Abstract
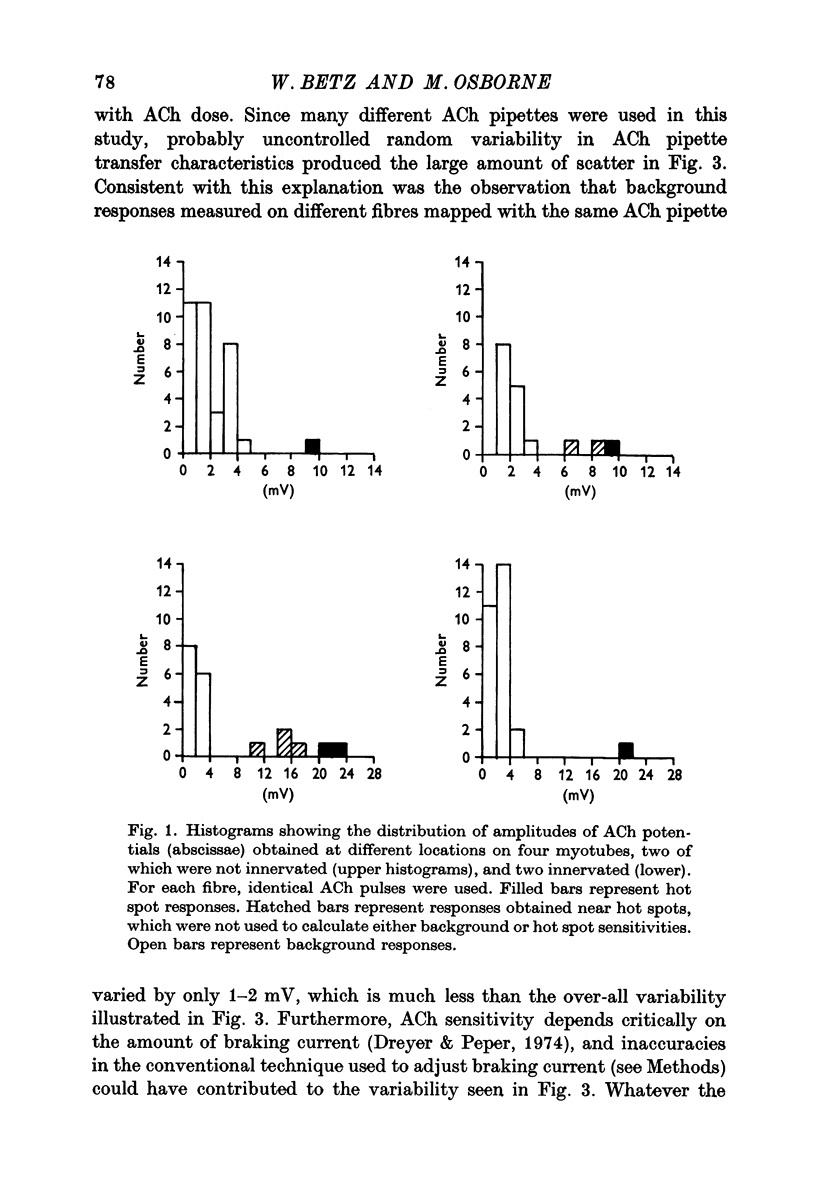
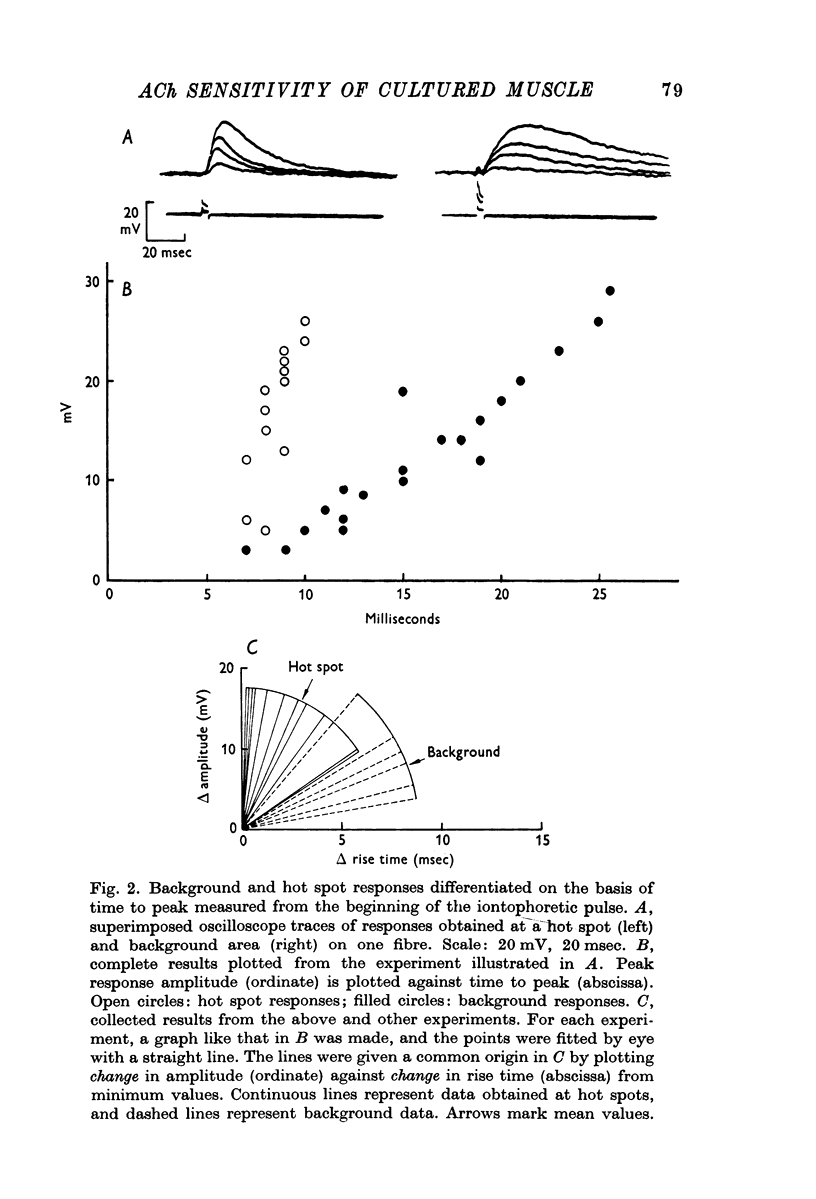
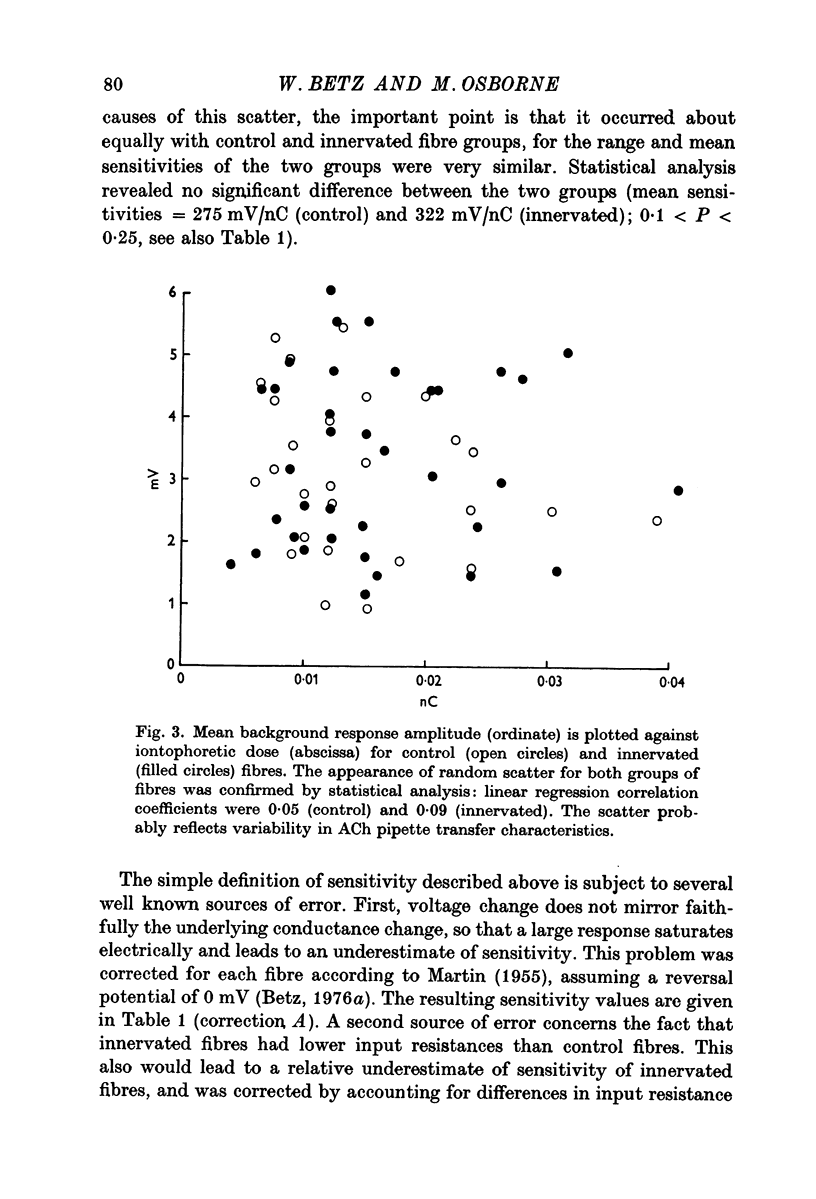
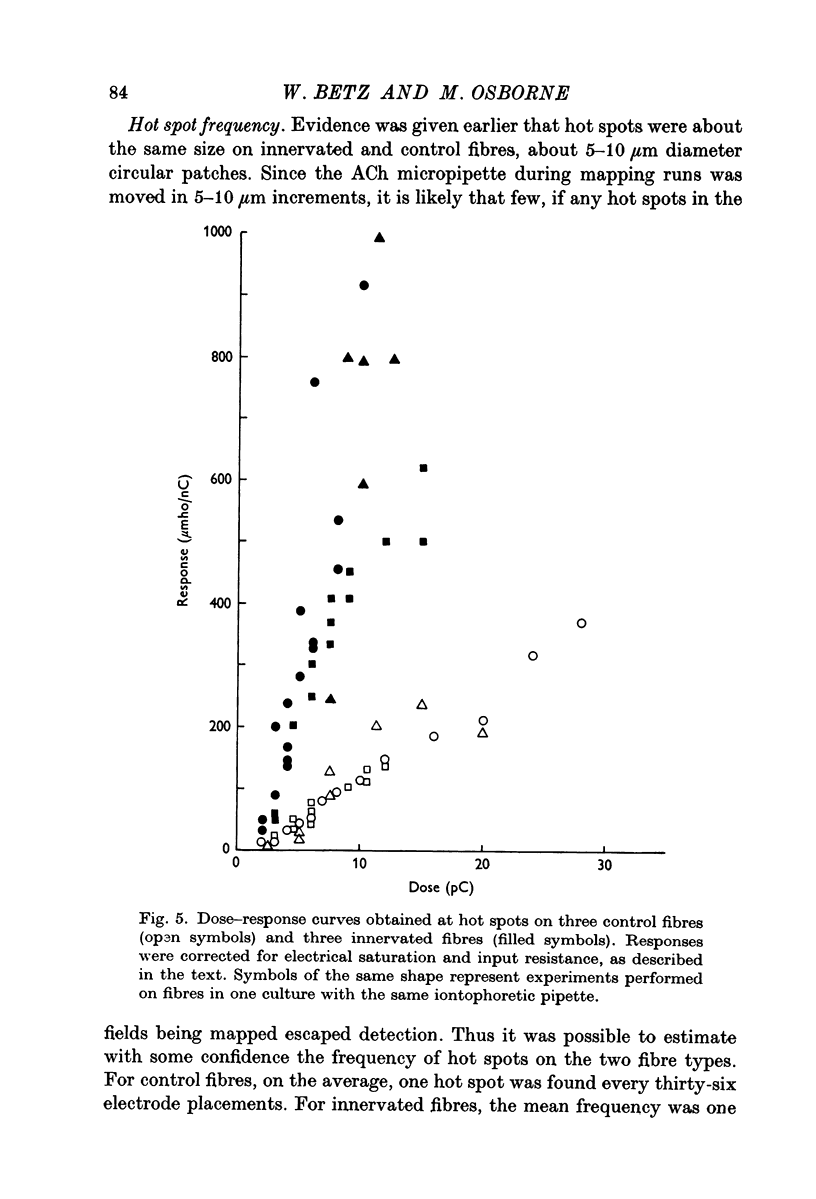
1. Chick embryo skeletal muscle fibres were grown in culture. The acetylcholine (AACh) sensitivity of non-innervated fibres was compared with that of fibres innervated in vitro by chick embryo ciliary ganglion neurones. 2. The general pattern of ACh sensitivity was unchanged by innervation: ACh hot spots were superimposed on a background of uniform ACh sensitivity. 3. Quantitative comparisons revealed two differences between non-innervated and innervated fibres. First, hot spots were encountered about one third more often on innervated fibres. Secondly, about one-third of the hot spots on innervated fibres had significantly higher ACh sensitivities than the remainder, which were similar to those on control fibres. 4. Apossible explanation of these results is that nerves which form synapses induce the appearanceof end-plates which have higher ACh sensitivities than the pre-existing ACh hot spots.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz W. Functional and non-functional contacts between ciliary neurones and muscle grown in vitro. J Physiol. 1976 Jan;254(1):75–86. doi: 10.1113/jphysiol.1976.sp011222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. The formation of synapses between chick embryo skeletal muscle and ciliary ganglia grown in vitro. J Physiol. 1976 Jan;254(1):63–73. doi: 10.1113/jphysiol.1976.sp011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. On the localization of acetylcholine receptors. J Physiol. 1955 Apr 28;128(1):157–181. doi: 10.1113/jphysiol.1955.sp005297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K. Iontophoretic application of acetylcholine: advantages of high resistance micropipettes in connection with an electronic current pump. Pflugers Arch. 1974 Apr 22;348(3):263–272. doi: 10.1007/BF00587417. [DOI] [PubMed] [Google Scholar]
- Feltz A., Mallart A. An analysis of acetylcholine responses of junctional and extrajunctional receptors of frog muscle fibres. J Physiol. 1971 Oct;218(1):85–100. doi: 10.1113/jphysiol.1971.sp009605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Berg D. K., Cohen S. A., Frank E. Enrichment of nerve--muscle synapses in spinal cord--muscle cultures and identification of relative peaks of ACh sensitivity at sites of transmitter release. Cold Spring Harb Symp Quant Biol. 1976;40:347–357. doi: 10.1101/sqb.1976.040.01.034. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Cohen S. A. The distribution of acetylcholine sensitivity over uninnervated and innervated muscle fibers grown in cell culture. Dev Biol. 1973 Mar;31(1):147–162. doi: 10.1016/0012-1606(73)90326-6. [DOI] [PubMed] [Google Scholar]
- Hooisma J., Slaaf D. W., Meeter E., Stevens W. F. The innervation of chick striated muscle fibers by the chick ciliary ganglion in tissue culture. Brain Res. 1975 Feb 21;85(1):79–85. doi: 10.1016/0006-8993(75)91009-4. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. R. The effect of membrane capacitance on non-linear summation of synaptic potentials. J Theor Biol. 1976 Jun;59(1):179–187. doi: 10.1016/s0022-5193(76)80031-8. [DOI] [PubMed] [Google Scholar]
- Robbins N., Yonezawa T. Developing neuromuscular junctions: first signs of chemical transmission during formation in tissue culture. Science. 1971 Apr 23;172(3981):395–398. doi: 10.1126/science.172.3981.395. [DOI] [PubMed] [Google Scholar]


