 | Sm10λpir(pSL479) Apr | 16 |
| AC-E481 | Sm10λpir(pSL481) Apr | 16 |
| AC-E482 | Sm10λpir(pSL482) Apr | This work |
| AC-E1078 | Sm10λpir(pSL148) Apr | This work |
| AC-E1082 | Sm10λpir(pSL108) Apr | This work |
| AC-E1211 | Sm10λpir(pAT1211) Apr | This work |
| V. cholerae El Tor biotype | ||
| AC-V66 | C6709-1 lacZ::res-tet-res Smr Tcr | 2 |
| Bah-2 | E7946 ΔattRS SmrlacZ | 22 |
| AC-V282 | C6709-1 ΔvieSAB Smr Tcr | 17 |
| AC-V279 | AC-V66 ΔvieAB Smr Tcr | 17 |
| AC-V494 | AC-V66 ΔtoxT Smr Tcr | 16 |
| AC-V752 | AC-V66 vieS::mTn5Kn2 Smr Tcr Knr | 18 |
| AC-V765 | AC-V66 ΔvieSAB Smr Tcr | This work |
| AC-V323b | AC-V66 ΔvieB Smr Tcr | This work |
| AC-V1079 | AC-V66 ΔvieS Smr Tcr | This work |
| AC-V1221 | AC-V66 ΔvieA-HTH Smr Tcr | This work |
| AC-V553 | AC-V66 ctxA::tnpR135 Smr Tcr Apr | 16 |
| AC-V1023 | AC-V752 ctxA::tnpR135 Smr Tcr Apr | 18 |
| AC-V1083 | AC-V323b ctxA::tnpR135 Smr Tcr Apr | This work |
| AC-V1097 | AC-V66 ctxA::tnpR168 Smr Tcr Apr | This work |
| AC-V1098 | AC-V752 ctxA::tnpR168 Smr Tcr Apr | This work |
| AC-V1151 | AC-V1079 ctxA::tnpR135 Smr Tcr Apr | This work |
| AC-V1093 | AC-V765 tcpA::tnpR135 Smr Tcr Apr | This work |
| AC-V1117 | AC-V66 pMMB67EH Smr Tcr Apr | This work |
| AC-V1110 | AC-V765 pMMB67EH Smr Tcr Apr | This work |
| AC-V1118 | AC-V66 pAT1114 Smr Tcr Apr | This work |
| AC-V1120 | AC-V765 pAT1114 Smr Tcr Apr | This work |
| Plasmids | ||
| pIVET5 | oriR6K mobRP4 tnpR-lacZY Apr | 2 |
| pSL479 | pIVET5::tcpA′::tnpR135 | 16 |
| pSL481 | pIVET5::ctxA′::tnpR135 | 16 |
| pSL482 | pIVET5::ctxA′::tnpR168 | This work |
| pCVD442 | oriR6K mobRP4 sacB Apr | 6 |
| pSL108 | pCVD442::ΔvieB | 17 |
| pSL148 | pCVD442::ΔvieS | This work |
| pAT1211 | pCVD442::ΔvieA-HTH | This work |
| pCR-Script | f1(+) ori Apr | Stratagene |
| pMMB67EH | IncQ broad-host-range cloning vector, Apr | 21 |
| pAT1113 | pCR-Script::vieSAB | This work |
| pAT1114 | pMMB67EH::vieSAB | This work |
| pGEM-T | f1 ori Apr | Promega |
| pAT853 | pGEM-T::′ctxA′ | This work |
| pAT854 | pGEM-T::′ctxB′ | This work |
| pAT856 | pGEM-T::′rpoB′ | This work |
| pDSM701 | pGEM-T::′toxT′ | This work |
TABLE 2.
Sequences of primers used in this study
| Primer | DNA sequence (5′→3′)a |
|---|---|
| CtxF1 | GCTCTAGATCGAGTCAGAGCAATCCGAG |
| CtxR1 | GAAGATCTGCATATGAAAATGATGATAA |
| VieSF4 | GCGAAAAGGAAATGGTATGAA |
| VieSR4 | AGCTGTTTTTTGATCATAATACTGCAATGGTGACTGT |
| VieSF5 | CACCATTGCAGTATTATGATCAAAAAAACAGCTCAGTG |
| VieSR5 | TGATACTGATGAAGCCACTGT |
| AHTHF1 | GCAGCTTTCCGTCAACATC |
| AHTHR1 | CTCTCGGTACTACTCTTCTTGTTGCGCCTGT |
| AHTHF2 | CAAGAAGAGTAGTACCGAGAGTTATGGCTG |
| AHTHR2 | TCCCCAACCGATAAGCAC |
| F18 | AATTGAGCAGTGTGGCTTGCAT |
| AHTHR3 | TGTTCCGATTCCGCTTGTC |
| F13 | TCGTCGTCAAAGCGAATACTT |
| R14 | CTGGTGCGGGCAGTCATAGAGT |
| F36 | CAACAACAGAGTGGTTTG |
| VieSR1 | ACATGCATGCTCTTAGCTTGAGCTTCAGGC |
| LSABF | TAGTCAGGTACCAGCAGTAATTGGACGAGCGAAAAGG |
| LSABR | TAGTCAGCATGCTTCAGAACAAAACCGAGGCAATG |
| CTAF | CTGTTAAACAAAGGGAGCATT |
| CTAR | GTGGGCACTTCTCAAACTAAT |
| CTBF | ATGCACATGGAACACCTCAA |
| CTBR | GCATGAGGCGTTTTATTATTC |
| RpoBF | CAGTTTGGGTCACTTATCAGC |
| RpoBR | ATTGGAAATACAGAACGAAAA |
| ToxTF | TTCAATTATCTTACTCAA |
| ToxTR | ACAAATATCTGCCCAACG |
| SP6 | GCACCCCAGGCTTTACAC |
| T7 | GGGTTTTCCCAGTCACGA |
Restriction enzyme sites are underlined.
The plasmid pAT1113 was constructed by cloning a 7.8-kbp fragment that includes the entire vieSAB locus and approximately 800 bp upstream of the putative VieS start codon into the SrfI site of pCR-Script (Stratagene). The vieSAB locus was amplified by PCR from AC-V66 genomic DNA with the primers LSABF and LSABR, which contain KpnI and SphI restriction sites at their 5′ ends, respectively. The PCR was performed with a 10:1 mixture of Taq and Pfu polymerases and extensions of 6 min plus 15 s per cycle at 68°C. The 7.8-kb insert was removed from pAT1113 by digestion with KpnI and SphI, gel purified, and subsequently ligated into similarly digested pMMB67EH to generate pAT1114.
Plasmids for generating gene deletions in V. cholerae were constructed in pCVD442, which encodes the sacB gene for counterselection (6). Plasmids pSL148 and pAT1211, used to generate in-frame deletions of vieS and vieA-HTH, respectively, were constructed by splicing by overlap extension (SOE) PCR (23). For plasmid pSL148, upstream and downstream fragments of approximately 600 bp each were amplified by PCR from AC-V66 genomic DNA by using Pfu polymerase and primer pairs vieSF4 and vieSR4 and vieSF5 and vieSR5, respectively. Primers vieSR4 and vieSF5 were designed with complementary sequence at their 5′ ends, allowing the upstream and downstream fragments to be annealed together, and amplified by PCR with primers vieSF4 and vieSR5. The resulting 1.2-kbp product was ligated into SmaI-digested pCVD442 by blunt-end ligation. For plasmid pAT1211, upstream and downstream fragments of approximately 900 bp were amplified by PCR from AC-V66 genomic DNA with Pfu polymerase and the primer pairs AHTHF1 and AHTHR1 and AHTHF2 and AHTHR2, respectively. The upstream and downstream fragments were annealed together and amplified by PCR with primers AHTHF1 and AHTHR2. The resulting 1.8-kbp product was treated with Taq polymerase and ligated into pGEM-T (Promega). The 1.8-kbp insert was removed from pGEM-T by digestion with SalI and SphI and ligated into similarly digested pCVD442.
Strains AC-V323b, AC-V1079, and AC-V1221, harboring in-frame deletions of vieB, vieS, and the vieA helix-turn-helix domain, respectively, were constructed by allelic exchange in the AC-V66 background. Plasmids pSL108 (ΔvieB), pSL148 (ΔvieS), and pAT1211 (ΔvieA-HTH) were conjugated into AC-V66 by mating with the appropriate Escherichia coli Sm10λpir strain, as previously described (17). After one passage in LB broth in the absence of antibiotic selection, sucrose-resistant colonies were screened for deletion alleles by colony PCR with the primer pairs F13 and R14 for ΔvieB, F36 and vieSR1 for ΔvieS, or F18 and AHTHR3 for ΔvieA-HTH. Strains carrying transcriptional fusions between the ctxA promoter and tnpR135 or tnpR168 were constructed by mating the V. cholerae strains AC-V66, AC-V752, AC-V323b, and AC-V1079 with E. coli strain AC-E481 or AC-E482, respectively, followed by selection for Smr Apr exconjugates in which the suicide plasmid had integrated into the V. cholerae chromosome by a single crossover in the ctxA promoter region. Strain AC-V1093, carrying the tcpA::tnpR135 transcriptional fusion, was constructed by mating the ΔvieSAB strain AC-V765 with the E. coli strain AC-V479 and selecting for Smr Apr exconjugates. The V. cholerae strain AC-V765 was constructed by transduction of the res-tet-res cassette from AC-V66 into the ΔvieSAB strain AC-V282 by using the CP-T1ts phage, as previously described (11).
V. cholerae strains AC-V1110, AC-V1117, AC-V1118, and AC-V1120 carrying the low-copy-number vector pMMB67EH or the derivative pAT1114 were generated by electroporation. Strains AC-V66 and AC-V765 were made electrocompetent by growing bacteria to mid-log phase in LB broth, washing the cells twice with ice-cold 2 mM CaCl2, and freezing them at −80°C in 10% glycerol. Electrocompetent bacteria were transformed with 20 ng of plasmid DNA at 2.1 kV in a 0.2-cm-diameter cuvette (Bio-Rad) and grown for 1 h at 37°C with shaking prior to selection.
Transcription induction assay.
Induction of transcriptional fusions to tnpR during infection of the small intestine of infant mice was measured as previously described (16). Strains carrying the ctxA::tnpR fusions were grown overnight at 37°C with shaking in LB broth containing streptomycin (50 μg/ml), ampicillin (30 μg/ml), and tetracycline (1 μg/ml). Cultures were diluted 10−3 in LB broth, and ∼106 CFU was intragastrically inoculated into 5-day-old CD-1 mice. At various times postinoculation, the small intestines were removed and homogenized in LB broth containing 20% glycerol, and bacteria were recovered by plating serial dilutions on LB broth containing streptomycin. The resulting colonies were replica plated to LB broth containing tetracycline to determine the percentage of Tcs CFU.
RPAs.
RNase protection assays (RPAs) were performed with total RNA isolated from strains grown in AKI broth (13). Briefly, cultures of each strain grown overnight in LB broth were diluted 10−3 into 10 ml of AKI broth in 25-ml glass culture tubes. After 4 h of growth under static conditions at 37°C, 3 ml of the culture was removed to a fresh 18-ml glass tube and shifted to growth with shaking at 37°C for an additional 3 h. RNA was harvested from 1 ml of cells with the RNeasy kit (QIAgen) as previously described (20). Riboprobe templates for ctxA, ctxB, rpoB, and toxT were generated by PCR amplification from AC-V66 genomic DNA with Taq polymerase and the following primer pairs: CTAF and CTAR (287 bp), CTBF and CTBR (300 bp), RpoBF and RpoBR (211 bp), and ToxTF and ToxTR (409 bp). The PCR products were ligated to pGEM-T (Promega) to generate plasmids pAT853, pAT654, pAT856, and pDSM701. Insert orientation was determined by PCR, and templates for in vitro transcription were generated by PCR from the plasmids by using the appropriate reverse primer and either the T7 or SP6 primer. Riboprobes were synthesized by in vitro transcription with the Maxiscript kit (Ambion) and 50 μCi of [32P]UTP (NEN) and gel purified on 4% denaturing polyacrylamide gels. RPAs were performed with the RPAII kit (Ambion) according to the manufacturer's instructions with 1 to 2 μg of total sample RNA. Products were separated on 4% denaturing polyacrylamide gels and exposed to Kodak phosphor screens. Data were collected with a phosphorimager and analyzed with the ImageQuant program (Molecular Dynamics).
Western blot analysis.
Western blots were performed with culture supernatants from strains grown in AKI broth, as described above. Culture supernatants were filter sterilized with 0.45-μm-pore-diameter Durapore filters (Millipore) and subsequently concentrated approximately 10-fold with YM10 spin columns (Centricon) (5,000 × g, 1.5 h). The Sigma Micro Protein Determination kit was used to measure total protein in concentrated supernatants. Whole-cell extracts were prepared by pelleting equivalent numbers of cells based on optical density at 600 nm (OD600) and boiling cells in 1× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer for 5 min. Equivalent amounts of total protein were separated on SDS-PAGE gels (12% polyacrylamide) and electrophoretically transferred to nitrocellulose membranes (Hybond). The CT-A and -B subunits were detected simultaneously with a mixture of rabbit polyclonal antisera against the purified proteins and the ECL enhanced chemiluminescence detection system (Amersham Pharmacia). For quantitative Western blots, known amounts of purified CT-B subunit (List Biological Laboratories) were added to concentrated supernatants from strain Bah-2. Reactive bands were detected by exposure to X-ray film (Kodak) and quantitated by measuring light transmittance on a Kodak Image Station 440. A standard curve was generated for the purified CT-B samples relating band intensity to the known CT-B protein concentration. The equation of this line was used to determine the concentration of the B subunit in culture supernatants from observed band intensities.
RESULTS
VieSAB affects ctxA induction in vivo.
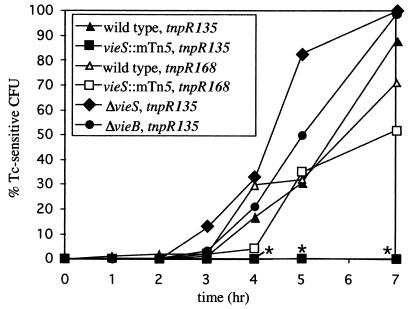
We previously reported that an mTn5 transposon insertion in vieS (Fig. 1), which is the putative sensor kinase of the VieSAB three-component signal transduction system, prevents induction of ctxA expression in the infant mouse model of cholera (18). As shown in Fig. 2, ctxA expression is induced in the wild-type C6709-1 background beginning 3 to 4 h after introduction into the animal (solid triangles). In contrast, ctxA induction is never observed in the vieS::mTn5 background, even after 24 h of infection (solid squares [data not shown]). In these experiments, ctxA expression was analyzed in a qualitative manner by using a transcriptional fusion between the ctxA promoter and the severe-ribosome-binding-site mutant, tnpR135. To determine whether disruption of vieS completely inhibits ctxA transcription or merely reduces expression to a level undetectable with the tnpR135 allele, we utilized a second ribosome-binding-site mutant, tnpR168, which is translated more efficiently than the tnpR135 allele, allowing lower levels of transcription initiation to be detected (16). The ctxA::tnpR168 transcriptional fusion was constructed and introduced into wild-type and vieS::mTn5 strain backgrounds containing the res-tet-res cassette to generate strains AC-V1097 and AC-V1098, respectively. Infant mice were inoculated with strains AC-V1097 and AC-V1098, and bacteria isolated from small intestine homogenates were tested for induction of ctxA expression over time by measuring the percentage of Tcs CFU. As shown in Fig. 2, transcription initiation at the ctxA promoter could be detected in the vieS::mTn5 mutant by using the tnpR168 allele, although expression was marginally delayed and reduced in comparison to that of the wild-type control (compare open triangles and open squares). When strains were analyzed for induction of the ctxA::tnpR fusions after 7 h of growth in vitro in AKI broth (a CT-inducing condition), similar results were obtained; induction of ctxA transcription in the vieS::mTn5 background was never observed with the tnpR135 reporter, but was wild type with the tnpR168 reporter (data not shown). These results suggest that transcription does still occur from the ctxA promoter in a vieS::mTn5 mutant background during growth in vivo, but that the frequency of transcription initiation is reduced compared to that in the wild type.
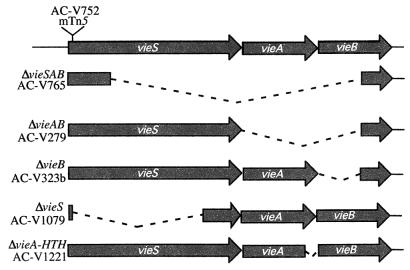
FIG. 1.
Genetic organization of the V. cholerae vieSAB locus. The site of the mTn5 insertion in strain AC-V752 is indicated at the top. Dashed lines indicate deleted sequences. Relevant strains isogenic with AC-V66 are listed next to the corresponding deletion.
FIG. 2.
Induction kinetics of the ctxA::tnpR fusion in various vieSAB mutant strain backgrounds. Infant CD-1 mice were intragastrically infected with the indicated strains, and the percentage of Tcs CFU, indicated on the y axis, was determined by replica plating of bacteria isolated from intestinal homogenates generated at various times postinoculation, indicated on the x axis. The data shown are the mean of multiple experiments. Data at 4, 5, and 7 h were tested for statistical significance with an unpaired t test. Asterisks denote strains with reduced induction of the gene fusion (P < 0.05). Strains AC-V553, AC-V1023, AC-V1083, and AC-V1151 carry the ctxA::tnpR135 fusion, indicated by solid symbols. Strains AC-V1097 and AC-V1098 carry the ctxA::tnpR168 fusion, indicated by open symbols.
To determine which specific components of the VieSAB system are required for ctxA induction in vivo, a ctxA::tnpR135 transcriptional fusion was introduced into strains harboring various in-frame deletions in the vieSAB locus (Fig. 1). Strains AC-V1079 and AC-V1083, which contain the ΔvieS and ΔvieB mutations, respectively, were constructed. These strains both carry the ctxA::tnpR135 fusion integrated at the ctxA locus and remain Tcr during growth in LB broth due to inactivity of the fusion. Both the ΔvieS and ΔvieB strains exhibit wild-type induction of ctxA expression during growth in vivo, suggesting that neither VieS nor VieB alone is essential for activation of ctxA expression under this condition (Fig. 2). The observation that the ΔvieS and vieS::mTn5 mutants do not have the same phenotype with respect to ctxA induction suggests that the mutations have different effects on expression of the downstream vieA and vieB genes.
Surprisingly, exconjugates produced by matings between AC-E481 carrying the ctxA::tnpR135 reporter and strains AC-V765, AC-V279, and AC-V1221, which harbor the ΔvieSAB, ΔvieAB, and ΔvieA-HTH mutations, respectively, were consistently Tcs after mating and colony purification on LB plates. These results suggest that ctxA transcription is induced in these backgrounds during growth on LB broth, which is normally a noninducing condition for ctxA expression. The results also indicate that the DNA binding activity of VieA specifically is required for proper regulation of ctxA transcription under these conditions. Since it was not possible to construct strains containing both the ctxA::tnpR135 reporter and an intact res-tet-res cassette in the ΔvieSAB, ΔvieAB, or ΔvieA-HTH strain background, we could not assess whether these mutations affect ctxA expression during in vivo growth by using the RIVET assay.
Transcription of ctxAB in vitro is reduced in a ΔvieSAB background.
Since suitable strains containing the ctxA::tnpR reporter could not be constructed, RPAs were performed to directly assess whether in-frame deletions in the vieSAB locus affect ctxAB transcription under the in vitro inducing condition in AKI broth. Because only modest differences in ctxA transcript levels were observed in initial experiments, an internal control transcript was probed in all subsequent experiments to allow normalization of ctxA transcript levels. The rpoB gene, which encodes the β subunit of RNA polymerase, was chosen for the internal control, because rpoB is expressed fairly constitutively, autoregulated at the transcriptional level (7), and has previously been used with success as a control in Northern blotting experiments (C. Squires, personal communication). Steady-state levels of rpoB transcript were stable during logarithmic-phase growth under the culture conditions we studied, and there was no detectable difference in rpoB transcript levels between RNA isolated from wild-type cells grown in LB broth or under AKI growth conditions (data not shown). In addition, the rpoB probe did not interfere with detection of the ctxA transcript (Fig. 3B, compare lanes 4 and 6).
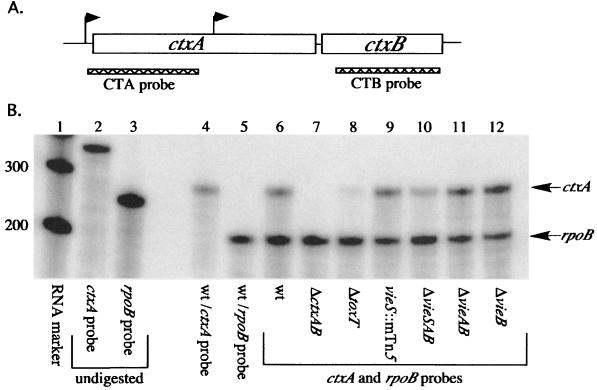
FIG. 3.
RPA for ctxAB transcript in various vieSAB mutant strain backgrounds. (A) Genetic organization of the V. cholerae ctxAB operon. Putative promoters are indicated by arrows. Probes designed to detect the ctxA and ctxB portions of the message are indicated by hatched bars drawn to scale. (B) RPA for ctxA message. Total RNA (1 μg) isolated from strains grown under the AKI inducing condition for 7 h as described in Materials and Methods was analyzed by using the ctxA-specific probe. A probe against rpoB was included as an internal loading control. Lanes: 1, RNA marker; 2 and 3, undigested ctxA and rpoB probes, respectively; 4 and 5, AC-V66 RNA ctxA probe only and rpoB probe only, respectively. Lanes 6 to 12 all contain both ctxA and rpoB probes. Lanes: 6, AC-V66; 7, Bah-2; 8, AC-V494; 9, AC-V752; 10, AC-V765; 11, AC-V279; 12, AC-V323b. wt, wild type. Protected bands of the expected sizes are indicated by arrows to the right. The sizes of the molecular weight markers in base pairs are given on the left. The blot shown is representative of three independent experiments.
RPAs were performed with 1 μg of total RNA isolated from the wild type and each vieSAB mutant strain grown under the AKI inducing condition for 7 h. Two negative controls were included in these experiments: Bah-2, an El Tor strain in which CTXΦ has been deleted and is thus ctxAB (22); and AC-494, a strain isogenic with AC-V66 in which the helix-loop-helix domain of ToxT, a known positive regulator of ctxA, has been deleted (16). The growth of all mutant strains was identical to that of the wild type under AKI conditions, as assessed by measuring the OD600 at hourly intervals (data not shown). When the ctxA riboprobe was used (Fig. 3A), an ∼287-bp protected band was observed in the wild type and all vieSAB mutant strains (Fig. 3B). This band corresponds to the ctxA transcript, because it is absent in RNA isolated from Bah-2 and reduced in intensity in RNA isolated from strain AC-494 (Fig. 3B, lanes 7 and 8). Because the ctxA transcript was observed in RNA isolated from all vieSAB mutant strains, the VieSAB system is not essential for induction of ctxA transcription in vitro in AKI broth. However, in three independent experiments, decreased ctxA transcript levels were observed in RNA isolated from the ΔvieSAB strain AC-V765 in comparison to that of the wild-type control (Fig. 3B, lanes 6 and 10). The ctxA band intensity was measured for each strain and normalized to the intensity of the rpoB internal control. When values for the vieSAB mutant strains were compared to those for the wild-type control, an approximately threefold reduction in ctxA transcript was consistently observed for the ΔvieSAB mutant strain AC-V765 (0.383 ± 0.101). This difference was determined to be significant by an unpaired t test (P < 0.001). There were no significant differences in ctxA transcript levels for any of the other vieSAB mutants tested, including the vieS::mTn5 strain AC-V752 and the ΔvieS strain AC-V1079 (Fig. 3B) (data not shown).
Although the genes for ctxA and ctxB are generally considered to be transcribed as an operon, differential transcription of the ctxB gene has previously been reported to occur from a promoter within the ctxA gene (9) (Fig. 3A). To test for possible differential transcriptional regulation of ctxA and ctxB by the VieSAB system, a second probe was designed to detect the ctxB transcript (Fig. 3A). The results obtained in RPAs with the ctxB riboprobe were essentially identical to those observed with the ctxA probe (data not shown). The ctxB transcript was absent or diminished in RNA prepared from the Bah-2 and AC-V494 negative controls. In two independent experiments, an approximately threefold reduction in the amount of ctxB transcript was observed for the ΔvieSAB strain AC-V765, which was significantly different from the level in the wild type by an unpaired t test (0.388 ± 0.092, P < 0.001). Significant differences in the concentration of ctxB transcript from the wild type were not observed for any other vieSAB mutant strain tested (data not shown). These results suggest that the VieSAB system is required for wild-type expression of both ctxA and ctxB in AKI broth and that the ctxA and ctxB genes are coregulated under the experimental conditions tested.
VieSAB system is required for maintenance of ctxAB transcription.
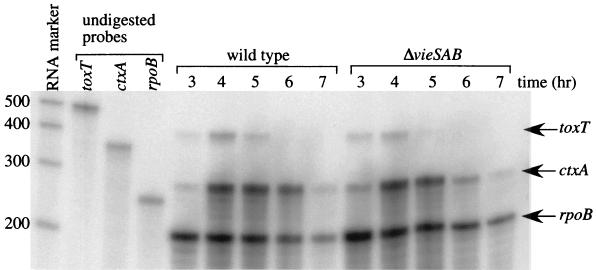
It has previously been reported that ctxA transcription is initiated in El Tor V. cholerae after 5 h of growth under AKI conditions and continues to be detectable by primer extension analysis after up to 10 h of growth (19). Failure to detect wild-type levels of ctxAB transcript in a ΔvieSAB strain background after 7 h of growth under the AKI inducing condition could be explained in several ways. The ΔvieSAB mutation might (i) delay the timing of ctxA transcription induction, (ii) reduce the level of ctxA transcription throughout growth, or (iii) shorten the time of maintenance of ctxA transcription after the initial induction. To distinguish among these possibilities, time course RPAs were performed with RNA isolated from AKI cultures of AC-V66 and AC-V765 (ΔvieSAB) at hourly intervals. Wild-type levels of ctxA transcript were observed at h 3 to 5 for the ΔvieSAB strain (Fig. 4), suggesting that the VieSAB system is not required for the initial induction of transcription from the ctxA promoter. In two independent experiments, however, a dramatic reduction in ctxA transcript was observed at 6 h for the ΔvieSAB strain, although rpoB transcript levels paralleled those of the wild type (Fig. 4). When band intensities were quantitated as described above, the amount of ctxA transcript at 6 h was reduced approximately 10-fold in the ΔvieSAB mutant in comparison to that in the wild type (0.123 ± 0.051, P < 0.05). Similar to previous experiments, at 7 h, we observed only a twofold reduction in the level of ctxA transcript in the ΔvieSAB mutant compared to the wild type (0.48 ± 0.05, P < 0.05); the 10-fold difference detected at 6 h was not sustained, because the concentration of ctxA transcript in the wild type declined dramatically between the two time points. These data suggest that the VieSAB system is not required for the initial induction of ctxA transcription or peak levels of transcript accumulation but rather is necessary for maintenance of ctxA transcription after induction.
FIG. 4.
Time course RPA to detect ctxA and toxT transcripts in the ΔvieSAB strain background. Strains AC-V66 (wild type) and AC-V765 (ΔvieSAB) were grown under the AKI inducting condition, and RNA was isolated at hourly time points as described in Materials and Methods. Total RNA (2 μg) from each time point was hybridized with radiolabeled riboprobes against ctxA, toxT, and rpoB. Protected bands of the expected sizes are indicated by the arrows to the right. The sizes of the molecular weight markers in base pairs are given on the left. The data shown are representative of two independent experiments.
VieSAB affects toxT transcription.
There are at least two ways in which the VieSAB system could affect ctxAB transcription. Either one of the response regulators VieA or VieB might bind directly to the ctxA promoter to activate transcription or the system might be required for wild-type expression of ToxT, a positive regulator of ctxA (3, 5, 12). To assess whether the VieSAB system might act indirectly on ctxAB by affecting toxT transcription, we included in the time course RPA experiments a riboprobe to detect toxT transcripts. If the VieSAB system affects ctxAB transcription indirectly through ToxT, reduced levels of toxT transcript should be observed. Previous work has shown that ToxT transcripts are detectable by primer extension at 4 to 5 h during growth under AKI conditions (19). In RNA isolated from the wild-type strain AC-V66, a protected band of 409 bp corresponding to the toxT transcript was detectable by RPA at 3 to 5 h (Fig. 4). In RNA isolated from the ΔvieSAB mutant strain AC-V765, toxT transcripts were also observed at 3 and 4 h of growth, but by 5 h, there was virtually no detectable transcript (Fig. 4). When toxT band intensities were quantitated and normalized to the rpoB control, a reproducible threefold decrease in toxT transcript was observed for the ΔvieSAB strain at 5 h (0.359 ± 0.102, P < 0.05). The levels of toxT transcript were not significantly different from those of the wild type at earlier time points. These data suggest that decreased ToxT expression may be responsible for reduced ctxAB transcription in the ΔvieSAB mutant. To test whether the reduced toxT transcript levels might also affect expression of another ToxT-regulated gene, tcpA, we utilized the RIVET system. A tcpA::tnpR135 fusion was introduced into the ΔvieSAB mutant background to generate strain AC-V1093, which was subsequently tested for resolution both in vitro under the AKI condition and in vivo. The ΔvieSAB mutation did not affect induction of the tcpA::tnpR135 fusion under either condition (data not shown).
Western analysis confirms reduced CT production in a ΔvieSAB background.
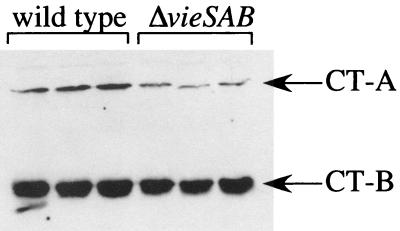
To test whether the decreased ctxAB transcript levels observed for the ΔvieSAB strain AC-V765 are relevant at the protein level, Western blotting was performed. The wild-type strain, AC-V66, and the ΔvieSAB strain, AC-V765, were grown for 7 h under the AKI inducing condition, and culture supernatants were analyzed for the presence of both the A and B subunits of CT. A reproducible decrease in the amount of both subunits was observed for the ΔvieSAB strain background in comparison to the wild type (Fig. 5). In this assay, the toxT mutant strain AC-494 produced no detectable CT, and consistent with the RPA data, mutants carrying other in-frame deletions in the vieSAB locus produced wild-type levels of CT protein (data not shown). Reduced CT production by the ΔvieSAB mutant could not be attributed to a defect in secretion, because CT was undetectable in whole-cell extracts by Western blotting (data not shown).
FIG. 5.
Western blot for CT. Strains AC-V66 (wild type) and AC-V765 (ΔvieSAB) were grown under the AKI inducing condition for 7 h, and CT was detected in equivalent amounts of total protein from concentrated, filtered supernatants by using a mixture of rabbit polyclonal antisera against the purified A and B subunits of CT. The experiment was performed in triplicate with independent cultures, and the results of all cultures are shown.
To quantitate the difference in CT protein production between the wild-type and ΔvieSAB strains, quantitative Western blots were performed. Known amounts of the CT-B subunit were added to concentrated supernatants of strain Bah-2, which is deleted for ctxAB, and band intensities on the Western blot were used to generate a standard curve. The quantities of CT-B subunit in concentrated culture supernatants from strains AC-V66 and AC-V765 were calculated from band intensities according to the standard curve. While the wild-type strain AC-V66 produced 0.20 ng of CT-B subunit per μg of total supernatant protein, the ΔvieSAB strain AC-V765 produced approximately 65% of this amount (0.13 ng of CT-B per μg of total supernatant protein). This result is consistent with the approximately twofold reduction in ctxAB transcript observed by RPA.
The ΔvieSAB mutation can be complemented in trans.
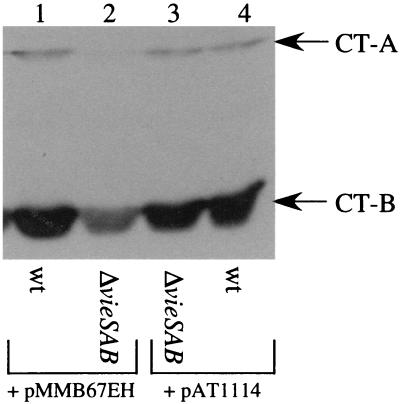
Since the defects in ctxAB transcription and CT protein production were observed only for the ΔvieSAB in-frame deletion mutant, it is formally possible that strain AC-V765 harbors a secondary mutation that affects expression of ctxAB. To demonstrate that loss of VieSAB causes decreased CT production by strain AC-V765, we complemented the ΔvieSAB mutation by providing the vieSAB locus in trans on plasmid pAT1114. This construct contains the vieSAB locus cloned on the stable, low-copy-number vector pMMB67EH. Both pAT1114 and pMMB67EH were introduced into the wild-type and ΔvieSAB strain backgrounds by electroporation, and the resulting strains were tested for production of CT under the AKI inducing condition by Western blotting of culture supernatants. Growth of plasmid-containing strains in AKI broth was indistinguishable from that of the wild type, as determined by measuring the OD600 at hourly intervals (data not shown). In addition, replica plating of bacteria isolated from AKI cultures to medium containing ampicillin demonstrated that both plasmids were maintained in >99% of the population, even in the absence of antibiotic selection. Reduced CT protein production was observed for the ΔvieSAB strain containing the pMMB67EH vector backbone (Fig. 6, lanes 1 and 2). The ΔvieSAB strain carrying the pAT1114 complementing plasmid, however, produced wild-type levels of CT protein (Fig. 6, lane 3). These data indicate that the ΔvieSAB in-frame deletion in strain AC-V765 is responsible for the defect in CT protein production.
FIG. 6.
Complementation of the ΔvieSAB mutation in trans. Strains were grown under the AKI inducing condition for 7 h, and the CT-A and -B subunits were detected by Western blotting as described in the legend to Fig. 5. The A subunit was evident in lane 2 upon longer exposure of the blot. Lanes: 1, AC-V1117; 2, AC-V1110; 3, AC-V1120; 4, AC-V1118. wt, wild type.
DISCUSSION
Bacterial pathogens often control expression of virulence factors in response to environmental conditions with two-component signal transduction systems (8, 25). In this report, we demonstrate that the V. cholerae VieSAB three-component signal transduction system is required for full expression of CT, the major pathogenicity factor of this diarrheal pathogen. The VieSAB system differs from conventional two-component systems, because, in addition to the VieS sensor kinase, it encodes two putative response regulators, VieA and VieB (17).
The VieSAB system was identified as a putative positive regulator of ctxAB by a selection strategy to isolate transposon insertion mutants that fail to induce expression of a ctxA::tnpR fusion during growth in the infant mouse small intestine (18). In this selection, a mutant was obtained with an mTn5 insertion near the 5′ end of the vieS gene. By using a ctxA::tnpR168 fusion, which has increased translational activity relative to the tnpR135 allele, we were able to detect induction of ctxA in the vieS::mTn5 mutant background. These results suggest that signaling through the VieSAB system is required for wild-type induction of ctxA expression during infection but is not absolutely essential. Consistent with this, in preliminary studies, we were not able to detect any significant difference between the wild-type and vieS::mTn5 strains in fluid accumulation assays in suckling mice (data not shown), although this assay (1) is not particularly sensitive.
Genetic evidence suggests that vieA may be expressed from a promoter distinct from the vieS promoter (17). The in vivo phenotype of the vieS::mTn5 mutant could therefore be due to expression of VieA in the absence of VieS and should be reproduced by an in-frame deletion of vieS. An in-frame deletion of vieS was constructed that removes the periplasmic, transmembrane, and histidine kinase domains, but leaves intact approximately 600 bp of sequence upstream of vieA. However, no detectable differences in ctxAB expression were observed between the wild-type and ΔvieS strains in any assay, either in vivo or in vitro. There are several possible explanations for the discrepancy between the ΔvieS and vieS::mTn5 mutant phenotypes. The mTn5 transposon insertion in vieS may be polar on the downstream vieA and vieB genes, leading to a reduction in ctxA transcription. This hypothesis is not favored because both vieA and vieB are believed to be expressed from unique promoters (17). A second possibility is that the putative vieA promoter has been deleted in the ΔvieS strain, but is still intact in the vieS::mTn5 mutant, allowing production of the VieA response regulator in the latter strain background. Finally, it is possible that transcription from the Knr gene promoter on the mTn5 transposon reads into the vieS gene, allowing production of a truncated VieS protein with altered signaling properties from an alternative translation start site.
Since we were not able to use RIVET to study ctxA induction in several vieSAB mutant strains, we instead examined directly whether in-frame deletions in the vieSAB locus affect ctxA transcription in vitro by RPA. An approximately threefold reduction in ctxAB transcript and a nearly twofold reduction in CT protein were observed for a ΔvieSAB strain, but other deletion mutations in the vieSAB locus had no effect on CT production. The defect observed for the ΔvieSAB strain was due to the in-frame deletion and not a secondary mutation, because we could complement the defect in trans. Although we observed a defect in ctxA transcription for the vieS::mTn5 strain by using RIVET, we did not detect any difference in the level of ctxAB message for this strain by RPA. It is possible that the ribosome-binding-site mutants of TnpR used in this study are sufficiently sensitive that we were able to detect a very minor reduction in ctxA transcription that was not observable by RPA.
Because a defect in CT expression is observed only when all components of the VieSAB system are deleted, there must be cross talk between VieSAB and another two-component system or other phosphodonor. One protein that might be involved in cross talk with VieSAB is VarA. VarA encodes a response regulator homologous to GacA, the global regulator of virulence factors in Pseudomonas aeruginosa. VarA is believed to act upstream of TcpP/H and ToxT to regulate ctxA expression (28). The cognate sensor kinase responsible for phosphorylation of VarA has not been identified. VarA may be phosphorylated by VieS, and this positive regulation could compensate for the loss of positive regulation by VieA in the ΔvieAB strain background. Similarly, it is possible that VieA can be phosphorylated by the cognate sensor for VarA and that this phosphorylation can compensate for loss of phosphorylation by VieS in a ΔvieS strain background. By this reasoning, reduced ctxA expression would be observed only if both vieS and vieA were deleted, as in the ΔvieSAB strain.
The VieSAB system may regulate ctxAB expression by affecting transcription of toxT, because reduced amounts of toxT transcript were observed in the ΔvieSAB mutant in comparison to the wild type by RPA. Since the initial induction of both ctxA and toxT is like that of the wild type in a ΔvieSAB strain background, signaling through ToxR, which sits atop the virulence gene regulatory cascade, is likely not affected by mutations in the VieSAB system. Decreased production of ToxT protein in the ΔvieSAB mutant may prevent this strain from maintaining wild-type levels of ctxA transcription after the initial induction, as observed in time course RPAs. One limitation of using RPAs to examine transcriptional regulation is that the assay measures steady-state levels of RNA and cannot distinguish between differences in promoter activity or RNA degradation, although we favor a role for VieSAB in promoter activity. We attempted to confirm that ToxT protein levels are reduced in the ΔvieSAB mutant by assessing the transcription of tcpA, another gene positively regulated by ToxT, by using the RIVET system. Induction of the tcpA::tnpR135 fusion was not affected by the ΔvieSAB mutation, suggesting either that there is no difference in the level of ToxT or that the amount of ToxT protein, although reduced, is still sufficient to activate tcpA transcription. Consistent with a reduction in expression of CT, but not TcpA, caused by deletion of vieSAB, the ΔvieSAB strain is not reduced in colonization of the infant mouse small intestine (17). It is known that TcpA is absolutely essential for colonization in this model (27), whereas CT is dispensable (26).
All RPAs presented in this work utilized an internal control probe against rpoB to confirm that equivalent amounts of RNA were analyzed. This probe should be useful as an internal control for any experiment, because it does not interfere with the ability of other probes to hybridize with the complementary mRNA. In addition, the medium in which V. cholerae is grown does not affect the level of rpoB transcript, because similar amounts were observed in RNA isolated from wild-type cells grown in LB or AKI broth. Finally, expression of rpoB is autoregulatory, so mutations in other regulatory systems should not affect rpoB transcription (7). One potential limitation of this internal control is that the level of rpoB transcript is growth rate dependent. As cells reach the end of exponential growth in AKI broth (7 h), the amount of rpoB transcript decreases relative to the total amount of RNA in the cell. Thus, to analyze a mutant compared to the wild type with this control, it is essential to harvest RNA from cells that are in the same growth phase. Because mutations in the vieSAB locus did not affect the growth rate of V. cholerae under the conditions tested, use of the rpoB control is reasonable.
While this work confirms that the VieSAB system contributes to regulation of CT expression, it raises many new questions. Although we have provided some evidence that the system regulates toxT expression, further work is needed to determine which response regulator is important for this function and to determine whether it acts directly at the ctxA promoter, the toxT promoter, or perhaps another promoter upstream of ToxT in the regulatory cascade. The VieA response regulator is the most likely candidate to regulate ctxAB expression, because differences in ctxA expression were observed under in vitro conditions in which vieB is not expressed (17).
The environmental signal or signals that VieS senses to upregulate expression of CT are also unclear. The AKI condition used to induce expression of the ToxR regulon in El Tor V. cholerae is a complex condition with many signals that could potentially upregulate toxin production, including nutrient availability, oxygen availability, and pH. The VieSAB system shares some homology with the BvgAS two-component signal transduction system of Bordetella pertussis, which regulates expression of virulence genes, including pertussis toxin. While in vitro conditions that inhibit the activity of the BvgAS pathway have been identified (growth at 25°C or in medium containing magnesium sulfate or nicotinic acid), the signals that activate this system during growth in the host have not been identified (4). A conserved motif search of VieS indicates that the protein contains two motifs similar to bacterial extracellular solute-binding motifs that bind amino acids. This observation suggests that the kinase activity of VieS may be activated by the binding of particular amino acids to the periplasmic domain. We have preliminary evidence to suggest that in the classical biotype of V. cholerae, CT protein production stimulated by the addition of amino acids to minimal medium is abrogated when the VieSAB system is inactivated (Anna D. Tischler and Andrew Camilli, unpublished data). We plan to continue studying this system by using the classical background to determine what specific amino acid or acids activate the VieS sensor kinase. Presumably, this amino acid inducing signal is also present in the complex AKI medium, which is an inducing condition for El Tor biotype V. cholerae, as well as in the small intestine, to allow for full induction of CT production.
Acknowledgments
We thank R. Finkelstein for the gift of antisera to the CT-A and -B subunits. In addition, we thank C. Squires and D. Merrell for critical reading of the manuscript.
This material is based on work supported under a National Science Foundation Graduate Research Fellowship to A.D.T. This research was also supported by NIH grants AI 40262 and AI 45746 to A.C. and the Center for Gastroenterology Research on Absorptive and Secretory Processes, NEMC (P30 DK34928).
REFERENCES
- 1.Baselski, V., R. Briggs, and C. Parker. 1977. Intestinal fluid accumulation induced by oral challenge with Vibrio cholerae or cholera toxin in infant mice. Infect. Immun. 15:704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camilli, A., and J. J. Mekalanos. 1995. Use of recombinase gene fusions to identify Vibrio cholerae genes induced during infection. Mol. Microbiol. 18:671-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Champion, G. A., M. N. Neely, M. A. Brennan, and V. J. DiRita. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323-331. [DOI] [PubMed] [Google Scholar]
- 4.Coote, J. G. 1991. Antigenic switching and pathogenicity: environmental effects on virulence gene expression in Bordetella pertussis. J. Gen. Microbiol. 137:2493-2503. [DOI] [PubMed] [Google Scholar]
- 5.DiRita, V. J., M. Neely, R. K. Taylor, and P. M. Bruss. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dykshoorn, D. M., R. St. Pierre, and T. Linn. 1996. Synthesis of the β and β′ subunits of Escherichia coli RNA polymerase is autogenously regulated in vivo by both transcriptional and translational mechanisms. Mol. Microbiol. 19:483-493. [DOI] [PubMed] [Google Scholar]
- 8.Dziejman, M., and J. J. Mekalanos. 1995. Two-component signal transduction and its role in the expression of bacterial virulence factors, p. 305-317. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, D.C.
- 9.Fando, R., J. L. Pérez, B. L. Rodriguez, J. Campos, A. Robert, L. García, A. Silva, and J. A. Benitez. 1997. Promoter activities in Vibrio cholerae ctxΦ prophage. Infect. Immun. 65:1561-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 11.Hava, D. L., and A. Camilli. 2001. Isolation and characterization of a temperature-sensitive generalized transducing bacteriophage for Vibrio cholerae. J. Microbiol. Methods 46:217-225. [DOI] [PubMed] [Google Scholar]
- 12.Higgins, D. E., E. Nazareno, and V. J. DiRita. 1992. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J. Bacteriol. 174:6974-6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwanaga, M., K. Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae 01 El Tor. Microbiol. Immunol. 30:1075-1083. [DOI] [PubMed] [Google Scholar]
- 14.Kolter, R., M. Inuzuka, and D. R. Helinski. 1978. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell 15:1199-1208. [DOI] [PubMed] [Google Scholar]
- 15.Krukonis, E. S., R. R. Yu, and V. J. DiRita. 2000. The Vibrio cholerae ToxR/TcpP/ToxT virulence cascade: distinct roles for two membrane-localized transcriptional activators on a single promoter. Mol. Microbiol. 38:67-84. [DOI] [PubMed] [Google Scholar]
- 16.Lee, S. H., D. L. Hava, M. K. Waldor, and A. Camilli. 1999. Regulation and temporal expression patterns of Vibrio cholerae virulence genes during infection. Cell 99:625-634. [DOI] [PubMed] [Google Scholar]
- 17.Lee, S. H., M. J. Achelichio, J. J. Mekalanos, and A. Camilli. 1998. Nucleotide sequence and spatiotemporal expression of the Vibrio cholerae vieSAB genes during infection. J. Bacteriol. 180:2298-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, S. H., S. M. Butler, and A. Camilli. 2001. Selection for in vivo regulators of bacterial virulence. Proc. Natl. Acad. Sci. USA 98:6889-6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medrano, A. I., V. J. DiRita, G. Castillo, and J. Sanchez. 1999. Transient transcriptional activation of the Vibrio cholerae El Tor virulence regulator ToxT in response to culture conditions. Infect. Immun. 67:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrell, D. S., and A. Camilli. 1999. The cadA gene of Vibrio cholerae is induced during infection and plays a role in acid tolerance. Mol. Microbiol. 34:836-849. [DOI] [PubMed] [Google Scholar]
- 21.Morales, V. M., A. Backman, and M. Bagdasarian. 1991. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39-48. [DOI] [PubMed] [Google Scholar]
- 22.Pearson, G. D., A. Woods, S. L. Chiang, and J. J. Mekalanos. 1993. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc. Natl. Acad. Sci. USA 90:3750-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senanayake, S. D., and D. A. Brian. 1995. Precise large deletions by the PCR-based overlap extension method. Mol. Biotechnol. 4:13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skorupski, K., and R. K. Taylor. 1997. Control of the ToxR virulence regulon in Vibrio cholerae by environmental stimuli. Mol. Microbiol. 25:1003-1009. [DOI] [PubMed] [Google Scholar]
- 25.Stock, J. B., A. J. Ninfa, and A. M. Stock. 1989. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol. Rev. 53:450-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor, R. K., V. L. Miller, D. B. Furlong, and J. J. Mekalanos. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. USA 84:2833-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thelin, K. H., and R. K. Taylor. 1996. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect. Immun. 64:2853-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong, S. M., P. A. Carroll, L. G. Rahme, F. M. Ausubel, and S. B. Calderwood. 1998. Modulation of expression of the ToxR regulon in Vibrio cholerae by a member of the two-component family of response regulators. Infect. Immun. 66:5854-5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu, R. R., and V. J. DiRita. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43:119-134. [DOI] [PubMed] [Google Scholar]