Abstract
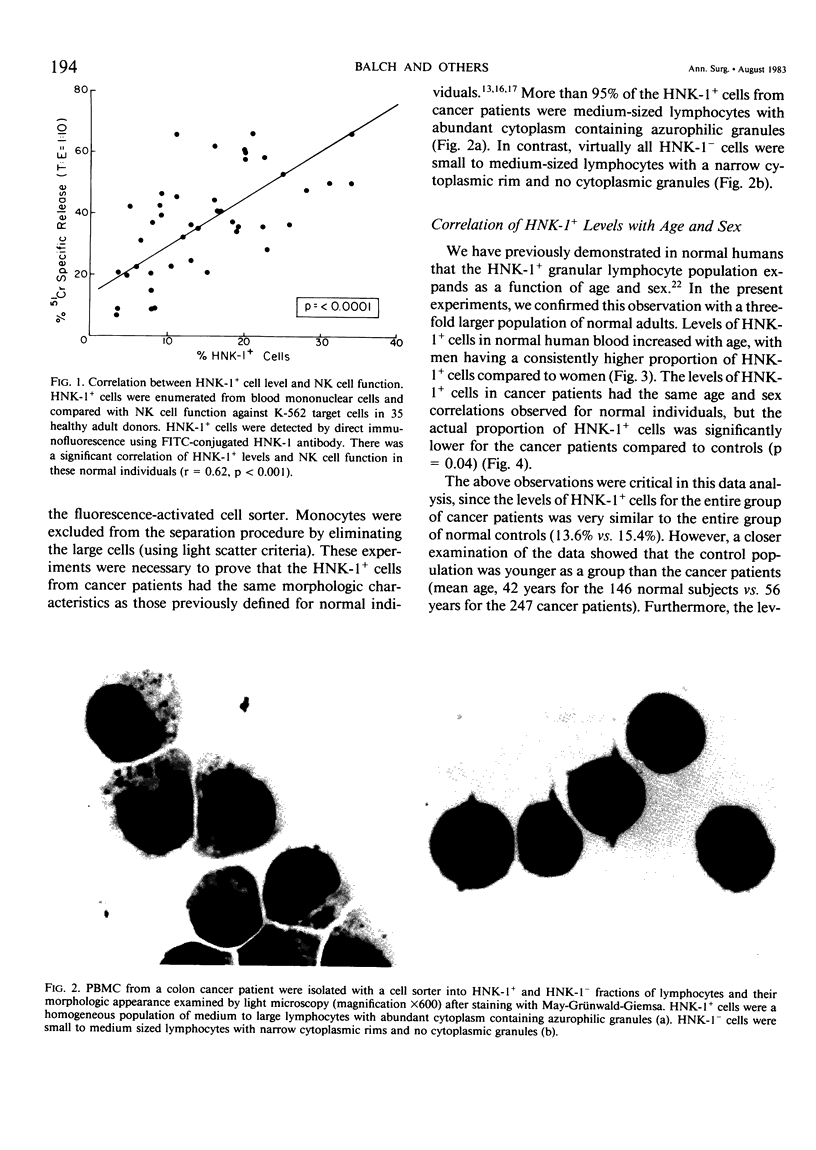
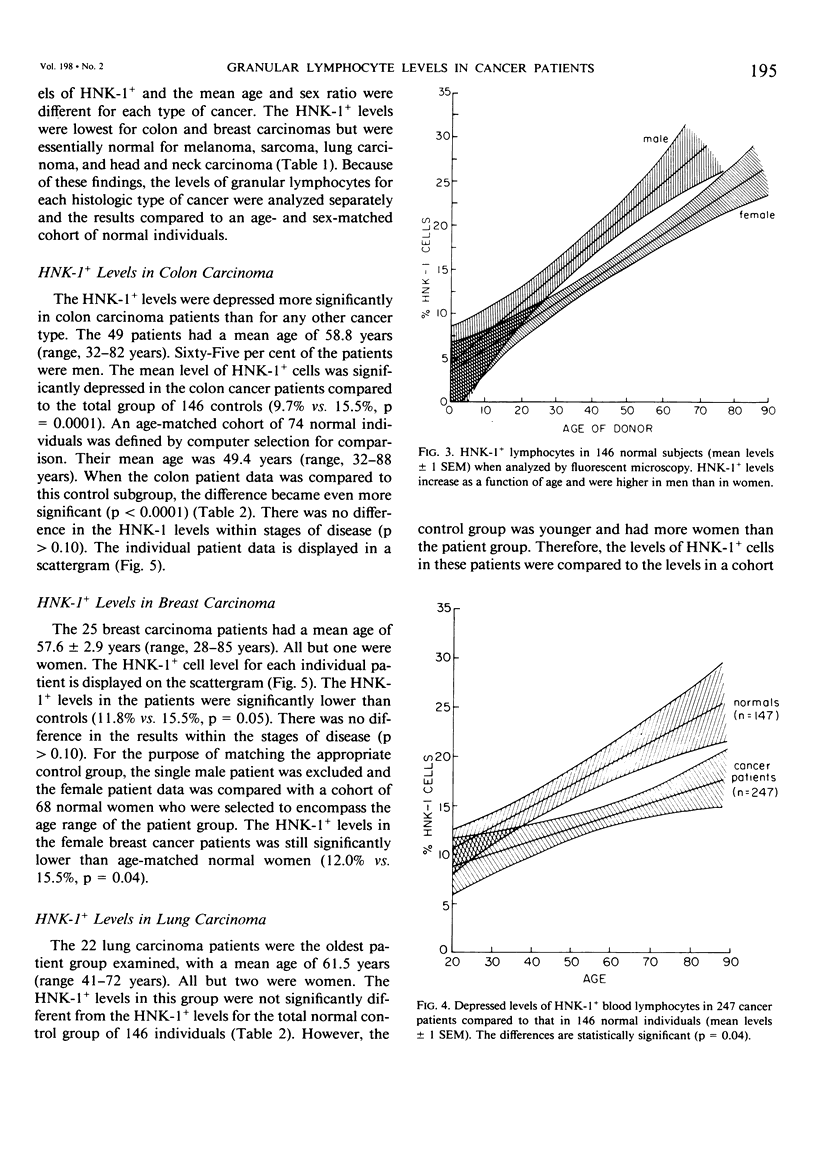
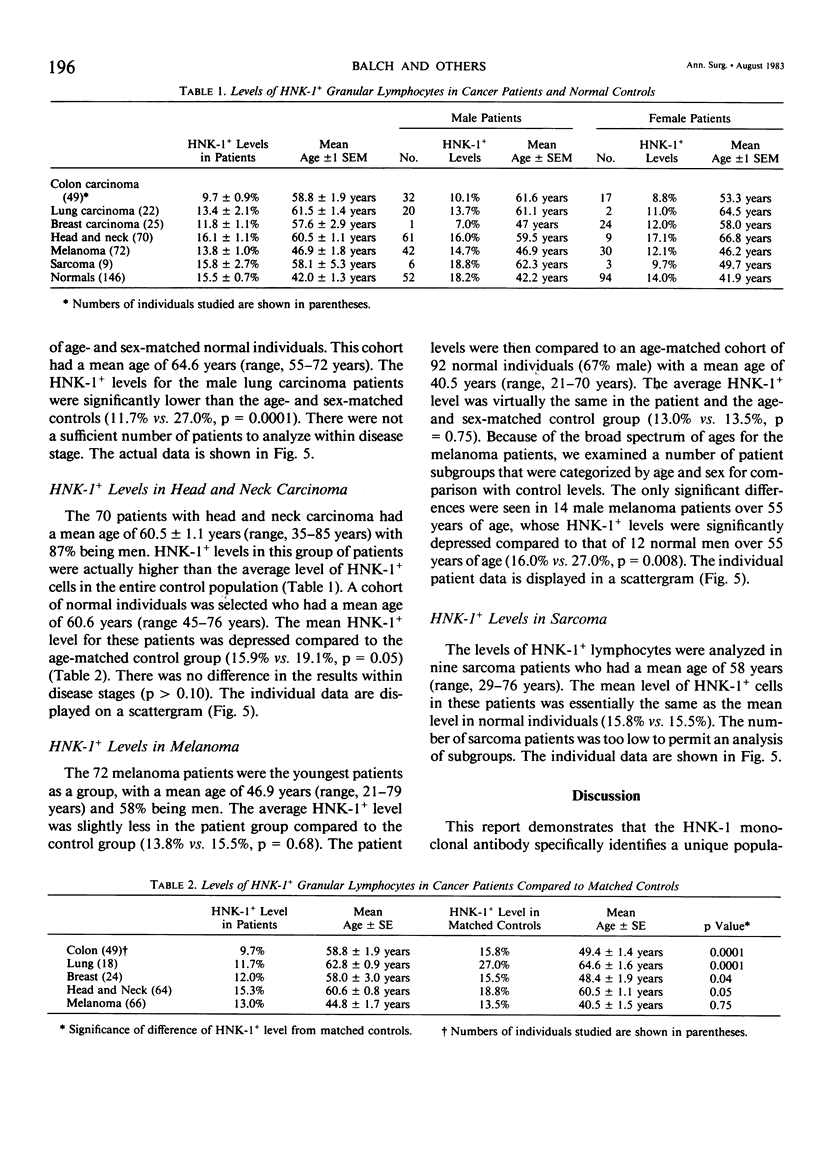
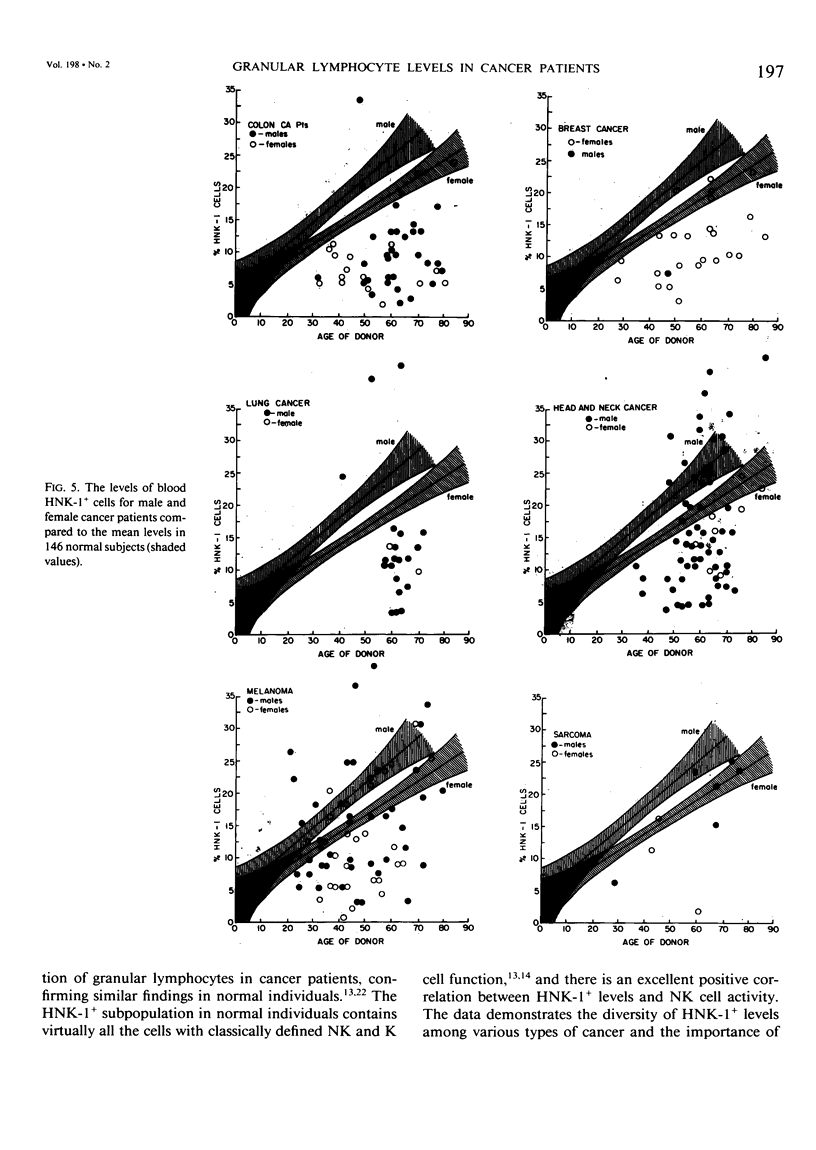
The HNK-1 (Leu-7) monoclonal antibody was used to enumerate and characterize the level of blood granular lymphocytes in 247 cancer patients. The results were compared to 146 control individuals. A fluorescence-activated cell sorter was used to purify blood HNK-1+ cells from cancer patients. The monoclonal antibody identified a homogeneous population of granular lymphocytes with greater than 95% purity. Conversely, virtually 100% of HNK-1- cells from cancer patients were agranular lymphocytes. These results were the same as previously observed in normal individuals, where the HNK-1+ cell fraction contained all the lymphocytes with spontaneous cytotoxicity in natural killer (NK) and killer (K) cell assays. The level of HNK-1+ cells in cancer patients correlated significantly with the patient's age and sex, with older individuals having higher levels and male patients containing a higher proportion than female patients. The levels in the cancer patients were significantly lower than normal controls (p = 0.04). When the results were subdivided by the histologic type of cancer, additional differences were noted. Compared to age and sex-matched controls, significantly depressed levels of HNK-1+ granular lymphocytes were observed in 49 patients with colon cancer (9.7% vs. 15.8%, p = 0.0001), 18 patients with lung carcinoma (11.7% vs. 27.0%, p = 0.0001), 24 patients with breast carcinoma (12.0% vs. 15.5%, p = 0.04) and 64 patients with head and neck carcinoma (15.9% vs. 19.1%, p = 0.05). However, there were no significant differences overall in the average HNK-1+ cell level of 66 patients with melanoma (13.0% vs. 13.5%, p = 0.75) and nine patients with sarcomas (15.8% vs. 14.3%, p = 0.71). Thus, this important subpopulation of granular lymphocytes with NK and K cell function was significantly depressed in most cancer patients. Accounting for the patient's age and sex and the histologic type of cancer was critical to interpreting the results.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. II. Distinguishing phenotypic and functional properties of natural killer cells from activated NK-like cells. J Immunol. 1982 Oct;129(4):1758–1761. [PubMed] [Google Scholar]
- Abo T., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. III. Interferon effects on spontaneous cytotoxicity and phenotypic expression of lymphocyte subpopulations delineated by the monoclonal HNK-1 antibody. Cell Immunol. 1982 Nov 1;73(2):376–384. doi: 10.1016/0008-8749(82)90464-6. [DOI] [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Characterization of HNK-1+ (Leu-7) human lymphocytes. I. Two distinct phenotypes of human NK cells with different cytotoxic capability. J Immunol. 1982 Oct;129(4):1752–1757. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Postnatal expansion of the natural killer and keller cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982 Jan 1;155(1):321–326. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abo T., Miller C. A., Gartland G. L., Balch C. M. Differentiation stages of human natural killer cells in lymphoid tissues from fetal to adult life. J Exp Med. 1983 Jan 1;157(1):273–284. doi: 10.1084/jem.157.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch C. M., Ades E. W., Loken M. R., Shore S. L. Human "null" cells mediating antibody-dependent cellular cytotoxicity express T lymphocyte differentiation antigens. J Immunol. 1980 Apr;124(4):1845–1851. [PubMed] [Google Scholar]
- Balch C. M., Dougherty P. A., Dagg M. K., Diethelm A. G., Lawton A. R. Detection of human T cells using anti-monkey thymocyte antisera. Tissue distribution and evidence for antigenic heterogeneity. Clin Immunol Immunopathol. 1977 Nov;8(3):448–460. doi: 10.1016/0090-1229(77)90008-3. [DOI] [PubMed] [Google Scholar]
- Canevari S., Fossati G., Della Porta G. Cellular immune reaction to human malignant melanoma and breast carcinoma cells. J Natl Cancer Inst. 1976 Apr;56(4):705–709. doi: 10.1093/jnci/56.4.705. [DOI] [PubMed] [Google Scholar]
- Catalona W. J., Ratliff T. L., McCool R. E. Discordance among cell-mediated cytolytic mechanisms in cancer patients: importance of the assay system. J Immunol. 1979 Mar;122(3):1009–1014. [PubMed] [Google Scholar]
- Fischer D. G., Hubbard W. J., Koren H. S. Tumor cell killing by freshly isolated peripheral blood monocytes. Cell Immunol. 1981 Mar 1;58(2):426–435. doi: 10.1016/0008-8749(81)90235-5. [DOI] [PubMed] [Google Scholar]
- Gathings W. E., Lawton A. R., Cooper M. D. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977 Nov;7(11):804–810. doi: 10.1002/eji.1830071112. [DOI] [PubMed] [Google Scholar]
- Hanna N., Burton R. C. Definitive evidence that natural killer (NK) cells inhibit experimental tumor metastases in vivo. J Immunol. 1981 Nov;127(5):1754–1758. [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Lavrin D. H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer. 1975 Aug 15;16(2):230–239. doi: 10.1002/ijc.2910160205. [DOI] [PubMed] [Google Scholar]
- Hersey P., Edwards A., Honeyman M., McCarthy W. H. Low natural-killer-cell activity in familial melanoma patients and their relatives. Br J Cancer. 1979 Jul;40(1):113–122. doi: 10.1038/bjc.1979.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersh E. V., Murphy S. G., Gutterman J. U., Morgan J., Quesada J., Zander A., Stewart D. Antibody-dependent cell-mediated cytotoxicity in human cancer: characterization of patient leukocyte activity and treatment effects. Cancer. 1982 Jan 15;49(2):251–260. doi: 10.1002/1097-0142(19820115)49:2<251::aid-cncr2820490210>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kiessling R., Petranyi G., Klein G., Wigzel H. Genetic variation of in vitro cytolytic activity and in vivo rejection potential of non-immunized semi-syngeneic mice against a mouse lymphoma line. Int J Cancer. 1975 Jun 15;15(6):933–940. doi: 10.1002/ijc.2910150608. [DOI] [PubMed] [Google Scholar]
- Klein M., Roder J., Haliotis T., Korec S., Jett J. R., Herberman R. B., Katz P., Fauci A. S. Chédiak-Higashi gene in humans. II. The selectivity of the defect in natural-killer and antibody-dependent cell-mediated cytotoxicity function. J Exp Med. 1980 May 1;151(5):1049–1058. doi: 10.1084/jem.151.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kärre K., Klein G. O., Kiessling R., Klein G., Roder J. C. Low natural in vivo resistance to syngeneic leukaemias in natural killer-deficient mice. Nature. 1980 Apr 17;284(5757):624–626. doi: 10.1038/284624a0. [DOI] [PubMed] [Google Scholar]
- Lamon E. W. The immune response to virally determined tumor associated antigens. Biochim Biophys Acta. 1974 Sep 9;355(2):149–176. doi: 10.1016/0304-419x(74)90002-x. [DOI] [PubMed] [Google Scholar]
- Lotzová E., Savary C. A., Gutterman J. U., Hersh E. M. Modulation of natural killer cell-mediated cytotoxicity by partially purified and cloned interferon-alpha. Cancer Res. 1982 Jun;42(6):2480–2488. [PubMed] [Google Scholar]
- Lotzová E. Several aspects of natural killer cell-mediated cytotoxicity in normal individuals and cancer patients. Cell Mol Biol Incl Cyto Enzymol. 1980;26(4):423–431. [PubMed] [Google Scholar]
- Lucero M. A., Fridman W. H., Provost M. A., Billardon C., Pouillart P., Dumont J., Falcoff E. Effect of various interferons on the spontaneous cytotoxicity exerted by lymphocytes from normal and tumor-bearing patients. Cancer Res. 1981 Jan;41(1):294–299. [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sessa C., Bolis G., Mangioni C. Natural killer activity of lymphoid cells isolated from human ascitic ovarian tumors. Int J Cancer. 1980 May 15;25(5):573–582. doi: 10.1002/ijc.2910250505. [DOI] [PubMed] [Google Scholar]
- Marx J. L. Natural killer cells help defend the body. Science. 1980 Nov 7;210(4470):624–626. doi: 10.1126/science.6159684. [DOI] [PubMed] [Google Scholar]
- Pross H. F., Baines M. G. Spontaneous human lymphocyte-mediated cytotoxicity againts tumour target cells. I. The effect of malignant disease. Int J Cancer. 1976 Nov 15;18(5):593–604. doi: 10.1002/ijc.2910180508. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Haliotis T., Klein M., Korec S., Jett J. R., Ortaldo J., Heberman R. B., Katz P., Fauci A. S. A new immunodeficiency disorder in humans involving NK cells. Nature. 1980 Apr 10;284(5756):553–555. doi: 10.1038/284553a0. [DOI] [PubMed] [Google Scholar]
- Roder J., Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979 Mar 29;278(5703):451–453. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- Seeley J. K., Masucci G., Poros A., Klein E., Golub S. H. Studies on cytotoxicity generated in human mixed lymphocyte cultures. II. Anti-K562 effectors are distinct from allospecific CTL and can be generated from NK-depleted T cells. J Immunol. 1979 Sep;123(3):1303–1311. [PubMed] [Google Scholar]
- Takasugi M., Ramseyer A., Takasugi J. Decline of natural nonselective cell-mediated cytotoxicity in patients with tumor progression. Cancer Res. 1977 Feb;37(2):413–418. [PubMed] [Google Scholar]
- Talmadge J. E., Meyers K. M., Prieur D. J., Starkey J. R. Role of NK cells in tumour growth and metastasis in beige mice. Nature. 1980 Apr 17;284(5757):622–624. doi: 10.1038/284622a0. [DOI] [PubMed] [Google Scholar]
- Timonen T., Saksela E., Ranki A., Häyry P. Fractionation, morphological and functional characterization of effector cells responsible for human natural killer activity against cell-line targets. Cell Immunol. 1979 Nov;48(1):133–148. doi: 10.1016/0008-8749(79)90106-0. [DOI] [PubMed] [Google Scholar]
- Troye M., Vilien M., Pape G. R., Perlmann P. Cytotoxicity in vitro of blood lymphocytes from bladder cancer patients and controls to allogeneic or autologous tumor cells derived from established cell lines or short-term cultures. Int J Cancer. 1980 Jan 15;25(1):33–43. doi: 10.1002/ijc.2910250105. [DOI] [PubMed] [Google Scholar]
- West W. H., Cannon G. B., Kay H. D., Bonnard G. D., Herberman R. B. Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells. J Immunol. 1977 Jan;118(1):355–361. [PubMed] [Google Scholar]



