Abstract
The efficiency of germ cell transplantation, the procedure of transferring germ cells from a donor male into the testes of recipient males, can be greatly increased by reduction of endogenous germ cells in recipient animals. To develop effective methods for suppression of endogenous spermatogenesis in potential pig and goat recipients, we either administered busulfan to pregnant sows or irradiated the testes of immature goats. Piglets from sows treated twice with busulfan (7.5 mg/kg) at days 98 and 108 of gestation showed reduced gonocyte numbers at 2, 4, and 8 weeks of age and reduced initiation of spermatogenesis at 16 weeks of age. For goats, groups of 3 kids at 1, 5, or 9.5 weeks of age received fractionated irradiation of the testes with 3 doses of 2 Gy on 3 consecutive days. At 2 months after irradiation, 5%–10% of seminiferous tubule cross sections contained pachytene spermatocytes, compared with 50%–100% in controls. At 3 months after irradiation, spermatozoa appeared in 20% of tubule cross sections in all treated goats and in 100% of tubules in control goats. By 6 months after irradiation, spermatogenesis had recovered in 60% of tubules in goats treated at 5 or 9.5 weeks of age but in only 29% of tubules after treatment at 1 week of age. Therefore, late gestation in utero treatment of pigs with low doses of busulfan and testicular irradiation of goats at 1 week of age will result in a reduction in the endogenous germ cell population that could facilitate donor cell colonization after germ cell transplantation.
Keywords: Irradiation, busulfan, testis, transgenesis, large animals
Transplantation of germ cells harvested from the seminiferous tubules of a donor male by microinjection into the testes of recipient males leads to establishment of donor-derived spermatogenesis (Brinster and Avarbock, 1994; Brinster and Zimmerman, 1994). This system provides a unique model for the study of spermatogenesis, restoration of fertility, and manipulation of spermatogonial stem cells.
Genetic modification of farm animals through transplantation of genetically altered male germ cells is a promising alternative strategy (Brinster, 2002) to the currently inefficient and costly methods of generating transgenic farm animals (reviewed in Niemann and Kues, 2003; Keefer, 2004). Transgenic pigs play a central role as a potential source of tissues and organs for xenotran-plantation to humans and are of increasing interest as models for biomedical research (Niemann and Kues, 2003). Similarly, transgenic dairy goats are of significant economic value for the production of biopharmaceutical proteins in their milk (Gavin, 1996; Niemann and Kues, 2003; Keefer, 2004). We recently developed a technique for germ cell transplantation and genetic manipulation of the donor cells before transplantation in pigs (Honaramooz et al, 2002). We demonstrated that the technique is also applicable in goats (Honaramooz et al, 2003b), and transplantation of male germ cells from transgenic donor goats to the testes of unrelated prepubertal recipient goats results in donor-derived fertility and progeny (Honaramooz et al, 2003b). In these earlier studies, unlike in rodents, immunocompetent recipient pigs and goats did not reject allogeneic germ cells (Honaramooz et al, 2002a,2003b), making the practical application of the approach more feasible in large animals.
Although donor germ cells can colonize normal recipient testes, colonization efficiency depends on successful competition of transplanted germ cells with endogenous germ cells for available stem cell niches (Shinohara et al, 2001, 2002). This, in turn, is dependent upon the relative number of stem cells in populations of donor cells and ease of access of transplanted cells to the stem cell niche located at the basal membrane of the recipient seminiferous tubules. The immature recipient testis facilitates access of transplanted germ cells to the basal membrane, as it lacks the hindering multiple layers of germ cells in its tubules and provides a more favorable environment than adult testes for engraftment and expansion of donor germ cells (Shinohara et al, 2001). In our previous studies, we used prepubertal pig and goat recipients (Honaramooz et al, 2002a, 2003b), resulting in about 7% donor-derived progeny (Honaramooz et al, 2003b). Efficiency of donor cell engraftment can be improved if the recipient testes have little or no endogenous spermatogonia, increasing both the stem cell niche availability and accessibility (Brinster et al, 2003). In rodents, animals chosen from strains inherently lacking spermatogenesis (eg, the dominant white spotting W mutation; Silvers, 1979; Geissler et al, 1988) are perfectly suited as recipients for germ cell transplantation (Ogawa et al, 2000; Shinohara et al, 2001). However, similar mutant animals are not readily available in farm animals, and in the single report of germ cell transplantation into a farm animal with genetically impaired spermatogenesis, an azoospermic Klinefelter bull was determined not to be a useful recipient model (Joerg et al, 2003).
An alternative to the use of recipient animal models with congenital germ cell deficiency is removal of endogenous germ cells by cytoablative methods to facilitate further access to, and the availability of, the stem cell niche. Several options are available, including pretreatment with busulfan (Brinster and Avarbock, 1994; Brinster and Zimmerman, 1994), irradiation (van den Aardweg et al, 1983; Shuttlesworth et al, 2000), cold ischemia (Yong et al, 1988), or hyperthermic treatment of the testes (Rockett et al, 2001; McLean et al, 2002). These options for germ cell depletion have not been systematically studied for use in farm animals; however, the most practical methods may be local irradiation of the testes or treatment with sterilizing drugs. Testicular irradiation for ablation of endogenous germ cells has been used in mice (van den Aardweg et al, 1983; Creemers et al, 2002; Giuili et al, 2002), rats (Shuttlesworth et al, 2000), monkeys (Schlatt et al, 2002), bulls (Izadyar et al, 2003), and rams (Oatley et al, 2005). Radiation dose and frequency of administration varied widely between studies and a critical evaluation of irradiation protocols in farm animals has not been reported. Although irradiation has the advantage of being a focal treatment, this method requires an expensive therapeutic radiation source that is not universally available; therefore, cytotoxic treatment with busulfan can be used as an alternative to irradiation for depletion of germ cells. Busulfan, a DNA-alkylating agent that destroys proliferating cells, is frequently used in adult rodents to deplete recipient germ cells before germ cell transplantation (Brinster and Avarbock, 1994; Brinster and Zimmerman, 1994; Ogawa et al, 1999; Brinster et al, 2003; Moisan et al, 2003). However, the sterilizing dose of busulfan is species- and strain-specific and treatment can be lethal due to severe bone marrow depression. In young and adult male rats, high sensitivity to the toxic effects of busulfan results in incomplete removal of germ cells and compromised testicular health (Ogawa et al, 1999). Treatment of pregnant female rats with a single dose of busulfan between 13 and 18 days of gestation resulted in birth of live male pups that were permanently infertile (Hemsworth et al, 1963). Similarly, male progeny born to female mice treated with busulfan on day 12.5 of gestation provided suitable recipients for germ cell transplantation (Brinster et al, 2003; Moisan et al, 2003).
Administration of busulfan to pregnant females is particularly useful for ablation of endogenous germ cells in litter-bearing species like mice, rats, and pigs, where treatment of a single female can result in multiple potential recipient males. In contrast, in species, such as goats, cattle, and monkeys, that only carry 1 or 2 fetuses, this approach is less efficient. In these species, however, the anatomical positioning of scrotal testes makes local irradiation of the testes, with shielding of the body of the animal, a more practical approach. Therefore, the aim of the present study was to develop protocols for germ cell ablation using busulfan treatment in pigs and testicular irradiation in goats.
Materials and Methods
Busulfan Treatment in Pigs
Experiment 1: Postnatal Treatment
In initial experiments, male Duroc pigs were treated 4 times at 5, 9, 13, and 17 weeks of age (prepubertal) with 0, 5, 10, or 15 mg/kg of busulfan (dissolved at 80–100 mg/mL in dimethyl sulfoxide [DMSO], n = 4/dose, IM). Pigs were castrated at 21 weeks of age, testis weight was recorded, and testes were fixed for histology to determine the abundance of gonocytes/spermatogonia per 1000 Sertoli cells.
Experiment 2: In Utero Treatment
As an alternative approach, pregnant Yorkshire-cross sows (n = 2) were treated once either at day 102 or 105 of gestation with 7.5 mg/kg busulfan (in DMSO, SC). The resulting male piglets and control male pigs born to untreated sows were castrated at 2, 4, or 8 weeks of age (2 treated and 2 control pigs per time point). Body weight and testis weights were recorded and testis tissue was examined histologically for the presence of germ cells.
In a second experiment, 4 sows were treated at both day 98 and day 108 of gestation with busulfan (7.5 mg/kg in DMSO, SC). Male piglets (n = 4) from 1 litter were hemicastrated at 10 days of age and the second testis harvested at 6 months of age. Six piglets from an untreated sow served as controls. Three of the sows were treated more than once (2 consecutive litters in 1 sow and 3 consecutive litters in 2 sows) for a total of 8 replicates. Testes from 4 treated and 4 control pigs each were analyzed at 2, 4, or 8 weeks of age, 2 treated and 2 controls at 16 weeks of age, and 2 treated and 1 control at 32 weeks of age, as described above.
In 1 litter born to a sow treated at days 98 and 108 of gestation and a control litter, body weight was recorded weekly for the first 5 weeks of life, blood samples were obtained at 3, 7, and 12 weeks of age, and a complete blood count was performed. In an additional treated and control litter, body weights were compared at 8 months of age.
Testis Irradiation in Goats
Male Alpine and Nubian goats were subjected to fractionated irradiation of the testes under short-acting anesthesia (tiletamine/ zolazapam, Fort Dodge Animal Health, Fort Dodge, Iowa; 3.3 mg/kg IV). Measurements were made of the length, width, and thickness of the testes within the scrotum. Animals were placed in dorsal recumbency and the testes were isolated from the rest of the body by placing a 7- × 7-cm lead shield with an opening just large enough to accommodate the scrotum, underneath of the scrotum in line with the beam-shaping device. Groups of 3 goats were treated at 1, 5, or 9.5 weeks of age with 3 daily low doses of 2 Gray (Gy, the international standard unit of energy for the absorbed dose of radiation) of fractional radiation delivered to the isolated testes by megavoltage electrons (Siemens Mevatron 77 (6 MV) linear accelerator). Testes were harvested at 2, 3, or 6 months after irradiation and tissue was processed for histology. A minimum of 150 tubule cross sections per testis were scored for the presence of germ cells and progression of spermatogenesis. Results were compared with those obtained from age-matched untreated control animals.
Experiments were approved by the Animal Care and Use Committees of the University of Pennsylvania, Southern Illinois University at Carbondale, and Tufts University.
Histological Analysis
Multiple pieces of testis tissue were fixed in Bouin solution overnight, washed in 70% EtOH, and processed for histology. Tissue sections from samples collected before puberty were analyzed for the presence of gonocytes/spermatogonia, and the number of gonocytes/spermatogonia per 1000 Sertoli cells was counted at 400× magnification. In samples collected from pubertal or post-pubertal animals, tubule cross sections were scored for the presence or absence of meiotic or postmeiotic germ cells. We used a blinded approach and a random sampling scheme for the analysis. Presence of germ cells was checked in all seminiferous tubules in all slides. The cell counts were performed on random fields covering a minimum of 25% of the tissue cross sections, and all cells in the randomly selected fields were counted.
Statistical Analysis
Where indicated, measured parameters were compared between treated and control animals by 1-way analysis of variance or unpaired Student’s t test. Differences associated with a P value less than .05 were considered to be significant.
Results
Effects of Busulfan Treatment in Pigs
Experiment 1: Postnatal Treatment
Treatment of pre-pubertal pigs with 0, 5, 10, or 15 mg/kg of busulfan resulted in a dose-dependent decrease in testis weight (129.8 ± 53.1, 105 ± 30.9, 70.5 ± 19.8, 46.2 ± 23.8 g, respectively; mean ± SD, n = 4/dose) with a significant (P < .05) reduction in the highest dose group, but systemic toxicity (50% and 25% mortality in the 15 mg/kg and 10 mg/kg groups, respectively) was considered to be unacceptable. Therefore, postnatal treatment of pigs was not explored further.
Experiment 2: In Utero Treatment
Timing of administration of busulfan to sows in late gestation was chosen to coincide with a period of male germ cell proliferation in the fetus (van Straaten and Wensing, 1977; França et al, 2000), when multipotent primordial germ cells differentiate into gonocytes (de Rooij, 1998).
In male piglets born to sows treated only once with busulfan at day 102 or day 105 of gestation, body weight, testis weight, and number of gonocytes per 1000 Sertoli cells were not different (P > .05) from controls at 2, 4, and 8 weeks of age (n = 2/group/age).
In contrast, in male pigs born to sows treated twice (day 98 and day 108 after breeding), testis weight (1.7 ± 0.3 g in treated [n = 4] vs 3.3 ± 0.4 g in controls [n = 6] at 10 days of age; mean ± SD, P < .05) and the number of gonocytes per 1000 Sertoli cells in the seminiferous tubules was significantly reduced (P < .05) compared with control pigs at 10 days and 2, 4, and 8 weeks of age (Table; Figure 1). At 16 weeks of age, less than 8% of seminiferous tubule cross sections contained meiotic germ cells in busulfan-treated boars, compared with 100% of tubules in age-matched controls. At 25 weeks of age, testes weights were still lower in treated animals than in control boars (203.3 ± 14.0 g in treated boars [n = 4] vs 282.1 ± 52.7 in controls [n = 6]; mean ± SD, P < .05). At 32 weeks of age, spermatogenesis had recovered in 72% of tubule cross sections in treated pigs compared with 100% of cross sections in control testes.
Effect of in utero treatment with busulfan (7.5 mg/kg) on days 98 and 108 on germ cell numbers in male piglets (experiment 2)
| Gonocytes or Spermatogonia/1000 Sertoli Cells (Mean ± SD)
|
||||
|---|---|---|---|---|
| Group | 10 days | 2 weeks | 4 weeks | 8 weeks |
| Treatment | 9.9 ± 2.5* | 21.8 ± 7.8* | 9.8 ± 1.3* | 13.8 ± 4.5* |
| Control | 75.2 ± 13.5 | 74.3 ± 12.2 | 51.0 ± 8.1 | 73.0 ± 15.5 |
Significant difference within age group (P < .05).
Figure 1.
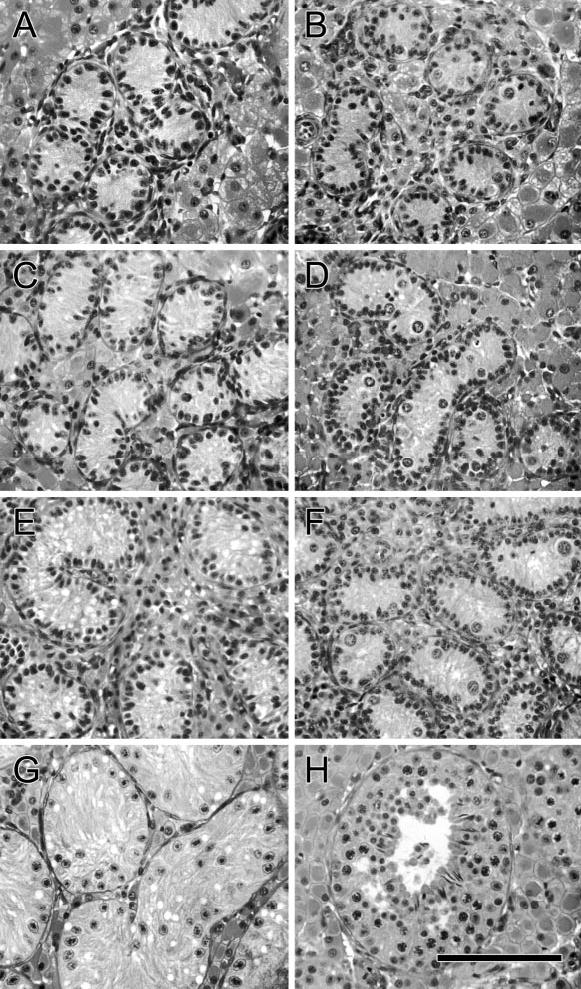
Histological appearance of testis tissue in piglets treated twice with busulfan in utero and controls. (A, C, E, G) Busulfan-treated pigs, (B, D, F, H) controls. (A, B) 2 weeks of age, (C, D) 4 weeks of age, (E, F) 8 weeks of age, (G, H) 16 weeks of age. Bar = 70 μm.
No adverse effects of treatment were clinically apparent in busulfan-treated sows. Litter size was within normal range for the herd, sows cycled normally and conceived on subsequent breedings.
Piglets born to treated sows were smaller than controls (1.4 ± 0.4 kg for 10 treated piglets from 1 litter vs 2.2 ± 0.2 kg for 6 piglets in a control litter; mean ± SD, P < .05). Weight gain over the first 6 weeks of life was slower than in untreated animals and, when compared at 8 months of age, body weight of pigs treated in utero was still lower (79.5 ± 3.9 kg, n = 4) than in untreated controls (91.0 ± 6.4 kg, n = 6, mean ± SD, P < .05). Blood samples taken at 3 weeks of age showed no evidence of anemia and platelet counts were adequate. Moderate poikilocytosis, mild to moderate anisocytosis and polychromasia, and few giant platelets and clumps were observed in blood from both treated and control pigs. Bone marrow from 1 treated animal examined at 7 weeks of age showed evidence of dyserythropoiesis. However, in blood samples collected at 7 and 12 weeks of age, no abnormalities were evident in the hemogram.
In some litters, piglets from treated sows exhibited bilateral cataracts that disappeared by 5 months of age. No other gross or histopathological abnormalities were observed in 2 pigs subjected to postmortem analysis after euthanasia at 4 and 7 weeks of age or in the sows at the end of the experiment.
Effects of Testis Irradiation in Goats
At 2 months after irradiation, 2%–4% of seminiferous tubule cross sections contained germ cells developed to the pachytene spermatocyte stage in goats irradiated at 1 or 5 weeks of age (n = 2), compared with greater than 50% of cross sections in age-matched controls (n = 4) (Figure 2). In a goat irradiated at 9.5 weeks of age (Figure 3), 10% of tubules contained spermatocytes, whereas controls (n = 2) showed complete spermatogenesis in all tubules. At 3 months after irradiation (n = 3), spermatozoa were apparent in 20% (range, 6%–33%) of tubule cross sections in all groups, compared with full spermatogenesis in all tubules in control goats (n = 6). By 6 months after irradiation, complete spermatogenesis was observed in 60% of tubules examined in goats treated at 5 or 9.5 weeks of age but in only 29% of tubules after irradiation at 1 week of age.
Figure 2.
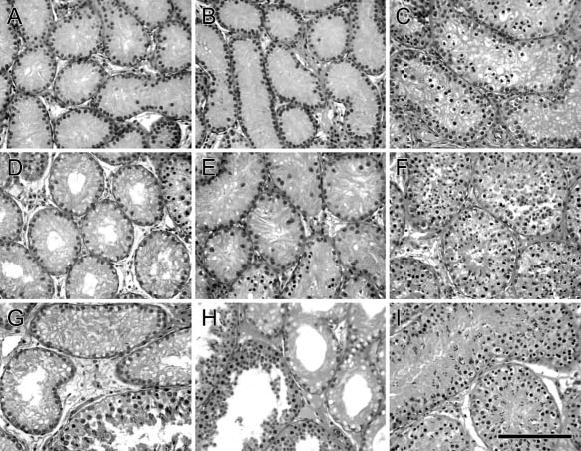
Histological appearance of goat testis tissue at 2, 3, and 6 months after fractionated irradiation compared with testes from age-matched control animals. (A, D, G) Testis tissue from goats irradiated at 1 week of age analyzed at 2, 3, and 6 months after irradiation. (B, E, H) Testis tissue from goats irradiated at 5 weeks of age analyzed at 2, 3, and 6 months after irradiation. (C, F, I) Testis tissue from 3-, 4-, and 7-month-old control goats. Bar = 70 μm.
Figure 3.
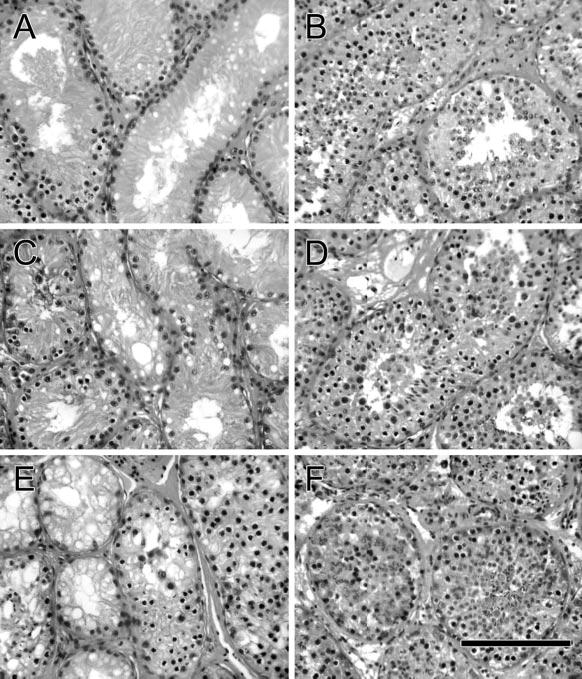
Histological appearance of testis tissue from goats irradiated at 9.5 weeks of age. (A, C, E) Analyzed at 2, 3, and 6 months after irradiation. (B, D, F) Testis tissue from 4-, 5-, and 8-month-old control goats. Bar = 70 μm.
Discussion
Germ cell transplantation represents a potentially powerful approach for introduction of genetic modifications into the germ line of domestic animal species where approaches routinely used in mice are not available or are inefficient. Recently, the technical aspects of germ cell transplantation in farm animals were addressed (Honaramooz et al, 2002, 2003b; Izadyar et al, 2003). Genetic manipulation of donor germ cells was accomplished (Honaramooz et al, unpublished) and donor germ cells formed fertile sperm after transplantation to immunocompetent recipients (Honaramooz et al, 2003b); however, full implementation of the technology requires the development of practically feasible protocols for preparation of recipients in each target species. As no previous work has been published in pigs and goats, this study was designed to provide protocols for depletion of germ cells in these species.
Here, we demonstrate that fetal busulfan treatment in pigs and local testicular irradiation in immature goats represent practical approaches to decrease endogenous spermatogenesis without severe adverse effects on the health of the recipient animals. Previous work in rodents demonstrated that maintenance of testicular health and subsequent donor-derived sperm production was improved when removal of germ cells was not complete and some endogenous spermatogenesis was allowed to occur (Brinster and Avarbock, 1994; Ogawa et al, 1999). While busulfan treatment of adult mice results in almost complete removal of endogenous spermatogenesis, subsequent fertility of recipient animals after germ cell transplantation is lower than in recipients prepared by fetal exposure to busulfan where ablation of endogenous germ cells is incomplete (Shinohara et al, 2002). Therefore, partial recovery of endogenous spermatogenesis in the treated pigs and goats in this study should prove advantageous and can be interpreted as an indication that the cytoablative treatments were not detrimental to the testicular environment.
Sensitivity to the toxicity of busulfan appears to be species specific. Adult mice can be treated with cytotoxic doses of busulfan (at concentrations as high as 30–100 mg/kg, depending on the strain) that result in almost complete removal of endogenous germ cells without significant systemic toxicity. This is in contrast with the situation in rats (Ogawa et al, 2000) and in pigs, as demonstrated in our preliminary experiments. However, the only previous report on postnatal busulfan treatment in pigs used multiple treatments with a much higher dose (40–100 mg/kg) than in the current study. No information was given on systemic effects of this dose on pigs, while high mortality in mouse recipients treated at the same dose was reported (Kim et al, 1997). It is unclear whether use of a different solvent in this study and the current study could account for the differences in toxicity. Because of the systemic toxicity of busulfan treatment in postnatal pigs observed in the current study, fetal busulfan treatment was chosen as it suppressed endogenous spermatogenesis in mice and rats born to treated females without significant mortality (Hemsworth and Jackson, 1963; Brinster et al, 2003; Moisan et al, 2003). We could demonstrate that busulfan-treated sows had normal litter sizes and suffered no ill systemic effects or depression of fertility following treatment. Piglets born to treated sows were smaller than those from control litters but had survival rates comparable with controls. Bone marrow analysis indicated evidence of disturbed erythropoiesis often associated with the use of alkylating drugs; however, piglets did not show obvious hematologic changes different from control animals. Cataracts presumably caused by the administration of DMSO (Rengstorff et al, 1972; Rubin, 1975), used as the solvent for the water-insoluble busulfan, to the pregnant sows were no longer apparent by the time animals reached sexual maturity. Therefore, systemic effects of fetal busulfan treatment on animal health were transient and did not appear to interfere with development. While we did not observe differences in treatment effect between the 2 swine breeds used, it may nonetheless be prudent to re-evaluate dosages for pigs of other genetic backgrounds.
In the current study, some loss of Sertoli cells due to fetal busulfan treatment or irradiation of the growing testis cannot be ruled out (de Rooij et al, 2002). Therefore, assessment of the number of germ cells relative to the number of Sertoli cells, as presented in the Table, may underestimate the effect of treatment. Loss of Sertoli cells could also contribute to a reduction in mature testis size in addition to the effect of spermatogenic suppression. While Sertoli cells in 1-week-old goats may have been more susceptible to radiation damage than those in the older goats, no difference in Sertoli cell number or morphology was observed between the 3 age groups at the time points analyzed.
For the irradiation protocol chosen in the present study for immature goat testes, we hypothesized that fractionated low-dose irradiation (3 × 2 Gy) would provide the desired effect on germ cells while minimizing potential detrimental effects on testicular somatic cells. A previous study in adult monkeys used only a single dose of 2 Gy, with the intent of mimicking testicular damage occurring in humans undergoing radiation therapy for cancer treatment rather than producing efficient suppression of endogenous spermatogenesis, resulting in reduction of testis volume to about 40% (Schlatt et al, 2002). In contrast, in a recent report on germ cell transplantation in cattle, a single dose of 10–14 Gy was required for efficient suppression of endogenous spermatogenesis in 5-month-old bull calves (Izadyar et al, 2003) and a single dose of 12 Gy has been used in adult rams (Oatley et al, 2005). While comparing results across studies in different animal species and ages is difficult, all reported protocols appear to reduce endogenous spermatogenesis. The dose and treatment strategy examined in the present study resulted in the desired effect of germ cell depletion; however, further studies critically comparing different protocols within a given species and age will be necessary to conclusively determined which strategy is optimal. The current study did not investigate the use of testicular irradiation in pigs because it is less practical than in utero treatment with busulfan. Therefore, its potential utility in pigs would require future investigation.
Successful transplantation of germ cells in domestic animals requires accessibility of the rete testis and presence of a lumen in the seminiferous tubules to accommodate the infused donor cell suspension. In the pigs and goats studied here, lumen formation in the seminiferous tubules becomes evident by about 12 weeks of age. Previous work in rodents has shown that sexually immature males present a superior testis environment for donor cell engraftment compared with adult recipients (Shinohara et al, 2001) and the limited success of homologous germ cell transplantation in cattle was attributed to advanced recipient age (Izadyar et al, 2003). We demonstrated previously that donor-cell colonization was successful in pre-pubertal pigs and goats (Honaramooz et al, 2002a, 2003b). Therefore, depletion of endogenous germ cells by fetal busulfan treatment or testicular irradiation in the first months of life followed by transplantation of germ cells at 3–4 months of age will assure that transient detrimental effects of treatment on the testicular environment have subsided, the seminiferous tubules are accessible for transplantation, and the testis environment is particularly suitable for donor-cell engraftment and proliferation during entry into puberty and completion of sexual maturation. Currently, no methods are available to reliably quantify the extent of donor cell colonization in a large animal recipient testis, as is in rodents (Dobrinski et al, 1999). However, based on previous reports evaluating recipient preparation strategies in rodents (Ogawa et al, 1999; Creemers et al, 2002; Brinster et al, 2003), we expect the results presented here will prove beneficial for future application to germ cell transplantation in these large animal species.
In conclusion, the treatment protocols developed in the current study to deplete endogenous germ cells will provide an essential step to make germ cell transplantation technology a viable, efficient alternative for genetic manipulation of pigs and goats.
Acknowledgments
We thank Dr Alex Travis for critical review of the manuscript, Mark Lewis for help with animal care, and James Hayden, RBP, for preparation of figures. We would like to dedicate this manuscript to the memory of Dr Lonnie D. Russell for his involvement in the preliminary studies in pigs.
Footnotes
Supported by grants 1R01 RR 17359-01 and 1 R41 HD044780-01 from the National Institutes of Health, the Illinois Council on Agricultural Research, and the Commonwealth and General Assembly of Pennsylvania. Portions of this study were presented at the annual meeting of the Society for the Study of Reproduction in Vancouver, August 1–4, 2004.
References
- Brinster RL. Germ cell transplantation. Science. 2002;296:2174–2176. doi: 10.1126/science.1071607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster CJ, Ryn BY, Avarbock MR, Karagenc L, Brinster RL. Restoration of fertility by germ cell transplantation requires efficient recipient preparation. Biol Reprod. 2003;69:412–420. doi: 10.1095/biolreprod.103.016519. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Zimmerman JW. Spermatogenesis following male germ cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creemers LB, Meng X, den Ouden K, van Pelt AMM, Izadyar F, Santoro M, Sariola H, de Rooij DG. Transplantation of germ cells from glial cell line-derived neurotrophic factor-overexpressing mice to host testes depleted of endogenous spermatogenesis by fractionated irradiation. Biol Reprod. 2002;66:1579–1584. doi: 10.1095/biolreprod66.6.1579. [DOI] [PubMed] [Google Scholar]
- de Rooij DG. Stem cells in the testis. Int J Exp Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij DG, van de Kant HJG, Dol R, Wagemaker G, van Buul PPW, van Duijn-Goedhart A, de Jong FH, Broerse JJ. Long-term effects of irradiation before adulthood on reproductive function in the male rhesus monkey. Biol Reprod. 2002;66:486–494. doi: 10.1095/biolreprod66.2.486. [DOI] [PubMed] [Google Scholar]
- Dobrinski I, Ogawa T, Avarbock MR, Brinster RL. Computer assisted image analysis to assess colonization of recipient seminiferous tubules by spermatogonial stem cells from transgenic donor mice. Mol Reprod Dev. 1999;53:142–148. doi: 10.1002/(SICI)1098-2795(199906)53:2<142::AID-MRD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- França LR, Silva VAJ, Chiarini-Garcia H, Garcia SK, Debeljuk L. Cell proliferation and hormonal changes during postnatal development of the testis in the pig. Biol Reprod. 2000;63:1629–1636. doi: 10.1095/biolreprod63.6.1629. [DOI] [PubMed] [Google Scholar]
- Gavin WG. Gene transfer into goat embryos. In: Houdebine LM, ed. Transgenic Animals: Generation and Use. Lausanne, Switzerland: Harwood, Academic Press Inc; 1996:19–21.
- Geissler EN, Ryan MA, Housman DE. The dominant-white spotting(W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55:185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Giuili G, Tomljenovic A, Labrecque N, Oulad-Abdelghani M, Rassoulzadegan M, Cuzin F. Murine spermatogonial stem cells: targeted transgene expression and purification in an active state. EMBO Rep. 2002;3:753–759. doi: 10.1093/embo-reports/kvf149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsworth BN, Jackson H. Effect of busulphan on the developing gonad of the male rat. J Reprod Fertil. 1963;5:187–194. doi: 10.1530/jrf.0.0050187. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Behboodi E, Blash S, Megee SO, Dobrinski I. Germ cell transplantation in goats. Mol Reprod Dev. 003a;64:422–428. doi: 10.1002/mrd.10205. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echerlard Y, Dobrinski I. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003b;69:1260–1264. doi: 10.1095/biolreprod.103.018788. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biol Reprod. 2002;66:21–28. doi: 10.1095/biolreprod66.1.21. [DOI] [PubMed] [Google Scholar]
- Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, Colenbrander B, Oldenbroek JK, Van der Ploeg KD, Woelders H, Kal HB, De Rooij DG. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126:765–774. [PubMed] [Google Scholar]
- Joerg H, Janett F, Schlatt S, Mueller S, Graphodatskaya D, Suwattana D, Asai M, Stranzinger G. Germ cell transplantation in an azoospermic Klinefelter bull. Biol Reprod. 2003;69:1940–1944. doi: 10.1095/biolreprod.103.020297. [DOI] [PubMed] [Google Scholar]
- Keefer CL. Production of bioproducts through the use of transgenic animal models. Anim Reprod Sci. 2004;82:5–12. doi: 10.1016/j.anireprosci.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Kim J-H, Jung-Ha H-S, Lee H-T, Chung K-S. Development of a positive method for male stem cell-mediated gene transfer in mouse and pig. Mol Rep Dev. 1997;46:515–526. doi: 10.1002/(SICI)1098-2795(199704)46:4<515::AID-MRD10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- McLean DJ, Russell LD, Griswold MD. Biological activity and enrichment of spermatogonial stem cells in vitamin A-deficient and hyperthermia-exposed testes from mice based on colonization following germ cell transplantation. Biol Reprod. 2002;66:1374–1379. doi: 10.1095/biolreprod66.5.1374. [DOI] [PubMed] [Google Scholar]
- Moisan AE, Foster RA, Betteridge KJ, Hahnel AC. Dose-response of RAG2−/−/γc−/− mice to busulfan in preparation for spermatogonial transplantation. Reproduction. 2003;126:205–216. doi: 10.1530/rep.0.1260205. [DOI] [PubMed] [Google Scholar]
- Niemann H, Kues WA. Application of transgenesis in livestock for agriculture and biomedicine. Anim Reprod Sci. 2003;79:291–317. doi: 10.1016/s0378-4320(03)00169-6. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Tibary A, de Avila DM, Wheaton JE, McLean DJ, Reeves JJ. Changes in spermatogenesis and endocrine unction in the ram testis due to irradiation and active immunization against luteinizing hormone-releasing hormone. J Anim Sci. 2005;83:604–612. doi: 10.2527/2005.833604x. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Transplantation of male germ line stem cells restores fertility in infertile mice. Nat Med. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31:461–472. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- Rengstorff RH, Petrali JP, Sim VM. Cataracts induced in guinea pigs by acetone, cyclohexanone, and dimethyl sulfoxide. Am J Optom Arch Am Acad Optom. 1972;49:308–319. doi: 10.1097/00006324-197204000-00003. [DOI] [PubMed] [Google Scholar]
- Rockett JC, Mapp FL, Garges JB, Luft JC, Mori C, Dix DJ. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol Reprod. 2001;65:229–239. doi: 10.1095/biolreprod65.1.229. [DOI] [PubMed] [Google Scholar]
- Rubin LF. Toxicity of dimethyl sulfoxide, alone and in combination. Ann N Y Acad Sci. 1975;243:98–103. doi: 10.1111/j.1749-6632.1975.tb25348.x. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod. 2002;17:55–62. doi: 10.1093/humrep/17.1.55. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accomplished by dramatic changes in stem cell number and niche accessibility. Proc Natl Acad Sci U S A. 2001;98:6186–6191. doi: 10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Gem line stem cell competition in postnatal mouse testes. Biol Reprod. 2002;66:1491–1497. doi: 10.1095/biolreprod66.5.1491. [DOI] [PubMed] [Google Scholar]
- Shuttlesworth GA, de Rooij DG, Huhtaniemi I, Reissmann T, Russell LD, Shetty G, Wilson G, Meistrich ML. Enhancement of a spermatogonial proliferation and differentiation in irradiated rats by gonadotropin-releasing hormone antagonist administration. Endocrinology. 2000;141:37–49. doi: 10.1210/endo.141.1.7272. [DOI] [PubMed] [Google Scholar]
- Silvers WK. Dominant spotting, patch, and rump-white. In: The Coat Colors of Mice. New York, NY: Springer Verlag; 1979:206–241.
- van den Aardweg GJ, de Ruiter-Bootsma AL, Kramer MF, Davids JA. Growth and differentiation of spermatogenetic colonies in the mouse testis after irradiation with fission neutrons. Radiat Res. 1983;94:447–463. [PubMed] [Google Scholar]
- van Straaten HWM, Wensing CJG. Histomorphometric aspects of testicular morphogenesis in pig. Biol Reprod. 1977;17:467–472. doi: 10.1095/biolreprod17.4.467. [DOI] [PubMed] [Google Scholar]
- Yong GP, Goldstein M, Phillips DM, Sundaram K, Gunsalus GL, Bardin CW. Sertoli cell-only syndrome produced by cold testicular ischemia. Endocrinology. 1988;122:1074–1082. doi: 10.1210/endo-122-3-1074. [DOI] [PubMed] [Google Scholar]


