DNA is often the target of anti-cancer agents, which alkylate or oxidatively damage the biopolymer. Hydroxyl radical, metal–oxo complexes, such as that produced by bleomycin, and singlet oxygen are examples of agents that oxidatively damage DNA.1–4 Singlet oxygen, which selectively oxidizes deoxyguanosine, is an important reactive oxygen species in photodynamic therapy.3,5,6 The reactivity of a subset of DNA alkylating agents is distinguished from reactive oxygen species by their formation of interstrand cross-links (ISC’s). For instance, interstrand cross-links are believed to be the source of the cytotoxicity of the anti-cancer agents, mitomycin C and chlorambucil.7 Herein, we describe a process involving a modified nucleotide (2) that potentiates the effects of singlet oxygen by reacting with this reagent to form ISC’s.
We recently described a mechanism in which 5-(2′-deoxyuridinyl)methyl radical (1) forms an ISC with the opposing deoxyadenosine when it is photochemically generated from 2 in DNA (Scheme 1).8,9 In the course of investigating the mechanism for this process, we examined the effect of singlet oxygen on DNA containing 2. Filtered (λ ≥ 400 nm) aerobic photolysis (30 min) of 5′-32P-3 in the presence of 1–50 μM of the singlet oxygen sensitizer, Rose Bengal, produced ISC’s in as high as 48% yield (Figure 1B). Anoxic photolysis of a mixture of 5′-32P-3 and Rose Bengal (50 μM) produces ~3% ISC.16 These observations suggest that direct photolysis of the phenyl selenide (2), which generates ISC’s via 1 independent of O2, and in lower yield, is not the source of cross-links under these conditions. Furthermore, the anoxic results suggest that ISC’s do not result from a direct photoreaction between the sensitizer and DNA. Instead, the dependence of the rate of disappearance of monomeric 2 on D2O content suggests that singlet oxygen, whose lifetime is enhanced 10-fold in the deuterated solvent, is responsible for phenyl selenide consumption (Figure 2).10
Scheme 1.
Figure 1.
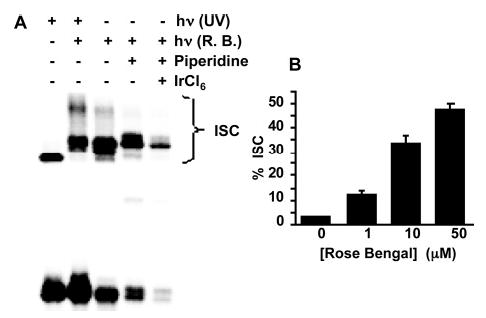
Formation of DNA interstrand cross-links (3, 10 nM) via UV or Rose Bengal sensitized aerobic photolysis. (A) Autoradiogram comparing ISC’s produced upon UV or Rose Bengal (50 μM) sensitized photolysis. (B) Effect of Rose Bengal concentration on ISC formation (30 min photolysis).
Figure 2.
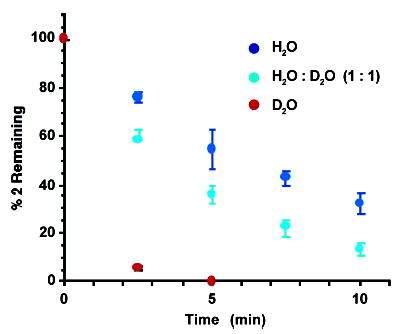
Effect of D2O on the consumption of monomeric 2 (50 μM) upon irradiation of Rose Bengal (10 μM).
Photolysis of an otherwise identical duplex containing dT in place of 2 produces ~2% ISC, indicating that the phenyl selenide (2) plays an integral role in their formation in 3.16 In contemplating a mechanism for this process, we recognized that phenyl selenides are oxidized to selenoxides by singlet oxygen in good yield, and allylic selenoxides undergo [2,3] sigmatropic rearrangements.11,12 Execution of these reactions in 2 would produce an electrophilic methide-type intermediate (5), akin to other molecules that alkylate DNA (Scheme 2).13,14 Evidence for this mechanism was gleaned from NMR analysis of the reaction of monomeric 2 with NaIO4 (Figure 3) or its photosensitization by Rose Bengal in deuterated phosphate buffer.16 Periodate is shown because it rapidly and completely oxidizes 2. The phenyl selenide (2) is completely consumed within 10 min and replaced by a diastereomeric mixture of the rearrangement product (5). Selenoxide 4 is not detected. Compound 5 reacts with the weak nucleophilic H2O over the course of 24 h to produce 5-hydroxymethyl-2′-deoxyuridine (6), which is identical to independently prepared material.15 Furthermore, ISC’s are efficiently formed with the opposing 2′-deoxyadenosine upon treatment of 3 with NaIO4, indicating that the methide (5) produces cross-links.16
Scheme 2.
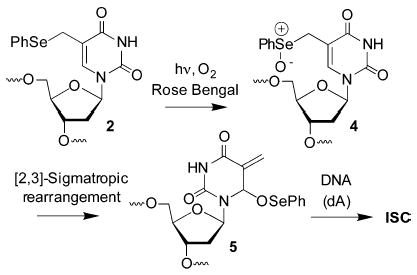
Figure 3.
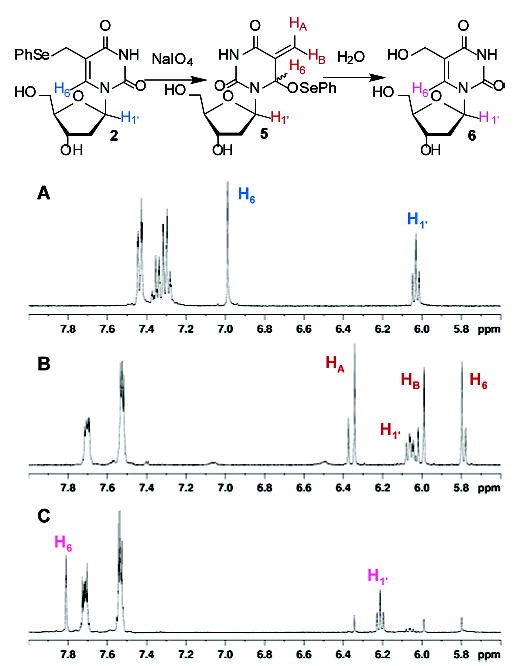
1H NMR analysis of the reaction of 2 (50 mM) with NaIO4 (50 mM) in deuterated phosphate buffer (50 mM, pD 7.4). (A) Before NaIO4 addition, (B) 10 min after NaIO4 addition, and (C) 24 h after NaIO4 addition.
When 5 is produced in duplex DNA, rotation about the N-glycosidic bond into the syn-conformation positions the exocyclic methylene to react with N1 of the opposing deoxyadenosine, which is the same position that 1 is believed to cross-link with. However, the cross-link products formed in the presence of singlet oxygen migrate more slowly than those produced via 1 (Figure 1A). Exposure of 5′-32P-3 to singlet oxygen (Figure 1A, lane 2) previously cross-linked via formation of 1 (Figure 1A, lane 1) indicates that the cross-links produced under singlet oxygen conditions contain additional damage. Oxidized deoxyguanosines within the ISC products were deemed to be the most likely lesions formed in addition to cross-links. The damaged purines were revealed by treatment of the ISC’s with piperidine and IrCl6 (Figure 1A, lanes 4 and 5).17–20 Piperidine treatment following oxidation with IrCl6 converts many of the ISC products into shorter, faster migrating fragments, indicating that not all of the cross-linked products also contain additional damaged nucleotides.16
The reaction of 2 with singlet oxygen is also distinguished from the radical pathway (1) by the effect of glutathione on ISC formation. ISC formation is unaffected by physiologically relevant glutathione concentrations (5 mM) when 3 is subjected to singlet oxygen.16 This observation reinforces those reported above, which suggest that the combination of the phenyl selenide derivative of thymidine (2) and singlet oxygen offers a novel and potentially important means for producing interstrand cross-links in DNA. The physiological importance of interstrand cross-links suggests that the incorporation of phenyl selenide 2 in DNA could be an effective adjuvant in photodynamic therapy.
Supplementary Material
Acknowledgments
We are grateful for support from the National Institute of General Medical Sciences (GM-054996).
Footnotes
Supporting Information Available: Experimental procedures for carrying out the experiments on 2 and 3. Autoradiograms showing ISC formation from 3 by NaIO4 and hydroxyl radical footprinting of these ISC’s, effect of glutathione on ISC formation, decomposition of singlet oxygen induced ISC’s by piperidine treatment, and control experiments. 1H NMR showing formation of 5 by Rose Bengal sensitized photolysis of 2. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Chen J, Stubbe J. Nat Rev Cancer. 2005;5:102–112. doi: 10.1038/nrc1547. [DOI] [PubMed] [Google Scholar]
- 2.von Sonntag, C. The Chemical Basis of Radiation Biology; Taylor & Francis: London, 1987.
- 3.Lee PCC, Rodgers MAJ. Photochem Photobiol. 1987;45:79–86. doi: 10.1111/j.1751-1097.1987.tb08407.x. [DOI] [PubMed] [Google Scholar]
- 4.McCallum JEB, Kaniyoshi CY, Foote CS. J Am Chem Soc. 2004;126:16777–16782. doi: 10.1021/ja030678p. [DOI] [PubMed] [Google Scholar]
- 5.Dolmans DE, Fukumura D, Jain R. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 6.Prat F, Hou CC, Foote CS. J Am Chem Soc. 1997;119:5051–5052. [Google Scholar]
- 7.Schärer OD. ChemBioChem. 2005;6:27–32. doi: 10.1002/cbic.200400287. [DOI] [PubMed] [Google Scholar]
- 8.Hong IS, Greenberg MM. J Am Chem Soc. 2005;127:3692–3693. doi: 10.1021/ja042434q. [DOI] [PubMed] [Google Scholar]
- 9.Monomeric compounds are referred to by the same number as when in 3.
- 10.Turro, N. J. Modern Molecular Photochemistry; Benjamin Cummings: Menlo Park, CA, 1978.
- 11.Krief A, Lonez F. Tetrahedron Lett. 2002;43:6255–6257. [Google Scholar]
- 12.Reich HJ, Yelm KE, Wollowitz S. J Am Chem Soc. 1983;105:2503–2504. [Google Scholar]
- 13.Douki T, Vadesne-Bauer G, Cadet J. J Org Chem. 2003;68:478–482. doi: 10.1021/jo026606i. [DOI] [PubMed] [Google Scholar]
- 14.Veldhuyzen WF, Shallop AJ, Jones RA, Rokita SE. J Am Chem Soc. 2001;123:11126–11132. doi: 10.1021/ja011686d. [DOI] [PubMed] [Google Scholar]
- 15.Sowers LC, Beardsley GP. J Org Chem. 1993;58:1664–1665. doi: 10.1021/jo00059a011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See Supporting Information.
- 17.Muller JG, Duarte V, Hickerson RP, Burrows CJ. Nucleic Acids Res. 1998;26:2247–2249. doi: 10.1093/nar/26.9.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Y, Muller JG, Luo W, Mayne CL, Shallop AJ, Jones RA, Burrows CJ. J Am Chem Soc. 2003;125:13926–13927. doi: 10.1021/ja0378660. [DOI] [PubMed] [Google Scholar]
- 19.Duarte VGD, Yamaguchi LF, Ravanat JL, Martinez GR, Medeiros MHG, DiMascio PD, Cadet J. J Am Chem Soc. 2000;122:12622–12628. [Google Scholar]
- 20.Ravanat JL, Di Mascio P, Martinez GR, Medeiros MHG, Cadet J. J Biol Chem. 2000;275:40601–40604. doi: 10.1074/jbc.M006681200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


