Abstract
The Tet(L) protein encoded in the Bacillus subtilis chromosome and the closely related Tet(K) protein from Staphylococcus aureus plasmids are multifunctional antiporters that have three cytoplasmic efflux substrates: a tetracycline-divalent metal (TC-Me2+) complex that bears a net single positive charge, Na+, and K+. Tet(L) and Tet(K) had been shown to couple efflux of each of these substrates to influx of H+ as the coupling ion. In this study, competitive cross-inhibition between K+ and other cytoplasmic efflux substrates was demonstrated. Tet(L) and Tet(K) had also been shown to use K+ as an alternate coupling ion in support of Na+ or K+ efflux. Here they were shown to couple TC-Me2+ efflux to K+ uptake as well, exhibiting greater use of K+ as a coupling ion as the external pH increased. The substrate and coupling ion preferences of the two Tet proteins differed, especially in the higher preference of Tet(K) than Tet(L) for K+, both as a cytoplasmic efflux substrate and as an external coupling ion. Site-directed mutagenesis was employed to test the hypothesis that some feature of the putative “antiporter motif,” motif C, of Tet proteins would be involved in these characteristic preferences. Mutation of the A157 in Tet(L) to a hydroxyamino acid resulted in a more Tet(K)-like K+ preference both as coupling ion and efflux substrate. A reciprocal S157A mutant of Tet(K) exhibited reduced K+ preference. Competitive inhibition among substrates and the parallel effects of the single mutation upon K+ preference, as both an efflux substrate and coupling ion, are compatible with a model in which a single translocation pathway through the Tet(L) and Tet(K) transporters is used both for the cytoplasmic efflux substrates and for the coupling ions, in an alternating fashion. However, the effects of the A157 and other mutations of Tet(L) indicate that even if there are a shared binding site and translocation pathway, some elements of that pathway are used by all substrates and others are important only for particular substrates.
The chromosomal tet(L) locus of Bacillus subtilis and the closely related tet(K) gene, which is predominantly found in plasmids of Bacillus species and of Staphylococcus aureus, encode proteins that confer resistance (Tcr) to tetracycline (TC) (35). Like other Tet efflux proteins, they catalyze efflux of a TC-divalent metal ion complex bearing a net single positive charge in exchange for protons, i.e., TC-Me2+/H+ antiport (mode 1) (17, 30, 50). Studies in our laboratory have shown that Tet(L) and Tet(K) are multifunctional antiporters that have additional catalytic modes (3-5, 17, 18, 30). First, they exhibit a monovalent cation (Na+ or K+)/H+ antiport mode (mode 2), with either Na+ or K+ serving as the cytoplasmic efflux substrate instead of the TC-Me2+ complex (5, 17, 18). In B. subtilis, this antiporter mode plays a physiological role in Na+ resistance and pH homeostasis (4, 30). We will use the term “efflux substrate” to denote TC-Me2+, Na+, or K+ that effluxes from the cytoplasmic side of the membrane, although, in experiments that use everted membrane preparations, the efflux substrate will actually be taken up from the extravesicular space. The monovalent cation/H+ antiport mode of Tet(L) (mode 2) exhibits apparent differences from Tet(K) in Na+ versus K+ affinity. The exclusion of Na+ by Tet(L), expressed in multicopy in a B. subtilis tet(L) deletion strain, was not greatly reduced by the presence of high [K+], whereas Na+ exclusion by Tet(K) was nearly abolished. This suggested that Tet(K) has a greater preference for K+ as a cytoplasmic efflux substrate, relative to Na+, than does Tet(L) (4, 30). The other Tet(L) and Tet(K) catalytic mode is an antiport mode in which Na+ or K+ efflux is coupled to entry of K+ as a coupling ion instead of to H+ (mode 3) (18, 30). The antiport reactions catalyzed by Tet(L) and Tet(K) are apparently electrogenic: e.g., H+ or K+ transported inward >TC-Me2+ or Na+ or K+ transported outward (17, 18). Therefore, there is net uptake of K+ when it serves as the external coupling ion even when it is also the cytoplasmic efflux substrate (18). We will use the term “coupling ion” to denote the H+ or K+ that moves inward across the external face of the membrane into the cytoplasmic side in exchange for an efflux substrate. Tet(K) exhibits more robust K+ transport than Tet(L) in the K+ uptake mode, as monitored by 86Rb+ uptake (18). Thus, the capacity to use K+ as a coupling ion mirrors the profiles of Tet(K) and Tet(L) with respect to Na+ and K+ preferences as a cytoplasmic substrate for efflux. The K+ uptake mode of Tet(L), mode 3, apparently has physiological importance as part of the capacity for K+ acquisition in B. subtilis (27, 30, 48).
The present study focused on three questions raised by the finding that K+ can serve as both an efflux substrate and as a coupling ion and that closely related Tet efflux proteins have different efficacy in catalyzing these fluxes of K+. First, we sought to examine whether K+ can be used as a coupling ion when TC-Me2+ is the efflux substrate just as it can when one of the monovalent cation substrates, Na+ or K+, is effluxed (18). If so, it was of interest to test the pH dependence of K+-86Rb+ uptake in exchange for TC-Me2+: i.e., whether it indicated that K+ competes with H+ as a coupling ion, especially at high pH. Replacement of H+ by K+ as a coupling ion for TC-Me2+ efflux at high pH could offer a benefit similar to that noted in connection with coupling antibiotic efflux to Na+ uptake in the alkaline environment of marine Vibrio parahaemolyticus (2, 37).
The second issue that is examined here was whether the earlier failure to observe cross-inhibition between TC-Me2+ and Na+ as efflux substrates (17) was reflective of distinct domains for the different substrates, as has been found for substrates of several (multi)drug efflux proteins (32, 36, 43, 52). As the importance of K+ as a substrate of Tet(L) and Tet(K) became evident, we wondered whether it was possible that there was a methodological rather than intrinsic basis for the earlier absence of TC-Me2+ versus monovalent cation substrate cross-inhibition in vesicle assays. In particular, since the standard assay buffer used for those assays of Tet(L) and Tet(K) activities was a potassium phosphate buffer, we sought to examine whether cross-inhibition among efflux substrates would be observed if an assay buffer without added K+ was employed.
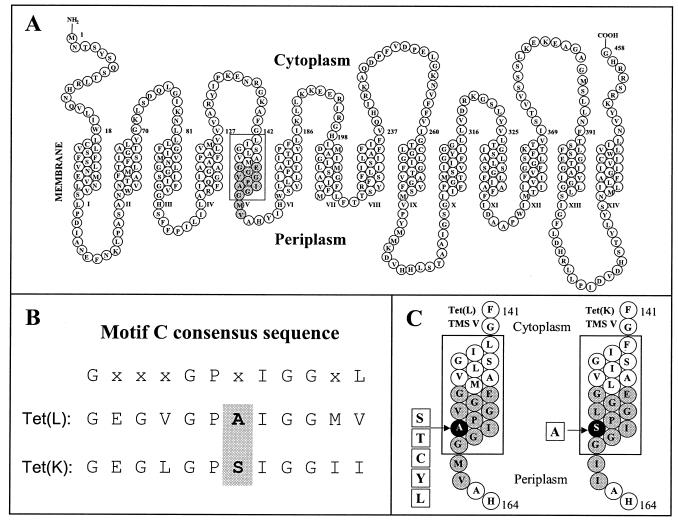
The third issue of interest was whether we could take advantage of the capacity of Tet(L) and Tet(K) to use 86Rb+-K+ as a coupling ion to begin to probe the basis for the difference in K+ preference between these closely related transporters. We pursued the hypothesis that a difference in motif C of Tet(L) and Tet(K) might be an important factor in their different substrate preferences. Motif C is located in the periplasmic end of transmembrane segment (TMS) V in both the 12- and 14-TMS Tet proteins (12, 41) (Fig. 1). It includes an essential glutamate residue (E152) in Tet(L) and Tet(K) (11, 27) and the consensus sequence GX8GX3GPX2GG. This consensus sequence is associated with antiporters, as opposed to symporters or uniporters; thus, motif C was hypothesized by others to be involved in the specific translocation mechanism of antiporters (24, 47).
FIG. 1.
The motif C regions of Tet(L) and Tet(K). The motif C (41) consensus sequence is shown in alignment with the sequences in the B. subtilis chromosomally encoded Tet(L) and Tet(K) regions. (A) Topological model of the whole Tet(L) protein, based on the data for Tet(K) (12, 22), with TMS V indicated by the rectangle and the position of motif C shaded in gray. (B) Alignment of the Tet(L) and Tet(K) motif C. (C) The motif C regions of Tet(L) and Tet(K), showing the site-directed mutants constructed and characterized in this study.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Escherichia coli DH5α (Gibco-BRL) was used for the preparation of everted vesicles and measurements of Tcr and transport. E. coli JM109 was used for M13 phage DNA manipulation, and strain CJ236 was used for preparation of uracil-containing single-stranded M13 phage DNA templates for site-directed mutagenesis (31). E. coli NM81, an Na+-sensitive strain with a deletion in nhaA (39), was used for measurements of Na+ transport. E. coli TK2420, with mutations in three K+ uptake systems (9, 10), was used for measurements of complementation for growth on low [K+] and for 86Rb+ transport experiments. E. coli strains were usually grown, with shaking, at 37°C in Luria broth (LBK) in which the NaCl was replaced by KCl (14). In some experiments, NaCl was added back at the concentrations indicated. For complementation experiments, E. coli TK2420 was grown in a defined medium (9) to which various concentrations of KCl were added. B. subtilis strain AG112 was prepared from the tet(L) deletion strain JC112 by replacing the chloramphenicol resistance cassette that disrupts the tet(L) locus in that strain (4) with a spectinomycin resistance cassette. B. subtilis strains were grown with shaking at 30°C in either TKM or TTM medium (4).The semidefined TKM and TTM media are, respectively, K+-replete and low K+ Tris-based malate-containing media to which no Na+ is added unless indicated for specific experiments. The plasmid used in the studies of Na+ resistance and transport was pGEM3Zf(+) (Promega), in which modest expression of tet genes occurred when they were cloned under control of the T7 promoter and studied in bacterial strains lacking T7 polymerase; those levels of expression were optimal for the Na+-related assays. For studies of Tc- and K+-related phenotypes and transport, the shuttle vector pBK15 (obtained from K. Zen) was used. The tet genes were cloned under the control of the ermC promoter of the vector. Basal levels of expression from this promoter yielded higher expression of tet genes than that obtained in the pGEM3Zf(+) constructs.
Construction and cloning of site-directed mutants.
A 1.4-kb DNA fragment encompassing tet(L) was amplified from wild-type B. subtilis BD99 (obtained from A. Garro) chromosomal DNA by PCR with primers 5′-GGAATTCCATATGAATACGTCTATATCACAG-3′ and 5′-CCCGGATCCTTTCACTCATTTA-3′ and cloned into the bacteriophage vector M13mp19 via BamHI and EcoRI sites. The sequence of the wild-type tet(L) open reading frame (ORF) was confirmed. Then the phage DNA was used as the template for site-directed mutagenesis according to the method of Kunkel et al. (31). The following are the oligonucleotides used to generate the indicated tet(L) mutants (lowercase underlined letters represent new codons introduced by site-directed mutagenesis): 5′-GAAGGTGTTGGGCCA(a/t)cgATTGGCGGAATGGTT-3′ for A157T/S, 5′-GAAGGTGTTGGGCCAt(g/a)tATTGGCGGAATGGTT-3′ for A157C/Y, and 5′-GAAGGTGTTGGGCCActtATTGGCGGAATGGTT-3′ for A157L. The whole tet(L) ORF was then cloned into vectors pBK15 and pGEM3Zf(+) via BamHI and EcoRI sites. For construction of an S157A mutant of tet(K), a 1.4-kb fragment encompassing the tet(K) gene was amplified from plasmid pT181 (obtained from R. Novick) by PCR with the following primers: 5′-GGGAATTCCATATGTTTAGTTTATATAAAAAATTT-3′ and 5′-CCCGGATCCCTATTCAAACTGCTTTTCA-3′. This fragment was cloned into M13mp19, followed by the same steps described above. The oligonucleotide used for the mutagenesis was 5′-GAAGGGTTAGGTCCTgcaATAGGGGGAATAATA-3′. All new constructs were verified by sequence analysis of the whole tet(L) ORF. The sequencing was performed by the Utah State Biotechnology Center (Logan, Utah) with an ABI-100 model 377 Sequencer.
Complementation and Tcr conferred upon E. coli strains.
For assays of Tcr, 10 μl of stationary-phase cultures of the E. coli DH5α transformants with various tet genes expressed from pBK15 was inoculated into 2 ml of LBK medium containing a TC concentration of 0, 2, 4, 8, 10, 12, 16, or 32 μg/ml. The data for growth (A600) at 15 h were plotted for determinations of the MIC (26). For assays of complementation of the K+ uptake defect of E. coli TK2420, 10 μl of a stationary-phase culture was inoculated into defined medium (9) containing KCl concentrations from 5 to 25 mM. For both assays, the A600 was recorded after incubation at 37°C for 15 h. All assays were carried out in duplicate in at least two independent experiments.
Preparation of membrane vesicles.
Everted membrane vesicles of E. coli strains were prepared as described previously (17), except that the buffer was 10 mM bis-[tris(hydroxymethyl)methylamino]-propane (BTP) (pH 7.5). Dithiothreitol was added, to 5 mM, in all steps of the preparation, but the final pellet was suspended in BTP without the sulfhydryl reagent. For assays of 86Rb+ uptake, right-side-out (RSO) vesicles of E. coli TK2420 were prepared by the method of Kaback (28) and preloaded as previously described (18).
Western analyses of membranes from E. coli transformants.
Membrane preparations (50 μg of protein) from E. coli DH5α cells harboring plasmids expressing wild-type or mutant tet genes were loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Western analyses of preparations from strains expressing various forms of tet(L) were carried out with an antibody raised against a synthetic peptide corresponding to the N terminus of Tet(L) (5) and developed by the enhanced chemiluminescence method (ECL kit; Amersham). Western analyses of strains expressing forms of tet(K) were analyzed with a new antibody prepared as described for the earlier one (5), but raised against a synthetic peptide that corresponded to the 19 residues at the N terminus of Tet(K). The signals were quantified by using ImageQuant software (Molecular Dynamics).
Transport assays. (i) Assays of Tet-dependent downhill TC-Co2+ efflux from RSO vesicles in exchange for K+ (mode 3).
For assays of net 86Rb+-K+ uptake driven by an outwardly directed gradient of TC-Co2+, RSO vesicles from E. coli TK2420 transformants were passively loaded at 4 C for 15 h with 5 mM TC and 5 mM CoCl2 or with 5 mM choline Cl (control) in Tris buffer at pH 7.5. Uptake was initiated by diluting 10 μl of these vesicles into 1 ml of 10 mM Tris-HCl, at the pH values indicated, containing 100 μM 86Rb+-KCl. For assays of [3H]TC efflux, at elevated pH in the presence or absence of K+, the same preparations of RSO vesicles were passively loaded at pH 7.5 with 100 μM [3H]TC and 200 μM CoCl2. Efflux was initiated by diluting 10 μl of these vesicles into 10 mM Tris-HCl at pH 8.3 in the presence or absence of 1 mM KCl. The binding controls for the RSO vesicle assays of 86Rb+ uptake were reactions that were carried out in the presence of 2% (wt/vol) toluene. Controls assessing active transport of either TC or Co2+ alone, in the absence of the other, are not shown, but were consistently negative.
(ii) TC- and energy (d-lactate)-dependent uptake of cytoplasmic efflux substrates in everted membrane vesicles (modes 1 and 2).
Energy-dependent [3H]TC, 22Na+, and 86Rb+-K+ transport as efflux substrates were assayed in everted vesicles of E. coli DH5α, E. coli NM81, and E. coli TK2420 transformants, respectively. The substrate concentrations were varied for kinetic experiments, as indicated. For characterization of mutant transporters, the efflux substrate concentrations used were: for TC transport, 25 μM [3H]TC plus 100 μM CoCl2; for Na+ transport, 10 mM 22Na+; and for K+ transport, 10 mM 86Rb+-K+. In all experiments in which mixtures of TC and Co2+ were used, the substrate is designated TC-Co2+ to represent the antibiotic-divalent cation complex. Similarly, in experiments in which mixtures of 86Rb and K+ were added as either the efflux substrate or the coupling ion (see below), the designation 86Rb+-K+ (or −KCl) is used to designate the mix used in the assay. The BTP buffer was at pH 7.5, and the electron donor was 2.5 mM Tris-d-lactate. Controls were reactions conducted without d-lactate addition and one in which both d-lactate and the uncoupler carbonyl cyanide-m-chlorophenylhydrazone (CCCP) (to a final concentration of 10 μM) were added. Substrate binding under the latter condition was subtracted from the values obtained in the presence of d-lactate.
(iii) Assays of TC- and energy (d-lactate)-dependent net uptake of 86Rb+, as an assay of the net K+ uptake mode when K+ is serving as the coupling ion (mode 3).
Assays of net 86Rb+-K+ uptake were performed as described previously (18); RSO vesicles were preloaded with either choline (control) or K+ at 100 μM and diluted into buffer containing the same 86Rb+-KCl concentration. Uptake of 86Rb+, dependent upon the electron donor (10 mM Tris-d-lactate), was measured. The choline control is particularly important for this assay, because it distinguishes a K+ leak through the transporter from net K+ uptake that is an antiporter function. A leak would be stimulated by d-lactate-dependent establishment of a transmembrane potential, but would be observed in vesicles that were loaded with choline instead of one of the efflux substrates of Tet(L) and Tet(K). K+ uptake as part of antiport would depend upon a trans efflux substrate. Such dependence was observed with every Tet protein used in this study. For all assays of transport, samples were taken at various time points, filtered on HAWP 02500 filters and GSWP 02500 filters (Millipore) for RSO and everted vesicle experiments, respectively. The filters were washed with reaction buffer and then dried; the radioactivity was counted by liquid scintillation spectrometry. All experiments shown in the figures represent the means of more than two experiments, with duplicate samples, with each of two independent vesicle preparations.
Na+ exclusion and K+ complementation assays in B. subtilis transformants.
Na+ exclusion experiments were conducted with cells of B. subtilis AG112 [Δtet(L)] harboring pBK15 or recombinant pBK15 expressing wild-type tet(L) or tet(K) or the A157T mutant tet(L). The cells were grown for 15 h in TTM or TKM medium at pH 8.3 in the presence of 100 mM NaCl labeled with 22Na+ (0.01 μCi/ml). The concentration of cytoplasmic Na+ was determined, as previously described (4), in a variation of the method of Harel-Bronstein et al. (20). Samples (5 ml of culture) were vacuum filtered onto 25-mm-diameter discs of Whatman GFF paper, washed with 10 ml of 100 mM Tris (pH 8.3) buffer, dried, and counted by liquid scintillation spectrometry. Parallel binding controls were conducted on cell samples in the presence of 10 μM gramicidin plus 2% butanol. After subtraction of the binding control, the cytoplasmic 22Na+ concentration per mg of cell protein was calculated. The molar concentration of Na+ was calculated with a cell water volume of 5 μl/mg of protein. The amounts of cell protein in these experiments and of vesicle protein in the experiments described above were determined by the method of Lowry et al. (33).
Complementation experiments were conducted with the same transformants to ascertain the comparative capacities of wild-type Tet(K) and the A157T mutant of Tet(L) to the previously determined ability of Tet(L) to complement the K+ acquisition deficit of the tet(L) deletion strain (48). Cells were grown on TTM medium prepared with a final concentration of 0.5 mM added K+. After 8 h, the A600 was read as described elsewhere (48).
RESULTS
Demonstration of TC-Co2+/86Rb+-K+ antiport, expanding mode 3 to all three efflux substrates.
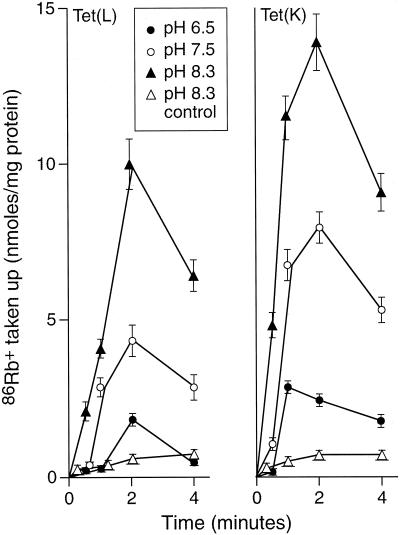
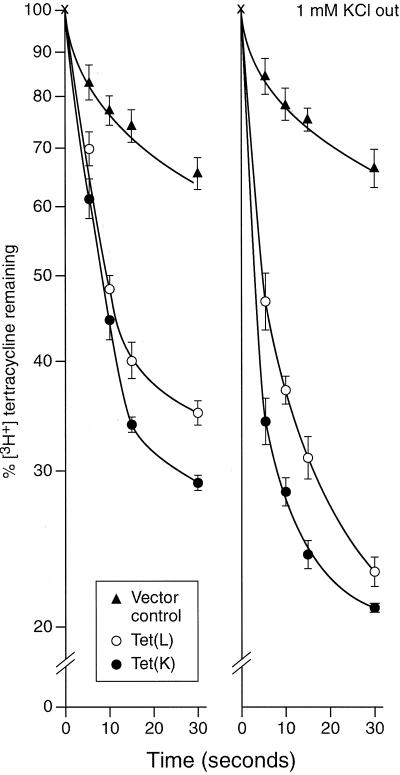
Assays were conducted to test whether K+ can serve as a coupling ion when TC-Co2+ is the efflux substrate. As shown in Fig. 2, an outwardly-directed TC-Co2+ gradient in preloaded RSO vesicles energized 86Rb+ accumulation in a highly pH-dependent fashion, with greater 86Rb+ uptake as the external pH increased. This uptake was more robust in Tet(K) vesicles than in Tet(L) vesicles. We then examined directly whether, at high pH, the presence of K+ as a coupling ion in the extravesicular buffer enhanced the capacity for TC exclusion in comparable preparations. The concentration of [3H]TC used for preloading the efflux substrate into the cytoplasmic side of the RSO vesicles (the intravesicular space) was reduced relative to the previous experiment in order to achieve a sufficiently high specific radioactivity, and K+ rather than a 86Rb+-K+ mix was used on the external (coupling ion) side in the +K+ reactions. As shown in Fig. 3, the rate of efflux from Tet(L)- or Tet(K)-containing vesicles was significantly higher than that in control vesicles. A somewhat faster rate for Tet(K) relative to Tet(L) was observed in the absence of added K+ (Fig. 3, left panel) even at the lower [TC] used in this experiment relative to that depicted in Fig. 2. For example, at 5 s, 39% efflux of [3H]TC had occurred from Tet(K) vesicles compared to 30% from Tet(L) vesicles (Fig. 3, left). The presence of K+ on the extravesicular side was accompanied by an increase in [3H]TC efflux (Fig. 3, right panel). The ratio of the percent [3H]TC efflux at 5 s in the +K+ versus −K+ condition was similar, at 1.8 and 1.7 for Tet(K) and Tet(L) vesicles, respectively. Thus, TC-Co2+-dependent K+ flux (Fig. 2) and K+-dependent increases in TC-Co2+ flux (Fig. 3) were both demonstrable.
FIG. 2.
Capacity of Tet(L) and Tet(K) to support 86Rb+ uptake by RSO vesicles of E. coli TK2420 upon generation of an outwardly directed gradient of TC-Co2+. The assay, conducted as described in Materials and Methods, was initiated by dilution of RSO vesicles that were preloaded with TC-Co2+ at pH 7.5 into buffers at the pH values indicated. Control experiments were conducted with vesicles that were incubated at pH 7.5, but not loaded with TC-Co2+ prior to dilution. These controls did not exhibit accumulation observed in the TC-Co2+-preloaded vesicles, as is shown for the control from the pH 8.3 experiment (▵). Toluene-treated vesicles were used as the binding control for this experiment.
FIG. 3.
Effect of K+ on the rate of Tet(L)- or Tet(K)-mediated [3H]TC efflux from RSO vesicles of E. coli TK2420. The experimental preparations were similar to those used in the experiments depicted in Fig. 2, except that the extravesicular pH was 8.3, the intravesicular TC was tritiated and at a somewhat lower concentration, and, when present at 1 mM in the outside buffer (right panel), nonradioactive KCl alone rather than an 86Rb+-KCl mix was added as a coupling ion.
Assays of kinetic properties of the (TC-Me2+)(Na+)(K+)/H+ antiports (modes 1 and 2) of wild-type Tet(L) and Tet(K).
Assays of cross-inhibition among cytoplasmic efflux substrates were conducted with BTP-buffered assay mixes, as described in Materials and Methods, in view of the possibility that the K+-replete buffers that are routinely used for assays of Tet proteins might have been a complicating factor in earlier experiments (17). The high [K+] might also have affected the Km determinations for TC as an efflux substrate in earlier studies by this and other laboratories. Km values for the three cytoplasmic efflux substrates of Tet(L) and Tet(K) were assayed in everted vesicles that were prepared in BTP buffer at pH 7.5. Since there was no added K+ in the intravesicular buffer, active uptake of the cytoplasmic efflux substrates by the everted membrane preparations was completely dependent upon the electrochemical proton gradient (acid and positive inside the everted vesicles) developed upon d-lactate addition, with intravesicular H+ serving as the coupling ions. The Km for TC was measured over a concentration range of 2.5 to 25 μM, with the Co2+ concentration maintained at 100 μM. As shown in Table 1, the Km for TC measured for Tet(L) was 7.5 μM, and that for Tet(K) was 14 μM. Both of those Km values were lower than values reported earlier in studies with buffers with high added [K+]. The earlier Km reported for Tet(L) was 14 μM (17), and that for Tet(K) was 40 μM (50). It had been inferred from Na+ exclusion experiments in whole cells that Tet(L) had a higher preference for Na+ versus K+ as a cytoplasmic efflux substrate than did Tet(K) (4). Km values were determined for Na+ with a concentration range of 5 to 100 mM for the monovalent cation and for K+ with a concentration range of 2 to 150 mM. The Km values determined for Na+ versus K+ supported the inference about relative preference. The Km for Na+ was somewhat higher for Tet(K), and that for K+ was substantially higher for Tet(L) (Table 1). As found earlier, the concentration range for optimal Na+ and K+ transport was much higher than that for the antibiotic-metal complex (17).
TABLE 1.
Kinetic parameters for Tet(L) and Tet(K)-mediated fluxes of 22Na+, 36Rb+-K+, and [3H]TC as cytoplasmic efflux substrates
| Protein |
Km of substratea:
|
||
|---|---|---|---|
| [3H]TC (μM) | 22Na+ (mM) | 36Rb+-K+ (mM) | |
| Tet(L) | 7.5 ± 2.5 | 20 ± 3 | 56 ± 4 |
| Tet(K) | 14 ± 3 | 33 ± 2 | 15 ± 2 |
The values are the averages of at least six separate determinations ± standard deviation.
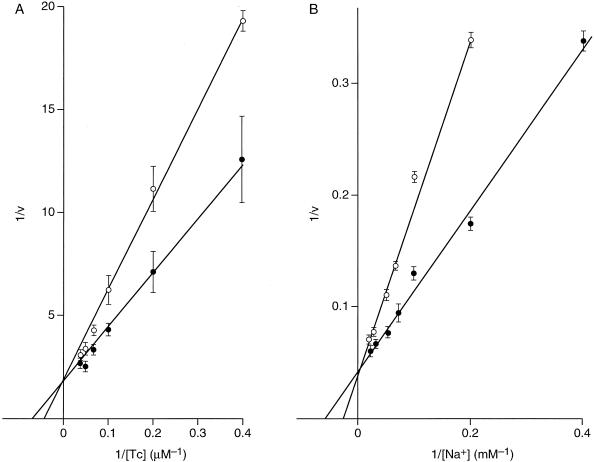
The transport of [3H]TC by everted vesicles containing either Tet(L) or Tet(K) was inhibited by added K+ at concentrations of 5 and 10 mM (data not shown). The signal/noise ratio in assays of Tet(L) made it difficult to reliably discern the pattern of inhibition, but results could be recorded for Tet(K). As shown in the double reciprocal plots in Fig. 4, for a single concentration of inhibitor, the effects of K+ on both [3H]TC-Co2+ (Fig. 4A) and 22Na+ (Fig. 4B) transport exhibited a competitive pattern.
FIG. 4.
Double reciprocal plots of experiments showing the effect of added K+ on TC and Na+ uptake by everted vesicles of E. coli Tet(K) vesicles. K+, Na+, and TC-Co2+ were serving as efflux substrates on the “cytoplasmic” side (outside of the everted system) of the vesicles. There was no intravesicular K+. Uptake of [3H]TC (A) or 22Na+ (B) was assayed in everted vesicles of transformants of E. coli DH5α and E. coli NM81, respectively, expressing tet(K). Assays were carried out as described in Materials and Methods, in the presence (○) or absence (•) of added extravesicular K+ at 5 mM (A) or 10 mM (B). Reciprocal plots of the data were plotted by using time points in the linear range (up to 1 min) after correction by subtraction of values for transport in the presence of CCCP. The results shown are the average of at least five separate determinations, and the error bars represent the standard deviation.
Growth phenotypes and Western analyses of motif C mutants in Tet(L).
Mutagenesis studies were focused on position A157 in Tet(L), the counterpart of which is S157 in Tet(K), because it appeared to be a strong candidate for a role in the better efficacy of Tet(K) as a K+ transporter. It is the next residue after the conserved and functionally important GP dipeptide at positions 155 to 156 in the antiporter motif C (47). Also, there are precedents for serine and threonine involvement in cation binding (1, 23, 42). In this study, the mutations shown in Fig. 1 were introduced into the 157 position of Tet(L) and Tet(K) by site-directed mutagenesis as described in Materials and Methods. As shown in Table 2, none of the site-directed mutations made in the A157 position of Tet(L) reduced the amount of protein incorporated into transformant membranes by 50% or more, although the amount of the A157T Tet(L) was significantly reduced relative to all the other mutants and the wild-type Tet(L). All of the mutants retained the ability to confer Tcr and to enhance K+ acquisition in a phenotype screen. The apparent differences among the transformants in that screen (Table 2) were principally that the three hydroxyamino acid substitutions led to a reduced MIC of TC: i.e., an increase in Tcs. The same substitutions as well as the cysteine substitution increased the efficacy of K+ acquisition: i.e., reduced the [KCl] required to support growth of E. coli TK2420.
TABLE 2.
Membrane incorporation and phenotypes conferred by Tet mutant proteins
| Protein | % Membrane assemblya | MIC of TC (μg/ml)b | [KCl] (mM)c |
|---|---|---|---|
| Vector | 2 | 21 | |
| Wild-type Tet(L) | 100 | 32 | 12 |
| Tet(L) A157S | 102 | 14 | 7 |
| Tet(L) A157T | 56 | 17 | 5 |
| Tet(L) A157C | 104 | 32 | 6 |
| Tet(L) A157Y | 107 | 10 | 3 |
| Tet(L) A157L | 90 | 28 | 16 |
| Wild-type Tet(K) | 12 | 7 | |
| Tet(K) S157A | 10 | 9 |
Membrane assembly was determined by Western analysis, shown as a relative percentage of the wild-type protein. Note that Western analyses for Tet(K) and Tet(K) S157A mutant [done with an antibody raised against the N-terminal peptide of Tet(K)] showed that they had similar levels of protein incorporation into the membrane, although incorporation was not comparable to that of the Tet(L) membrane assembly.
Minimal TC concentration at which there is no growth of E. coli DH5α after 15 h of incubation at 37°C.
Minimal concentration of KCl permitting the growth of E. coli TK2420 to 1.0 at A600 after 15 h of incubation at 37°C.
Assays of 86Rb+-K+ fluxes in vesicles from E. coli strains expressing mutant Tet(L) proteins.
Transport assays were undertaken to develop more quantitative information under assay conditions in which the ion compositions of the buffers were more controlled than in the different growth media, not all of which were defined. Also, since phenotypic data could not be reproducibly obtained from a phenotypic screen (25), transport experiments were needed to assess the Na+ translocation capacities of at least selected mutants. The assay measured energy (d-lactate)-dependent accumulation of 86Rb+-K+ into RSO vesicles that was dependent upon the presence of an efflux substrate inside the vesicle. K+ was used as the efflux substrate and was present at the same concentration as the 86Rb+-K+ outside. Choline was used as the nonefflux substrate control that was loaded into one set of vesicles. The choline control provides the means of assessing the dependence of energized 86Rb+ uptake on the presence of intravesicular (trans) efflux substrate.
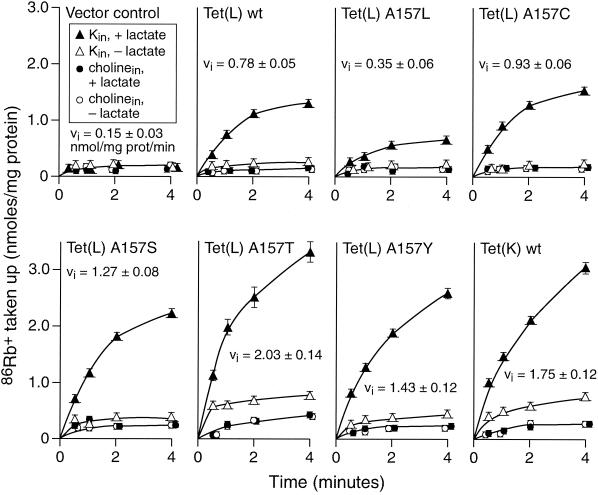
Consistent with the phenotype screen, all of the Tet(L) mutants with hydroxyamino acid substitutions at position 157 exhibited more Tet(K)-like rates in this assay of net 86Rb+-K+ uptake; the cysteine substitution had a qualitatively similar, but smaller effect (Fig. 5). The values shown are adjusted for differences in Tet protein in the membrane; preliminary determinations were used to ensure that the assays met the condition of linearity with protein concentration over the range of values involved in the correction. The results supported the hypothesis that a hydroxyamino acid at the 157 position was an important determinant of the greater preference of Tet(K), relative to Tet(L), for K+ as coupling ion. The results further indicated that the effect was permissive with respect to the size of the amino acid side chain and could be partially supported by cysteine at the same position. To see if the K+ preference on the cytoplasmic side, as an efflux substrate, was similarly affected by the same kind of mutation, the Km for 86Rb+-K+ of the A157T mutant of Tet(L) was determined in the everted vesicle protocol that had been used for the wild-type Tet proteins; this was again an assay of 86Rb+-K+/H+ antiport (mode 2), since the vesicles did not contain K+ to serve as a coupling ion in the everted assay system. Tet(L) A157T exhibited a Km of 22 ± 3 mM, far closer to the Km for Tet(K) than for Tet(L) (Table 1). In the RSO vesicle assay, the A157L mutant exhibited very little 86Rb+-K+ uptake (Fig. 5): i.e., an even greater deficit relative to wild-type Tet(L) than suggested by the phenotype screen (Table 2).
FIG. 5.
Energy and intravesicular cation dependence of 86Rb+ uptake, as a coupling ion, by RSO vesicles of E. coli TK2420 transformed with various tet plasmids. Vesicles were passively loaded with either 100 μM choline-Cl (control, nonefflux substrate) or KCl (efflux substrate). Uptake was initiated by diluting 25 μl of vesicles into 500 μl of 10 mM Tris-HCl (pH 7.5), containing a final concentration of 100 μM 86Rb+-KCl. To half of the reaction mixtures, 10 mM Tris-d-lactate was added to energize those vesicles. Samples were taken at the times indicated and treated as described in Materials and Methods. The initial velocities, vi, in these determinations are shown for the energized vesicles that contained intravesicular K+ (efflux substrate) and are the average of at least four separate determinations. The error bars show the standard deviation of the values. The subscript “in” denotes intravesicular location.
[3H]TC and 22Na+ efflux and net 86Rb+-K+ uptake assayed in vesicles of E. coli strains expressing Tet(K) S157A versus Tet(L) A157S.
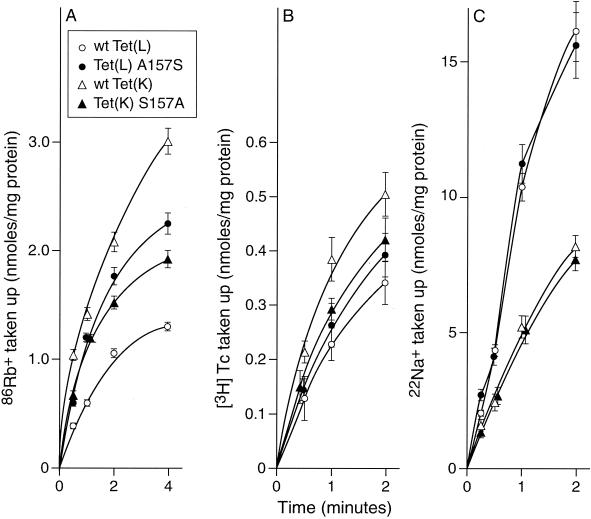
The mutation of A157 in Tet(L) to a hydroxyamino acid produced a transporter with a more Tet(K)-like substrate profile for K+ both as a coupling ion and as a cytoplasmic efflux substrate. This suggests that this residue is an important element in the differences in substrate preference between Tet(L) and Tet(K) with respect to Na+ versus K+ efflux and with respect to K+ as a coupling ion. We sought to examine whether the converse mutant, an S157A mutant of Tet(K), would support this conclusion. Western analyses showed that the mutant Tet(K) was incorporated into E. coli membranes at levels that were indistinguishable from that of wild-type Tet(K) and that the S157A mutant conveyed a pattern of phenotypes in the standard screen that was similar to that of the wild-type Tet(K) (Table 2). Since Tet(L) has been purified (5), but Tet(K) has not, the Western analyses using different antibodies to assess membrane incorporation of the two proteins could not be quantified relative to each other; however, comparisons were made with identical constructs and conditions for Tet(L) and Tet(K) wild-type and mutant strains. In transport assays of the net K+ uptake mode (Fig. 6A), the S157A mutant of Tet(K) clearly exhibited more Tet(L)-like behavior, showing activity that was in between the profiles for the two different wild-type Tet proteins and the initial rate of which was comparable to that of the A157S mutant of Tet(L). It was also of interest to determine the effect of the A157S mutation in Tet(L) and S157A mutation in Tet(K) on their patterns of transport of efflux substrates. Uptake of [3H]TC-Co2+ and 22Na+ were examined in everted vesicles in the absence of added K+ on either side of the membranes. As with uptake of K+ as coupling ion (Fig. 6A), the rate of TC-Co2+ uptake observed for the S157A mutant of Tet(K) was more Tet(L)-like than before and was similar to the A157S profile (Fig. 6B). With respect to [3H]TC transport, however, the shift toward the other transporter pattern was less pronounced. The transport phenotype of each single mutant still resembled its own parent more than the other Tet protein. In contrast to these effects of the reciprocal single mutations in the 157 position of Tet(L) and Tet(K) on the TC efflux (mode 1) and net K+ uptake (mode 3) modes, there was no effect on 22Na+ transport (mode 2) (Fig. 6C). The TC-Co2+ and Na+ transport capacities of the other Tet(L) A157T mutant were also examined and were found to be indistinguishable from those of the A157S mutant of Tet(L) (25).
FIG. 6.
Transport activities of the Tet(K) S157A mutant compared with those of wild-type Tet(L) and Tet(K) and the A157S mutant of Tet(L). (A) 86Rb+-K+ uptake (as coupling ion) by right-side-out vesicles was assayed as described in the legend to Fig. 5 in the presence of intravesicular K+ (as efflux substrate) and added d-lactate as energy source. The data shown here were corrected for binding. (B) [3H]TC uptake (as efflux substrate) in exchange for H+ was assayed as described in Materials and Methods, and the values shown are corrected for the TC bound by the vesicles of each type in CCCP-treated preparations. (C) 22Na+ uptake (as efflux substrate) in exchange for H+ was assayed in a BTP buffer-based reaction mixture as described in Materials and Methods, and the data were corrected for the binding in a CCCP-treated control for each construct. Since the bacterial mutant used for these assays has residual Na+/H+ antiporter activity, the activity for the vector control was also subtracted. Although not shown, other control assays of the same types shown for earlier assays were conducted and yielded values similar to those in the earlier assays.
22Na+ exclusion assay and complementation of the K+ uptake defect in whole cells of a tet(L) deletion strain of B. subtilis.
All of the experiments on the mutants had thus far been carried out in E. coli cells and membranes, a heterologous system. Would a change of A157 of Tet(L) to a hydroxyamino acid have similar effects on the K+-related phenotypes conferred by Tet(L) in B. subtilis, the natural biological setting for Tet(L)? First, exploration of K+ preference as a cytoplasmic substrate was probed with the same 22Na+ exclusion assay that was employed in earlier studies (4). In this assay, the relative K+ versus Na+ preference is assessed by the magnitude of the inhibitory effect of K+ on the capacity of transformants of B. subtilis AG112 [Δtet(L)] to exclude 22Na+. The Tet proteins used were the wild-type Tet(L) and Tet(K) proteins and the A157T mutant of Tet(L). The Tet(L) A157T mutant had exhibited a Tet(K)-like capacity for use of K+ as coupling ion (Fig. 5) and a Tet(K)-like Km for K+ as an efflux substrate in E. coli vesicles (Table 1). As shown in Table 3, the A157T mutant Tet(L) behaved just like Tet(K) in the whole-cell exclusion assay. Wild-type Tet(L) supported the capacity of a tet(L) deletion strain of B. subtilis AG112 to maintain a cytoplasmic Na+ level of about 26 mM during growth in the presence of 100 mM Na+, while the Na+ concentration in a control transformant was about 85 mM; it made no significant difference whether the K+ concentration in the medium was 1 mM or 100 mM. Both wild-type Tet(K) and the A157T mutant of Tet(L) enabled B. subtilis AG112 to exclude Na+ almost as well as wild-type Tet(L) when the K+ concentration was 1 mM. Perhaps even this low [K+] inhibited 22Na+ efflux by Tet(K) somewhat, resulting in the modestly higher intracellular [22Na+] relative to that maintained by Tet(L). Most impressive, however, was the finding that when the K+ concentration was 100 mM, the 22Na+ concentration in the cells containing wild-type Tet(K) or the A157T mutant of Tet(L) was about three times higher than that maintained by wild-type Tet(L).
TABLE 3.
Na+ exclusion by the A157T mutant of Tet(L) compared to that of wild-type Tet(L) and Tet(K)
| Transformant of B. subtilis AG112 | Cytoplasmic Na+ (mM)a
|
|
|---|---|---|
| 1 mM K+ | 100 mM K+ | |
| Vector control | 84.8 ± 2.3 | 82.7 ± 9.0 |
| tet(L) | 25.5 ± 9.6 | 25.6 ± 8.6 |
| tet(K) | 39.5 ± 3.9 | 77.5 ± 10.4 |
| tet(L) A157T | 34.7 ± 14.4 | 77.0 ± 7.0 |
Cells were grown for 15 h in medium containing 1 or 100 mM K+ supplemented with 100 mM 22NaCl. The values for cytoplasmic Na+ are the average of at least six separate determinations ± standard deviations.
A growth experiment was used to assess whether A157T Tet(L) also exhibited a Tet(K)-like profile with respect to K+ use as a coupling ion in the natural B. subtilis setting. This question was approached by testing whether Tet(K) promoted net K+ uptake better than wild-type Tet(L) in whole cells of B. subtilis AG112. This tet(L) deletion strain of B. subtilis had been found to grow less well than its wild-type parent on media containing low K+ concentrations, a deficit that was complemented by a plasmid expressing wild-type tet(L) (48). When tranformants of B. subtilis AG112 were grown on TTM medium containing 0.5 mM added K+, the following growth (as A600) data were collected at 8 h: 0.57 ± 0.01 for the control transformant, 0.67 ± 0.01 for the transformant with Tet(L) restored, 0.71 ± 0.1 for the transformant with Tet(K) restored, and 0.78 ± 0.01 for the transformant with Tet(L) A157T restored. Thus, the mutant Tet(L) behaved in the natural host in a manner that was comparable to that observed in E. coli with respect to its capacity to confer a capacity for net K+ uptake that was more comparable to Tet(K) than to Tet(L).
DISCUSSION
The present experiments demonstrate, for the first time, that K+ can serve as a coupling ion when TC-Co2+ is the efflux substrate for Tet(L)- and Tet(K)-mediated antiport. This extends mode 3, coupling to K+, to each of the three cytoplasmic efflux substrates. Several features of the TC-Co2+/K+ antiport were notable in the experiments in which an outwardly directed TC-Co2+ gradient supported Tet-dependent accumulation of 86Rb+ (Fig. 2). First, Tet(K) showed much more robust Rb+ uptake than Tet(L). This was consistent with earlier experiments in E. coli vesicles with monovalent cation efflux substrates (18). It was also consistent with the relative profiles of these two transporters with respect to net K+ uptake in the natural bacterial host (4). Second, as the pH was raised, Rb+ accumulation increased. This suggests that the protons and K+ ions are alternative coupling ions that exhibit competitive behavior, as has also been shown for the spore germination-associated Na+/H+-K+ antiport activity of Bacillus cereus GerN (45) and the Zn2+/H+-K+ antiport activity of B. subtilis CzcD (19). Tet-dependent TC-Co2+ or Na+ or K+ antiport in exchange with H+ and K+ is electrogenic, with more than one coupling ion taken up for each singly charged efflux substrate molecule that is transported outward (5, 18). The current studies do not show whether K+ can replace the full coupling ion complement or whether at least one proton remains involved even when K+ is also serving as a coupling ion. In either case, the utilization of K+ as a coupling ion is accompanied by an enhancement of TC-Co2+ efflux at high pH (Fig. 3). This suggests that the TC-Co2+/K+ antiport capacity enhances antibiotic resistance under conditions in which the proton motive force is reduced. Use of Na+ as a coupling ion is more typically associated with bypassing a low proton motive force (2, 37, 38). For antiporters that have toxic cations as their efflux substrates, use and uptake of K+ as a coupling ion may be an adjunct to the detoxification achieved by the efflux itself, since adequate or elevated cytoplasmic K+ levels are protective against the toxic cations (19, 37). Additionally, the expression of housekeeping antiporters that use K+ as a coupling ion at elevated pH may be a physiologically important part of the K+ acquisition strategies of cells (48). We have noticed during our use of the triple-K+-uptake mutant E. coli TK2420 that this strain grows better on media containing low [K+] as the pH is raised. The well-mined E. coli genome may contain additional dedicated K+ uptake systems that are yet to be discovered and that are activated at elevated pH. It is also possible that housekeeping antiporters that couple to K+ play a role in this alkali-stimulated K+ acquisition in E. coli like Tet(L) does in B. subtilis (48).
The capacity of Tet(L) and Tet(K) for coupling antiport to K+ and the differences in K+ preference between the two closely related transporters have enabled us to make progress towards our long-term goals of clarifying structure-function and mechanistic properties of these important transport proteins. Among the questions of importance with respect to multifunctional major facilitator superfamily antiporters (34), such as Tet(L) and Tet(K) are the following. Do the different substrates and different coupling ions use common or distinct binding sites and translocation pathways? If common binding sites, translocation pathways, or both are used, does every substrate use all of the same features of the site or pathway to the same extent, or is there variability among substrates? And is a single translocation pathway used for the efflux substrates and coupling ions in an alternating, ping-pong type of mechanism, as is found for many antiporters (8, 16, 21, 29, 40, 44), or are the efflux substrates and coupling ions translocated simultaneously through distinct translocation pathways, as in some other antiporters (6, 40)? Complete resolution of these major questions will require high-resolution structural data in addition to more detailed studies of binding and transport kinetics and more extensive mutagenesis studies in combination with approaches to provide additional structural information. However, the kinetic work and mutational work in this and other recent studies of Tet(L) and Tet(K) (11, 13, 25-27), taken together, do provide information that is relevant to these important issues.
With respect to common or distinct binding sites, the inverse relationship between the proton concentration and utilization of K+ as a coupling ion suggests that these coupling ions compete for a common binding site. Similarly, the competitive inhibition between K+ and each of the other cytoplasmic efflux substrates suggests that a common binding site is used for all three efflux substrates. Cross-inhibition between Na+ and K+ had been observed earlier (5). The competitive relationship between K+ and TC-Co2+, which was not observed earlier, could now be shown in transport assays of Tet(K) by using BTP buffer (Fig. 4A). Competition between K+ and TC-Co2+ as efflux substrates is also indicated by the phenotypes of the site-directed mutants of Tet(L). Those mutants in the A157 position of Tet(L) that had significantly enhanced capacity to complement the K+ uptake defect of E. coli TK2420 relative to wild-type Tet(L) (Table 2 and Fig. 5), also conferred lower Tcr, i.e., a lower MIC of TC, than wild type Tet(L) in the K+-replete LBK medium used for the MIC determinations (Table 2). This is consistent with competition between K+ and TC-Co2+ on the cytoplasmic side. Dosch et al. (7) observed a consonant effect in cells of a K+ uptake mutant of E. coli expressing tetC from pBR322. In the absence of added K+, the MIC of TC was lower than in its presence. These authors suggested that both the effect of K+ on the MIC of TC and the complementation of the E. coli mutant's K+ uptake phenotype by tetC expression reflected a transport capacity of TetC for K+. This is particularly interesting because it raises the likelihood that the K+ transport capacities exhibited by 14-transmembrane segment (TMS) Tet(L) and Tet(K) are also exhibited by at least one major 12-TMS Tet protein. On the other hand, no evidence exists currently for the same capacity in the related and most extensively studied Tet efflux protein, 12-TMS Tet(B) (46). We should note, though, that in our studies of the reciprocal mutant, the MIC of Tet(K) S157A was not higher than that of the wild-type Tet(K): i.e., was more Tet(L) like than the wild-type Tet(K) (Table 2). Such a finding would have been most consistent with the other findings and interpretations just discussed. Thus, some caution in interpretations based on MIC determinations is warranted. The complexities of evaluating Tet transporter function via an extended growth period may sometimes yield less perfect reflections of actual transporter function than assays of the early time points of transport.
Although a common binding site for efflux substrate binding is suggested for Tet(L) and Tet(K), it is most likely that different substrates depend more than others on specific properties of the site. Recent mutagenesis studies of Tet(L) included several mutations in motif A, a conserved motif in the cytoplasmic loop region between TMS II and III that has been implicated as having a role in TC-Co2+ binding in Tet(B) proteins (41, 49, 51). The two different mutations of Tet(L), G70R and D74C, that abolished efflux of TC-Co2+ also diminished Na+ efflux, but the effect on the monovalent cation substrate was much more modest than the effect on the more complex antibiotic-divalent metal substrate (27). Other residues have been identified that are required for either TC-Co2+ binding or translocation, but exhibit no apparent importance for either Na+ efflux or K+ uptake as a coupling ion (e.g., D200), which is near the cytoplasmic surface of Tet(L) (26).
It has been suggested that the 14-TMS Tet(L) and Tet(K) proteins and the 12-TMS Tet(B) protein have a three-dimensionally similar “catalytic core” that includes three carboxylates in distinct TMS regions (11). Recent mutagenesis studies of Tet(L) supported the notion of such a common set of crucial acidic residues (27). Mutational studies of these residues further indicated that they all had a crucial role in efflux of monovalent cation substrates of Tet(L) and net uptake of K+ as a coupling ion in addition to TC-Co2+ efflux (27). The participation of a core set of residues in transport of multiple efflux substrates and coupling ions is consistent with a ping-pong mechanism of antiport in which a single translocation pathway is used for both the efflux substrates and coupling ions. The finding that mutations at the A157 position of Tet(L) have parallel effects upon K+ preference as both an efflux substrate and coupling ion is further support for this type of model. In both E. coli cells and vesicles and in B. subtilis cells, assays that probed K+ efflux or K+ uptake all indicated that a hydroxyamino at position 157 enhanced the preference of Tet(L) and Tet(K) for K+. Even if future detailed kinetic studies support a ping-pong model, however, we expect that different features of the common translocation pathway are particularly important for proper positioning and movement of specific substrates of the transporter. This might be the basis for observations made by others on the apparent association of particular Tet(K) functions with regions of the molecule (15). In the present study, the chemistry of the residue at position 157 had a strong effect on the preference of Tet(L) for K+, but it had a significantly more modest effect on TC-Co2+ utilization as an efflux substrate and no effect on Na+ transport (Fig. 6). Thus far, no mutations that affect only Na+ efflux have been found in Tet(L). Possibly, the important contact points of this efflux substrate are close to the minimal set that is shared among all the substrates (and perhaps coupling ions) of Tet(L).
Acknowledgments
This work was supported by research grant GM52837 from the National Institute of General Medical Sciences.
REFERENCES
- 1.Blostein, R., A. Wilczynska, S. J. D. Karlish, J. M. Arguello, and J. B. Lingrel. 1999. Evidence that Ser775 in the alpha subunit of the Na,K-ATPase is a residue in the cation binding pocket. J. Biol. Chem. 272:24987-24993. [DOI] [PubMed] [Google Scholar]
- 2.Chen, J., Y. Morita, M. N. Huda, T. Kuroda, T. Mizushima, and T. Tsuchiya. 2002. VmrA, a member of a novel class of Na+-coupled multidrug efflux pumps from Vibrio parahaemolyticus. J. Bacteriol. 184:572-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng, J., A. A. Guffanti, and T. A. Krulwich. 1994. The chromosomal tetracycline resistance locus of Bacillus subtilis encodes a Na+/H+ antiporter that is physiologically important at elevated pH. J. Biol. Chem. 269:27365-27371. [PubMed] [Google Scholar]
- 4.Cheng, J., A. A. Guffanti, W. Wang, T. A. Krulwich, and D. H. Bechhofer. 1996. Chromosomal tetA(L) gene of Bacillus subtilis: regulation of expression and physiology of a tetA(L) deletion strain. J. Bacteriol. 178:2853-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng, J., D. B. Hicks, and T. A. Krulwich. 1996. The purified Bacillus subtilis tetracycline efflux protein TetA(L) reconstitutes both tetracycline-cobalt/H+ and Na+/H+ exchange. Proc. Natl. Acad. Sci. USA 93:14446-14451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dierks, T., E. Riemer, and R. Kramer. 1988. Reaction mechanism of the reconstituted aspartate/glutamate carrier from bovine heart mitochondria. Biochim. Biophys. Acta 943:231-244. [DOI] [PubMed] [Google Scholar]
- 7.Dosch, D. C., F. F. Salvacion, and W. Epstein. 1984. Tetracycline resistance element of pBR322 mediates potassium transport. J. Bacteriol. 160:1188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driessen, A. J. M., D. Molenaar, and W. N. Konings. 1989. Kinetic mechanism and specificity of the arginine-ornithine antiporter of Lactococcus lactis. J. Biol. Chem. 264:10361-10370. [PubMed] [Google Scholar]
- 9.Epstein, W., and B. S. Kim. 1971. Potassium transport loci in Escherichia coli K-12. J. Bacteriol. 108:639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein, W., E. Buurman, D. McLaggan, and J. Naprstek. 1993. Multiple mechanisms, roles and controls of K+ in Escherichia coli. Biochem. Soc. Trans. 21:1006-1010. [DOI] [PubMed] [Google Scholar]
- 11.Fujihira, E., T. Kimura, Y. Shiina, and A. Yamaguchi. 1996. Transmembrane glutamic acid residues play essential roles in the metal-tetracycline/H+ antiporter of Staphylococcus aureus. FEBS Lett. 391:243-246. [DOI] [PubMed] [Google Scholar]
- 12.Ginn, S. L., M. H. Brown, and R. A. Skurray. 1997. Membrane topology of the metal-tetracycline/H+ antiporter TetA(K) from Staphylococcus aureus. J. Bacteriol. 179:3786-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginn, S. L., M. H. Brown, and R. A. Skurray. 2000. The TetA(K) tetracycline/H+ antiporter from Staphylococcus aureus: mutagenesis and functional analysis of motif C. J. Bacteriol. 182:1492-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg, E. B., T. Arbel, J. Chen, R. Karpel, G. A. Mackie, S. Schuldiner, and E. Padan. 1987. Characterization of a Na+/H+ antiporter gene of Escherichia coli. Proc. Natl. Acad. Sci. USA 84:2615-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffith, J. K., D. H. Cuellar, C. A. Fordyce, K. C. Hutchings, and A. A. Mondragon. 1994. Structure and function of the class C tetracycline/H+ antiporter: three independent groups of phenotypes are conferred by TetA (C). Mol. Membr. Biol. 11:271-277. [DOI] [PubMed] [Google Scholar]
- 16.Gropp, T., N. Brustovetsky, M. Klingenberg, V. Muller, K. Fendler, and E. Bamberg. 1999. Kinetics of electrogenic transport by the ADP/ATP carrier. Biophys. J. 77:714-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guffanti, A. A., and T. A. Krulwich. 1995. Tetracycline/H+ antiport and Na+/H+ antiport catalyzed by the Bacillus subtilis TetA(L) transporter expressed in Escherichia coli. J. Bacteriol. 177:4557-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guffanti, A. A., J. Cheng, and T. A. Krulwich. 1998. Electrogenic antiport activities of the Gram-positive Tet proteins include a Na+(K+)/K+ mode that mediates net K+ uptake. J. Biol. Chem. 273:26447-26454. [DOI] [PubMed] [Google Scholar]
- 19.Guffanti, A. A., Y. Wei, S. V. Rood, and T. A. Krulwich. 2002. An antiport mechanism for a member of the cation diffusion facilitator family: divalent cations efflux in exchange for K+ and H+. Mol. Microbiol. 45:145-153. [DOI] [PubMed]
- 20.Harel-Bronstein, M., P. Dibrov, Y. Olami, E. Pinner, S. Schuldiner, and E. Padan. 1995. MH1, a second-site revertant of an Escherichia coli mutant lacking Na+/H+ antiporters (ΔnhaAΔnhaB), regains Na+ resistance and a capacity to excrete Na+ in a ΔμH+-independent fashion. J. Biol. Chem. 270:3816-3822. [DOI] [PubMed] [Google Scholar]
- 21.Hilgemann, D. W., D. A. Nicoll, and K. D. Phillipson. 1991. Charge movement during Na+ translocation by native and cloned cardiac Na+/Ca2+ exchanger. Nature 352:715-718. [DOI] [PubMed] [Google Scholar]
- 22.Hirata, T., E. Fujihira, T. Kimura-Someya, and A. Yamaguchi. 1998. Membrane topology of the Staphylococcal tetracycline efflux protein Tet(K) determined by antibacterial resistance gene fusions. J. Biochem. 124:1206-1211. [DOI] [PubMed] [Google Scholar]
- 23.Hohenester, E., J. W. Keller, and J. N. Jansonius. 1994. An alkali metal ion size-dependent switch in the active site structure of dialkylglycine decarboxylase. Biochemistry 33:13561-13570. [DOI] [PubMed] [Google Scholar]
- 24.Iwaki, S., N. Tamura, T. Kimura-Someya, S. Nada, and A. Yamaguchi. 2000. Cysteine-scanning mutagenesis of transmembrane segments 4 and 5 of the Tn10-encoded metal-tetracycline/H+ antiporter reveals a permeability barrier in the middle of a transmembrane water-filled channel. J. Biol. Chem. 275:22704-22712. [DOI] [PubMed] [Google Scholar]
- 25.Jin, J. 2002. A mutagenesis approach to structure-function relationships in tetracycline efflux protein Tet(L). Ph.D. dissertation. Mount Sinai School of Medicine, New York University, New York, N.Y.
- 26.Jin, J., A. A. Guffanti, C. Beck, and T. A. Krulwich. 2001. Twelve-transmembrane-segment (TMS) version (ΔTMS VII-VIII) of the 14-TMS Tet(L) antibiotic resistance protein retains monovalent cation transport modes but lacks tetracycline efflux capacity. J. Bacteriol. 183:2667-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin, J., and T. A. Krulwich. 2002. Site-directed mutagenesis studies of selected motif and charged residues and of cysteines of the multifunctional tetracycline efflux protein Tet(L). J. Bacteriol. 184:1796-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaback, H. R. 1971. Bacterial membranes. Methods Enzymol. 22:99-120. [Google Scholar]
- 29.Khananshvili, D. 1990. Distinction between the two basic mechanisms of cation transport in the cardiac Na+-Ca2+ exchange system. Biochemistry 29:2437-2442. [DOI] [PubMed] [Google Scholar]
- 30.Krulwich, T. A., J. Jin, A. A. Guffanti, and D. H. Bechhofer. 2001. Functions of tetracycline efflux proteins that do not involve tetracycline. J. Mol. Microbiol. Biotechnol. 3:237-246. [PubMed] [Google Scholar]
- 31.Kunkel, T. A., K. Bebenek, and J. McClary. 1991. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 204:125-139. [DOI] [PubMed] [Google Scholar]
- 32.Lewinson, O., and E. Bibi. 2001. Evidence for simultaneous binding of dissimilar substrates by the Escherichia coli multidrug transporter MdfA. Biochemistry 40:12612-12618. [DOI] [PubMed] [Google Scholar]
- 33.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 34.Marger, M. D., and M. H. Saier, Jr. 1993. A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem. Sci. 18:13-20. [DOI] [PubMed] [Google Scholar]
- 35.McMurry, L. M., and S. B. Levy. 2000. Tetracycline resistance in gram-positive bacteria, p. 660-677. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 36.Mitchell, B. A., I. T. Paulsen, M. H. Brown, and R. A. Skurray. 1999. Bioenergetics of the staphylococcal multidrug export protein QacA: identification of distinct binding sites for monovalent and divalent cations. J. Biol. Chem. 274:3541-3548. [DOI] [PubMed] [Google Scholar]
- 37.Morita, Y., A. Kataoka, S. Shiota, T. Mizushima, and T. Tsuchiya. 2000. NorM of Vibrio parahaemolyticus is an Na+-driven multidrug efflux pump. J. Bacteriol. 182:6694-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padan, E., and T. A. Krulwich. 2000. Sodium stress, p. 117-130. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. ASM Press, Washington, D.C.
- 39.Padan, E., N. Maisler, D. Taglicht, R. Karpel, and S. Schuldiner. 1989. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system(s). J. Biol. Chem. 264:20297-20302. [PubMed] [Google Scholar]
- 40.Palmieri, F., C. Indiveri, F. Bisaccia, and R. Kramer. 1993. Functional properties of purified and reconstituted mitochondrial metabolite carriers. J. Bioenerg. Biomembr. 25:93-97. [DOI] [PubMed] [Google Scholar]
- 41.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedersen, P. A., J. M. Nielsen, J. H. Rasmussen, and P. L. Jorgensen. 1998. Contribution to Tl+, K+, and Na+ binding of Asn776, Ser775, Thr774, Thr772, and Tyr771 in cytoplasmic part of fifth transmembrane segment in alpha-subunit of renal Na,K-ATPase. Biochemistry 37:17818-17827. [DOI] [PubMed] [Google Scholar]
- 43.Putman, M., L. A. Koole, H. W. van Veen, and W. N. Konings. 1999. The secondary multidrug transporter LmrP contains multiple drug interaction sites. Biochemistry 38:13900-13905. [DOI] [PubMed] [Google Scholar]
- 44.Sekler, I., R. S. Lo, and R. R. Kopito. 1995. A conserved glutamate is responsible for ion selectivity and pH dependence of the mammalian anion exchangers AE1 and AE2. J. Biol. Chem. 270:28751-28758. [DOI] [PubMed] [Google Scholar]
- 45.Southworth, T. W., A. A. Guffanti, A. Moir, and T. A. Krulwich. 2001. GerN, an endospore germination protein of Bacillus cereus, is an Na+/H+-K+ antiporter. J. Bacteriol. 183:5896-5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura, N., S. Konishi, S. Iwaki, T. Kimura-Someya, S. Nada, and A. Yamaguchi. 2001. Complete cysteine-scanning mutagenesis and site-directed chemical modification of the Tn10-encoded metal-tetracycline/H+ antiporter. J. Biol. Chem. 276:20330-20339. [DOI] [PubMed] [Google Scholar]
- 47.Varela, M. F., C. E. Sansom, and J. K. Griffith. 1995. Mutational analysis and molecular modelling of an amino acid sequence motif conserved in antiporters but not symporters in a transporter superfamily. Mol. Membr. Biol. 12:313-319. [DOI] [PubMed] [Google Scholar]
- 48.Wang, W., A. A. Guffanti, Y. Wei, M. Ito, and T. A. Krulwich. 2000. Two types of Bacillus subtilis tetA(L) deletion strains reveal the physiological importance of TetA(L) in K+ acquisition as well as in Na+, alkali, and tetracycline resistance. J. Bacteriol. 182:2088-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi, A., N. Ono, T. Akasaka, T. Noumi, and T. Sawai. 1990. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by a transposon, Tn10. The role of the conserved dipeptide, Ser65-Asp66, in tetracycline transport. J. Biol. Chem. 265:15525-15530. [PubMed] [Google Scholar]
- 50.Yamaguchi, A., Y. Shiina, E. Fujihira, T. Sawai, N. Noguchi, and M. Sasatsu. 1995. The tetracycline efflux protein encoded by the tet(K) gene from Staphylococcus aureus is a metal-tetracycline/H+ antiporter. FEBS Lett. 365:193-197. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi, A., Y. Someya, and T. Sawai. 1992. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by transposon Tn10. The role of a conserved sequence motif, GXXXXRXGRR, in a putative cytoplasmic loop between helices 2 and 3. J. Biol. Chem. 267:19155-19162. [PubMed] [Google Scholar]
- 52.Yu, J.-L., L. Grinius, and D. C. Hooper. 2002. NorA functions as a multidrug efflux protein in both cytoplasmic membrane vesicles and reconstituted proteoliposomes. J. Bacteriol. 184:1370-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]