Abstract
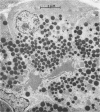
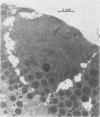
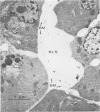
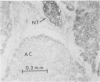
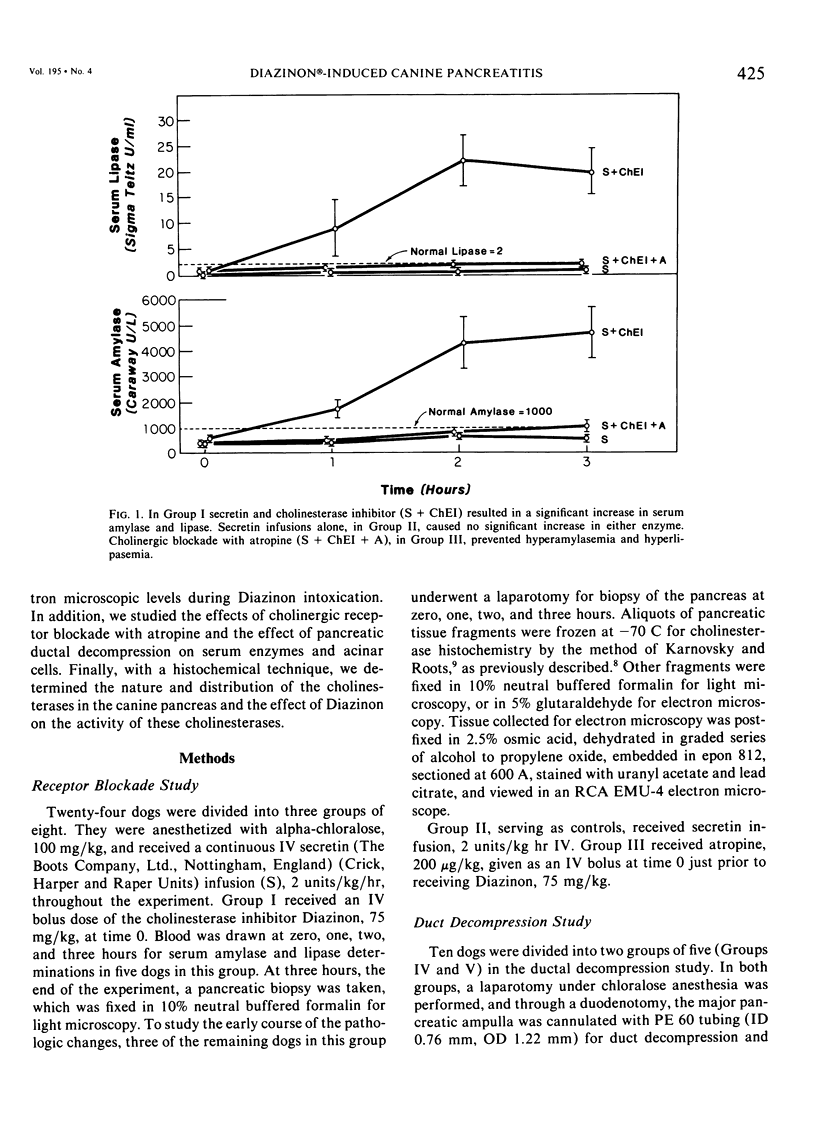
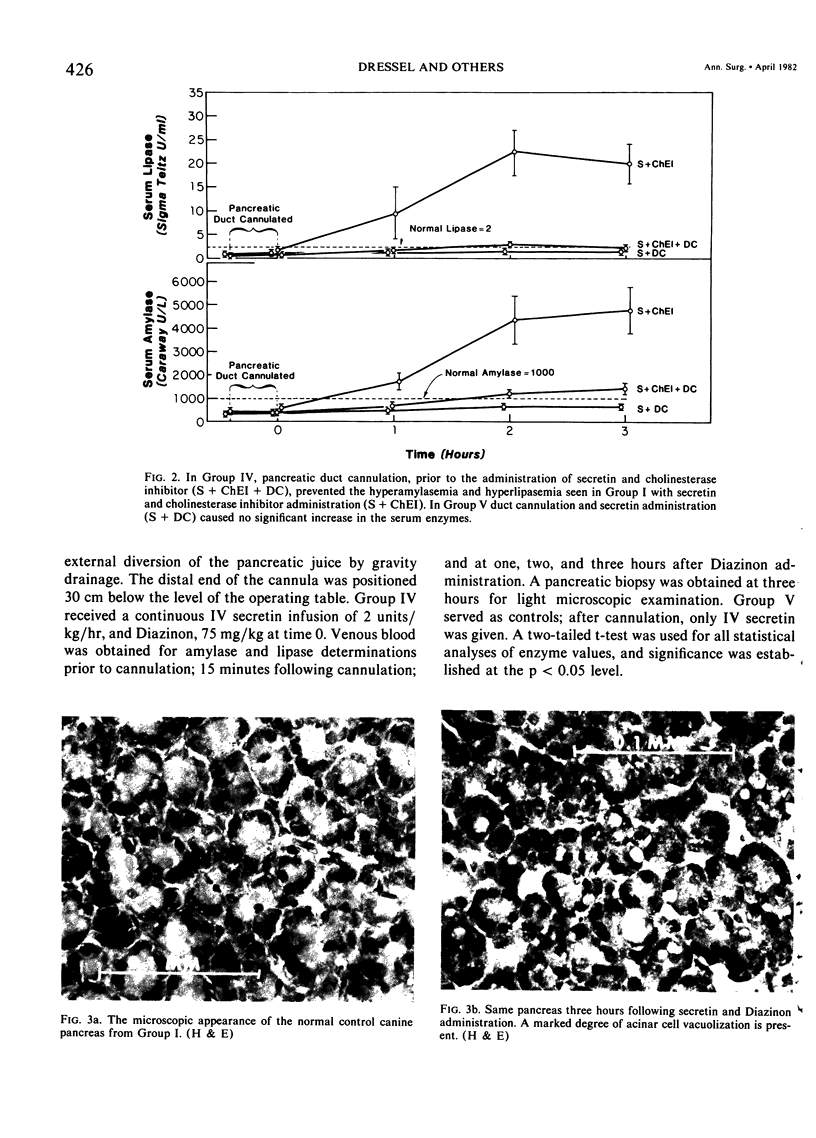
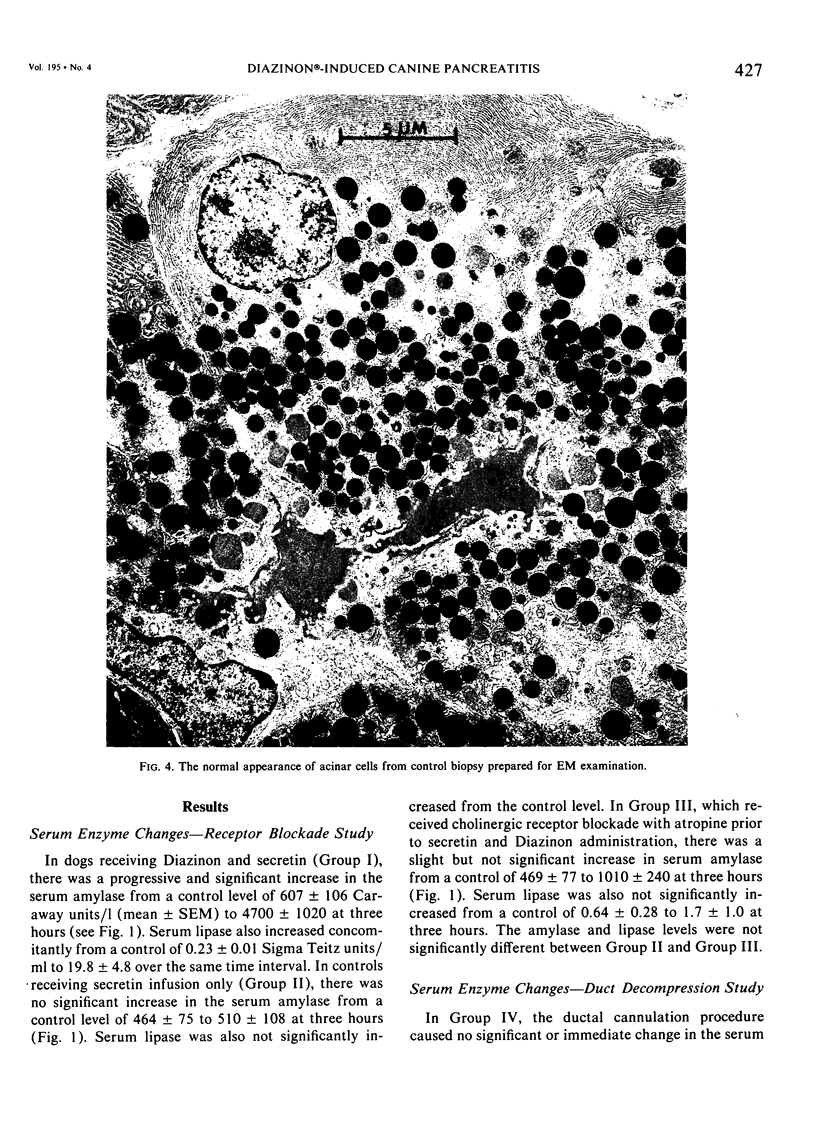
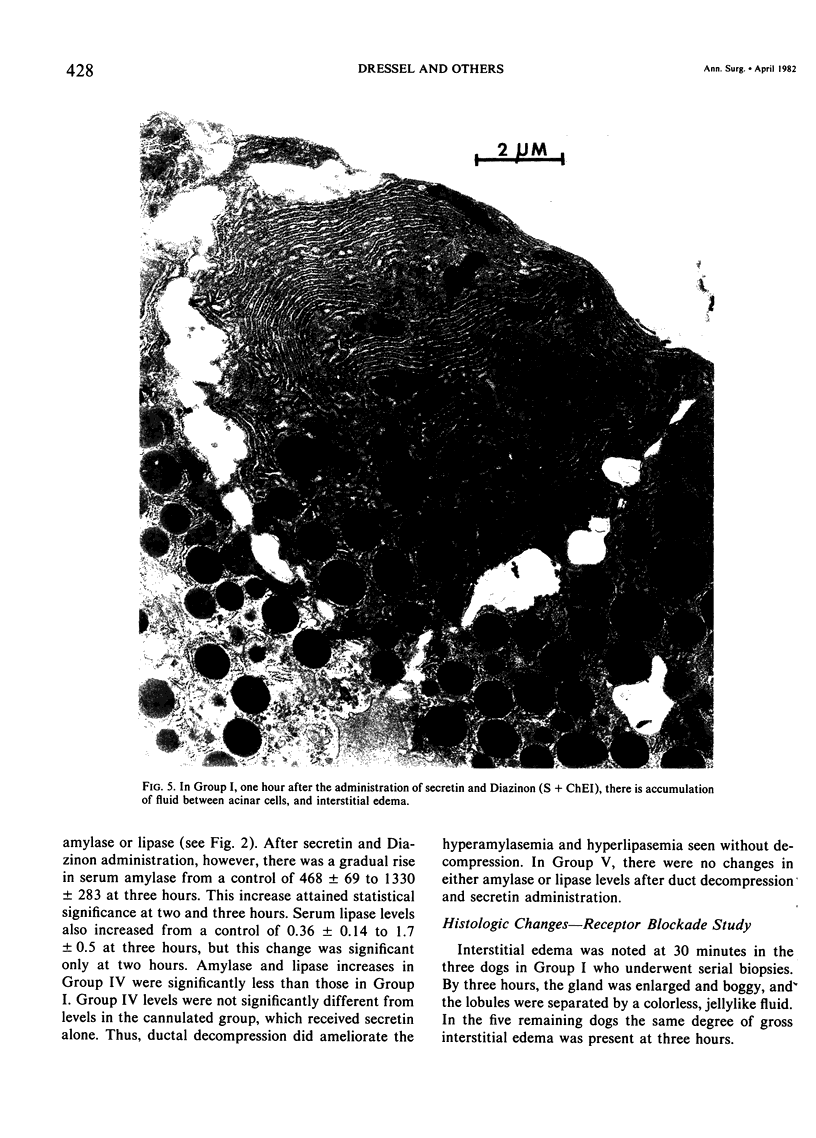
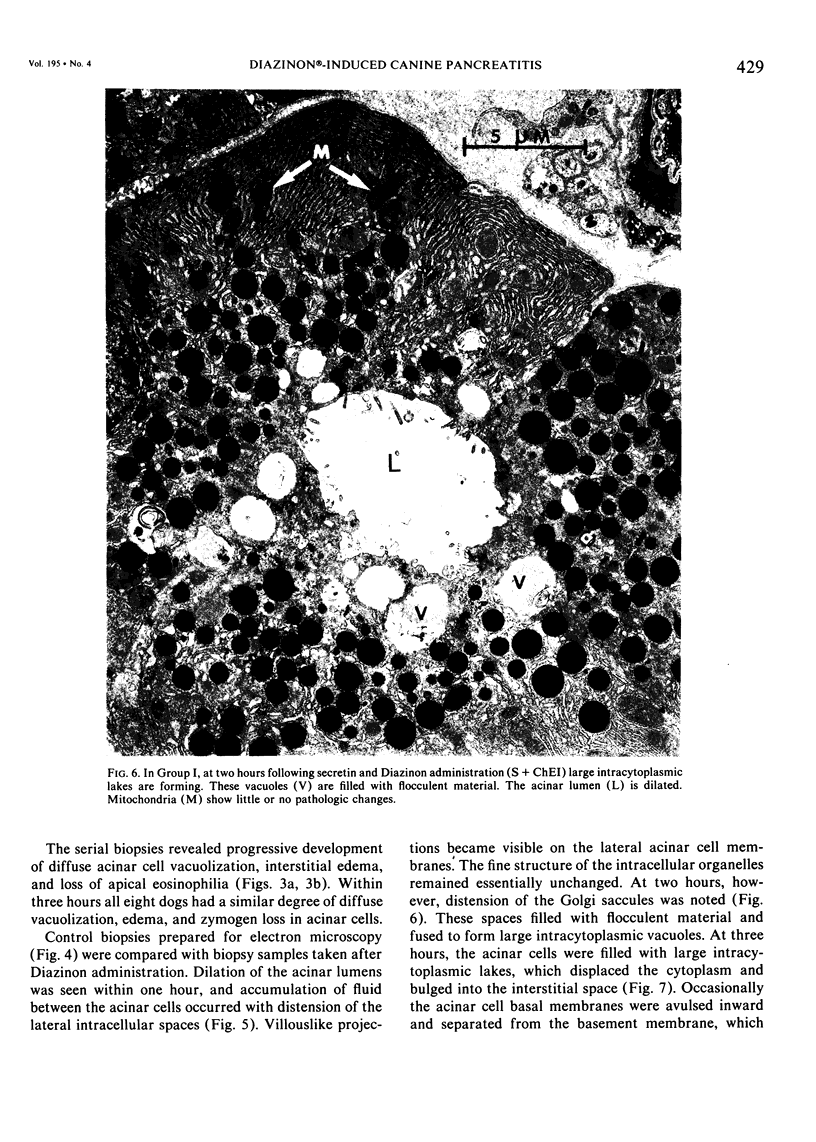
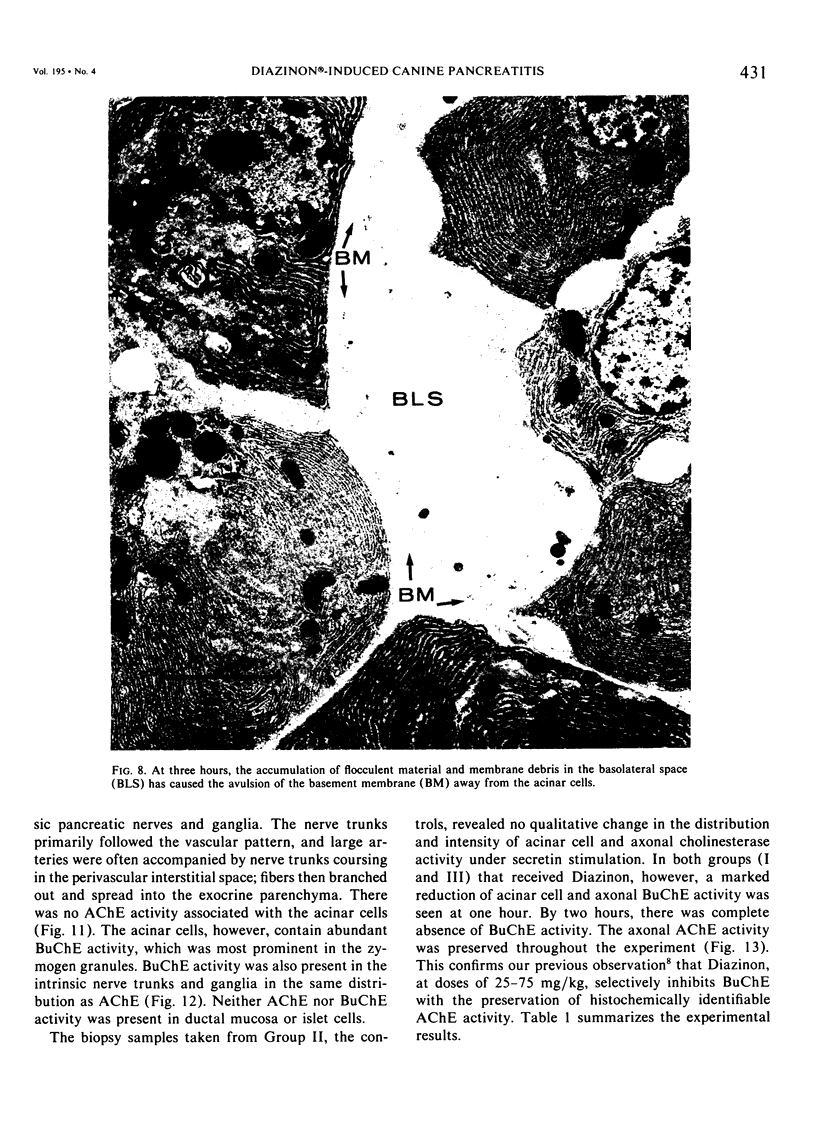
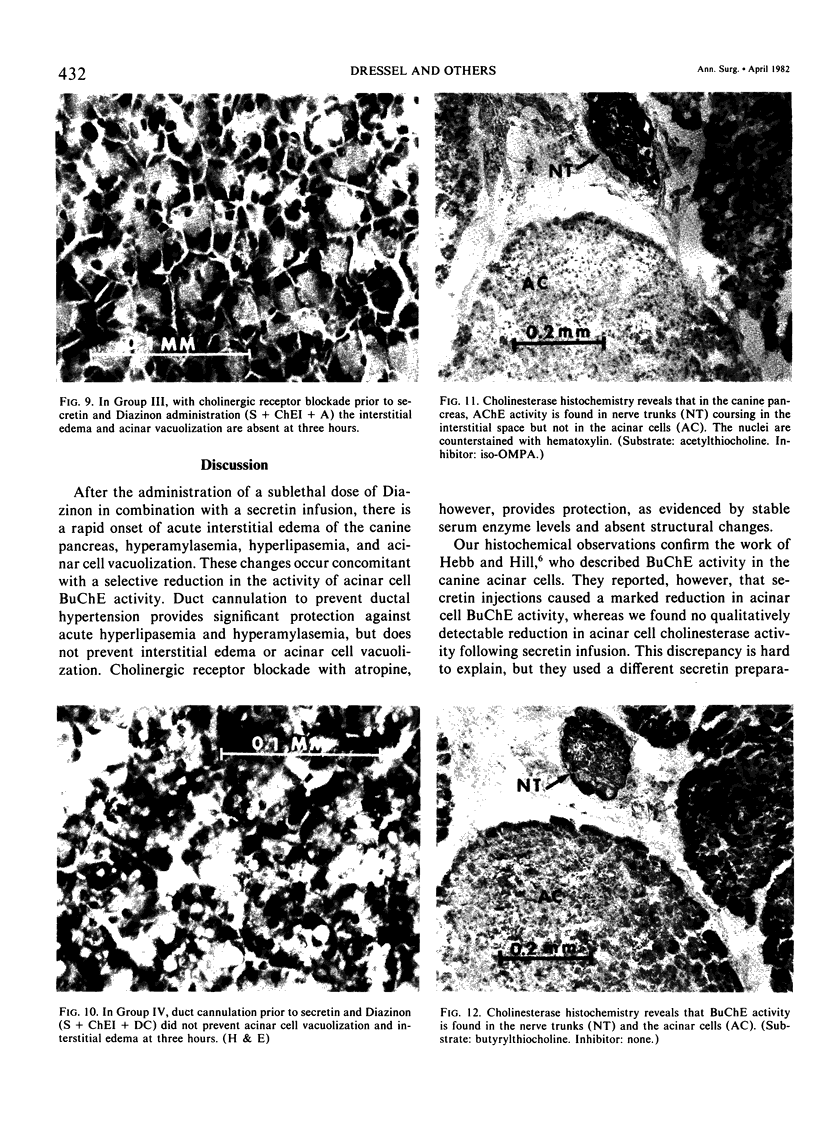
Three groups of eight dogs each were studied to evaluate the early evolution of the hyperamylasemia, hyperlipasemia, and acinar cell pathology at the light and electron microscopic levels during acute Diazinon-induced pancreatitis. Two more groups of five dogs each were evaluated for the effects of cholinergic receptor blockade with atropine and ductal decompression on the evolution of serum enzyme changes and acinar cell pathology. Group I dogs received a secretin infusion of 2 units/kg/hr, and a Diazinon infusion of 75 mg/kg, and demonstrated significant increases in serum amylase and lipase at one, two and three hours. Light microscopy revealed acinar cell vacuolization and progressive interstitial edema. Electron microscopy revealed the formation of large intracytoplasmic vacuoles filled with flocculent material, the fusion of these vacuoles with basolateral membrane, and the formation of interstitial edema. In both group II dogs (that received secretin alone) and Group III dogs (that received atropine, 200 micrograms/kg IV prior to secretin and Diazinon), the serum enzyme levels and histologic results were normal. In group IV dogs, pancreatic duct cannulation to prevent hypertension prevented the hyperamylasemia and hyperlipasemia, but not the acinar cell vacuolization and interstitial edema. This model for acute interstitial pancreatitis is apparently cholinergic-receptor mediated, the serum enzyme elevations are due primarily to ductal hypertension, and the acinar cell pathology is primarily due to cholinergic stimulation and occurs independent of ductal hypertension.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks P. A. Acute pancreatitis. Gastroenterology. 1971 Sep;61(3):382–397. [PubMed] [Google Scholar]
- Case R. M. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc. 1978 May;53(2):211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Dressel T. D., Goodale R. L., Borner J. W., Etani S. A study of the cholinesterases of the canine pancreatic sphincters and the relationship between reduced butyrylcholinesterase activity and pancreatic ductal hypertension. Ann Surg. 1980 Nov;192(5):614–619. doi: 10.1097/00000658-198011000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel T. D., Goodale R. L., Jr, Arneson M. A., Borner J. W. Pancreatitis as a complication of anticholinesterase insecticide intoxication. Ann Surg. 1979 Feb;189(2):199–204. doi: 10.1097/00000658-197902000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel T. D., Goodale R. L., Jr, Hunninghake D. B., Borner J. W. Sensitivity of the canine pancreatic intraductal pressure to subclinical reduction in cholinesterase acitivity. Ann Surg. 1979 Jul;190(1):6–12. doi: 10.1097/00000658-197907000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERB C., HILL K. J. Distribution of cholinesterases in the mammalian pancreas. Q J Exp Physiol Cogn Med Sci. 1955 Apr;40(2):168–175. doi: 10.1113/expphysiol.1955.sp001108. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Mendel B., Rudney H. Studies on cholinesterase: 1. Cholinesterase and pseudo-cholinesterase. Biochem J. 1943 Apr;37(1):59–63. doi: 10.1042/bj0370059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C., Hand A. R. Uptake and fate of luminally administered horseradish peroxidase in resting and isoproterenol-stimulated rat parotid acinar cells. J Cell Biol. 1978 Jan;76(1):207–229. doi: 10.1083/jcb.76.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Saito A., Kanno T. Concentration of pancreozymin as a determinant of the exocrine-endocrine partition of pancreatic enzymes. Jpn J Physiol. 1973 Oct;23(5):477–495. doi: 10.2170/jjphysiol.23.477. [DOI] [PubMed] [Google Scholar]