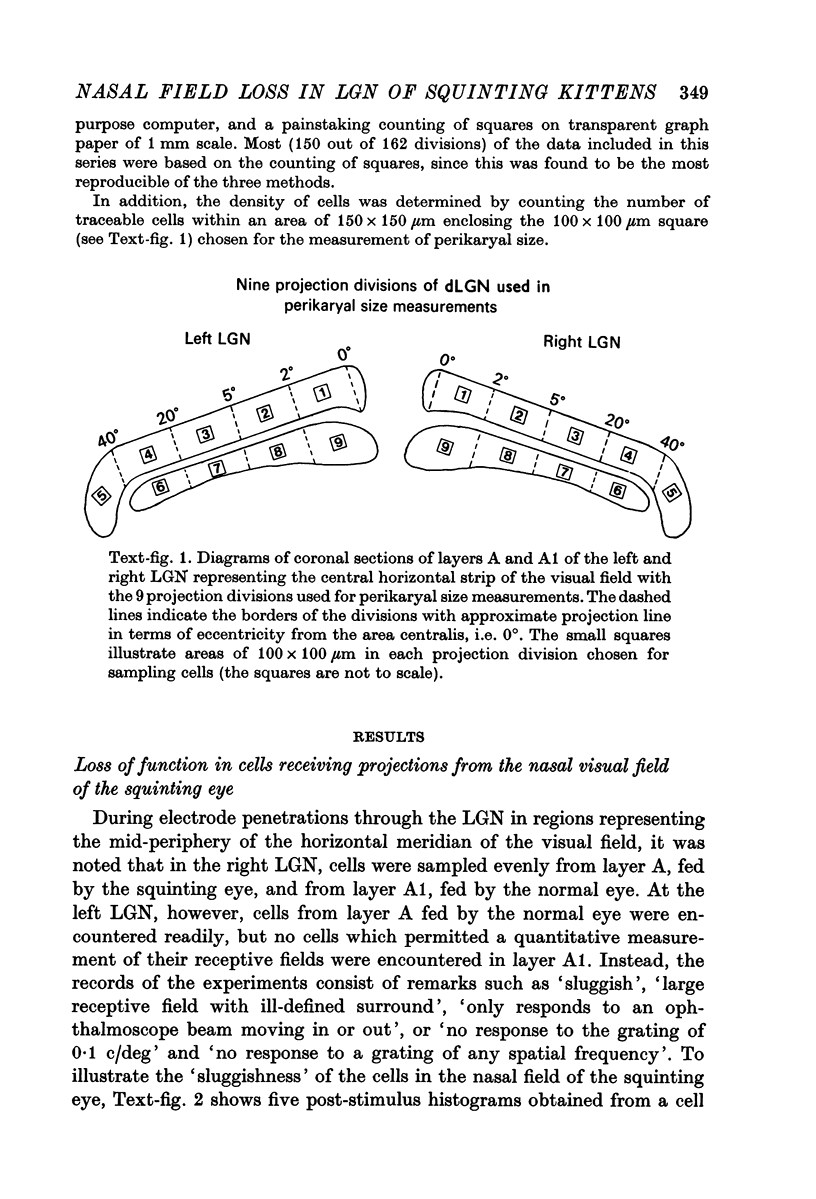
Abstract
1. Recordings of single cells were made in layers A and A1 of the lateral geniculate nucleus of kittens raised with convergent squint in one eye, and morphological studies of cells representing the different parts of the visual fields in these layers were also made from histological sections.
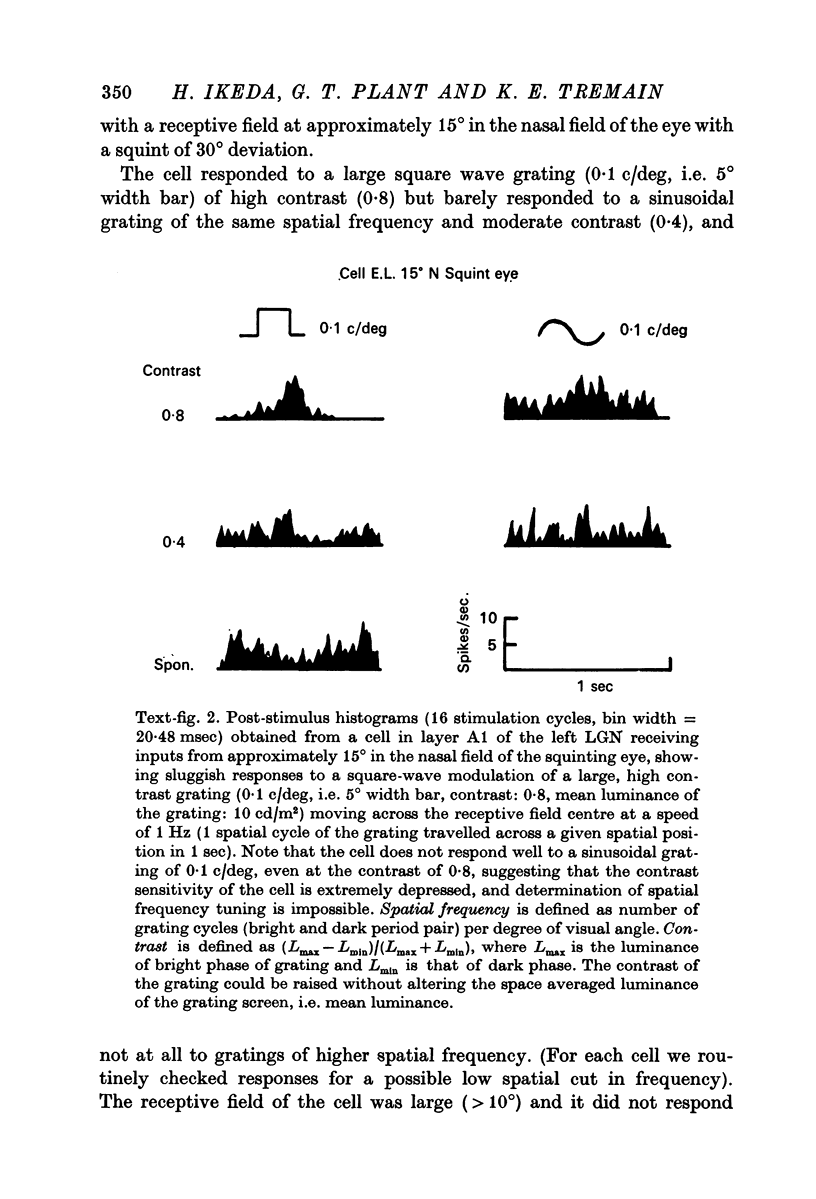
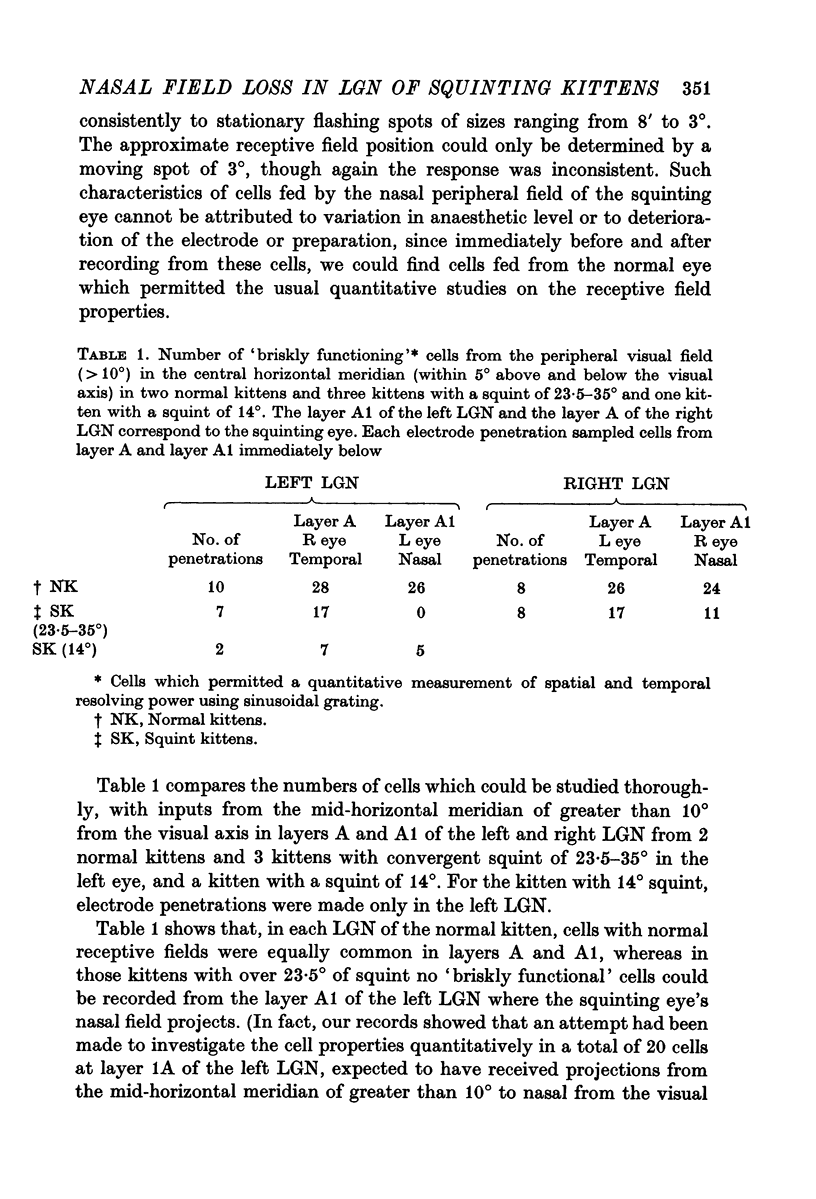
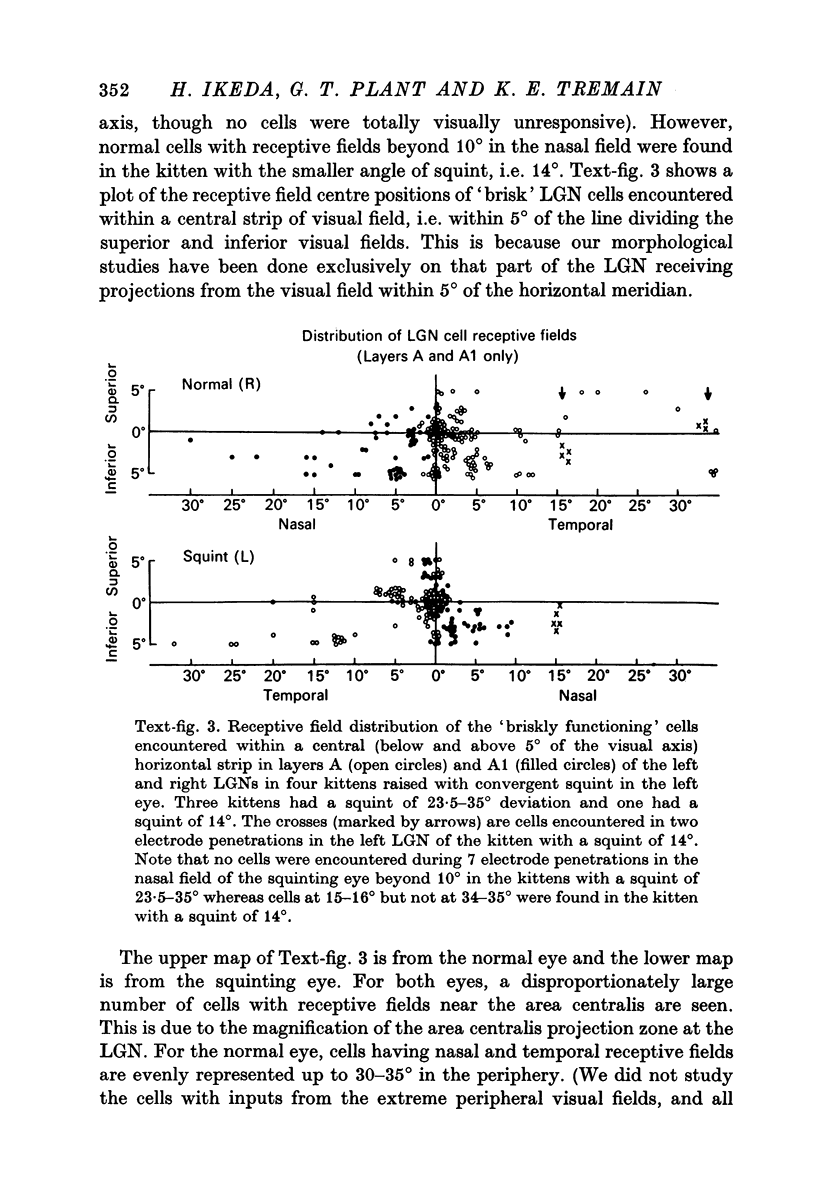
2. For the normal eye, cells receiving inputs from the nasal and temporal visual fields were evenly represented up to the periphery, whereas for the squinting eye, no cells which permitted quantitative studies of receptive field properties could be found in the periphery of the nasal field.
3. The loss of nasal field, represented by the loss of functional cells in the LGN layer A1 fed by the squinting eye, depended on the severity of the squint. The greater the angle of convergent squint, the greater the loss of nasal field represented by the loss of functional cells.
4. The cells fed by the squinting eye's temporal visual field retained their brisk function, although minor modifications in the receptive field organisation were apparent.
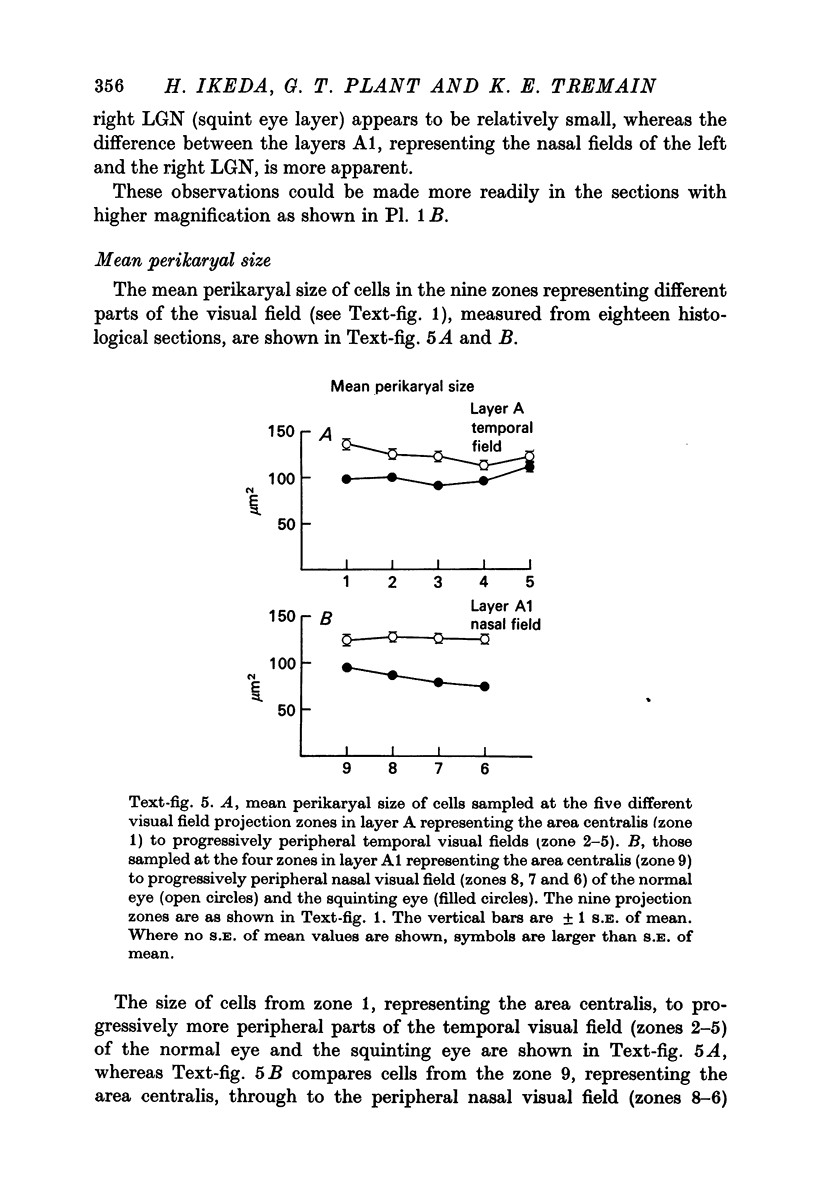
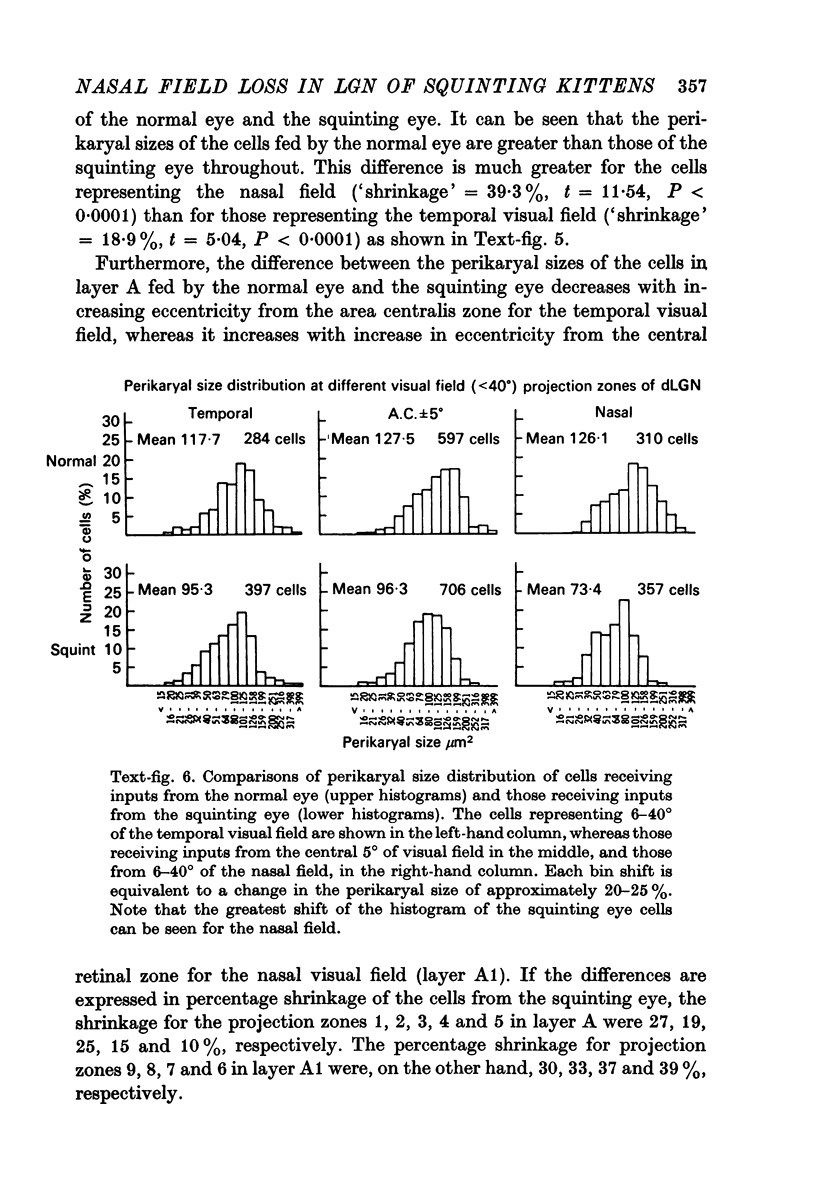
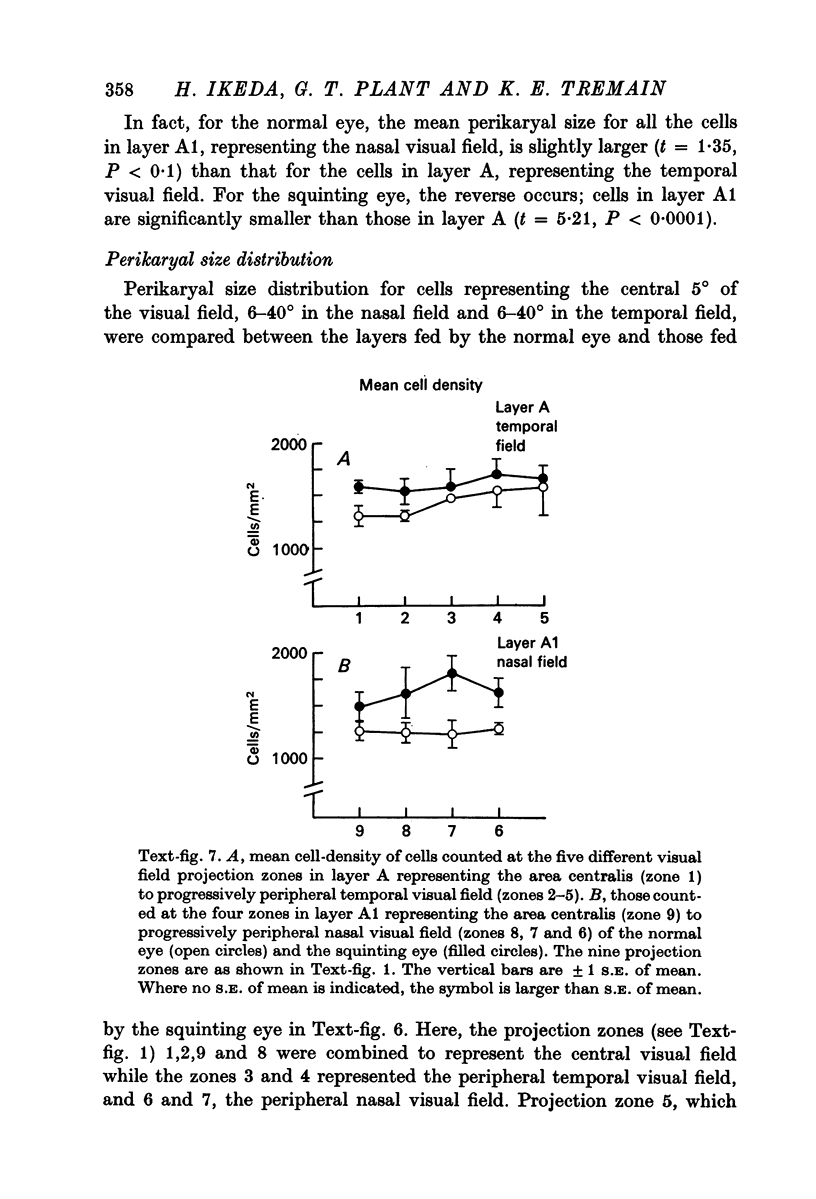
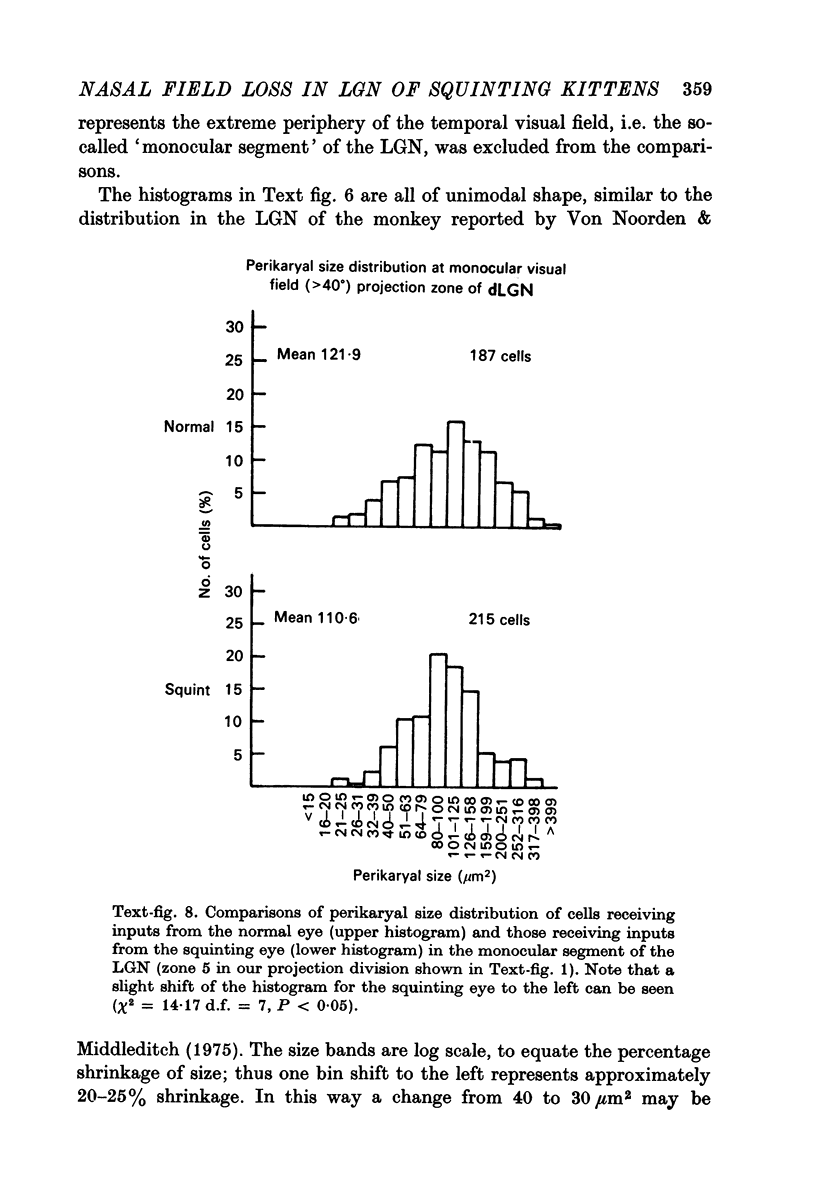
5. The mean perikaryal size was smaller and the cell-density higher for cells in layers fed by the squinting eye. As found for the functional loss of cells, the shrinkage of perikaryal size and the increase of cell-density was smallest in the zones fed by the temporal visual field, and greatest in the zones fed by the peripheral nasal visual field.
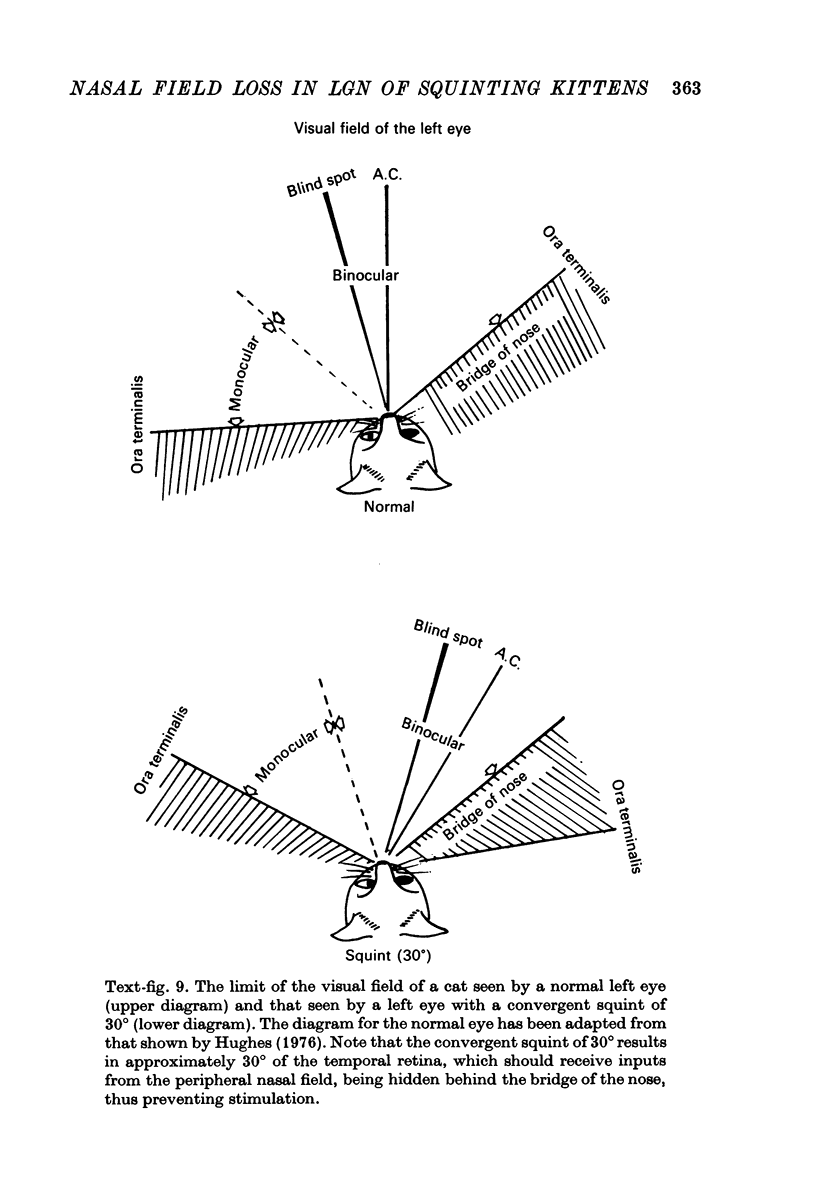
6. The functional and morphological changes in the cells in the LGN, which receive inputs from the nasal field of the squinting eye, are attributed to part of the temporal retina being hidden behind the bridge of the nose. It is proposed that this is a consequence of disuse atrophy, due to lack of stimulation during the sensitive period of development.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B. B., Wässle H. The morphological types of ganglion cells of the domestic cat's retina. J Physiol. 1974 Jul;240(2):397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOW K. L., RIESEN A. H., NEWELL F. W. Degeneration of retinal ganglion cells in infant chimpanzees reared in darkness. J Comp Neurol. 1957 Feb;107(1):27–42. doi: 10.1002/cne.901070103. [DOI] [PubMed] [Google Scholar]
- Chow K. L., Stewart D. L. Reversal of structural and functional effects of long-term visual deprivation in cats. Exp Neurol. 1972 Mar;34(3):409–433. doi: 10.1016/0014-4886(72)90038-6. [DOI] [PubMed] [Google Scholar]
- Duffy F. H., Burchfiel J. L., Conway J. L. Bicuculline reversal of deprivation amblyopia in the cat. Nature. 1976 Mar 18;260(5548):256–257. doi: 10.1038/260256a0. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Fisken R. A., Powell T. P. Effects of experimental deafferentation on cells in the lateral geniculate nucleus of the cat. Brain Res. 1973 Mar 30;52:363–369. doi: 10.1016/0006-8993(73)90672-0. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Kelly J. P. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- Guillery R. W., Stelzner D. J. The differential effects of unilateral lid closure upon the monocular and binocular segments of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1970 Aug;139(4):413–421. doi: 10.1002/cne.901390403. [DOI] [PubMed] [Google Scholar]
- Hammond P. Contrasts in spatial organization of receptive fields at geniculate and retinal levels: centre, surround and outer surround. J Physiol. 1973 Jan;228(1):115–137. doi: 10.1113/jphysiol.1973.sp010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hughes A. A supplement to the cat schematic eye. Vision Res. 1976;16(2):149–154. doi: 10.1016/0042-6989(76)90091-2. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Jacobson S. G. Nasal field loss in cats reared with convergent squint: behavioural studies. J Physiol. 1977 Sep;270(2):367–381. doi: 10.1113/jphysiol.1977.sp011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Properties of LGN cells in kittens reared with convergent squint: a neurophysiological demonstration of amblyopia. Exp Brain Res. 1976 May 10;25(1):63–77. doi: 10.1007/BF00237326. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Wright M. J. Sensitivity of neurones in visual cortex (area 17) under different levels of anaesthesia. Exp Brain Res. 1974;20(5):471–484. doi: 10.1007/BF00238014. [DOI] [PubMed] [Google Scholar]
- Kratz K. E., Spear P. D. Postcritical-period reversal of effects of monocular deprivation on striate cortex cells in the cat. J Neurophysiol. 1976 May;39(3):501–511. doi: 10.1152/jn.1976.39.3.501. [DOI] [PubMed] [Google Scholar]
- Maffei L., Bisti S. Binocular interaction in strabismic kittens deprived of Vision. Science. 1976 Feb 13;191(4227):579–580. doi: 10.1126/science.1251195. [DOI] [PubMed] [Google Scholar]
- Matthews M. R., Cowan W. M., Powell T. P. Transneuronal cell degeneration in the lateral geniculate nucleus of the macaque monkey. J Anat. 1960 Apr;94(Pt 2):145–169. [PMC free article] [PubMed] [Google Scholar]
- RASCH E., SWIFT H., RIESEN A. H., CHOW K. L. Altered structure and composition of retinal cells in darkreared mammals. Exp Cell Res. 1961 Nov;25:348–363. doi: 10.1016/0014-4827(61)90285-3. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J. The projection of the visual field to the lateral geniculate and medial interlaminar nuclei in the cat. J Comp Neurol. 1971 Sep;143(1):101–108. doi: 10.1002/cne.901430107. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Guillery R. W., Kaas J. H., Sanderson K. J. Behavioral, electrophysiological and morphological studies of binocular competition in the development of the geniculo-cortical pathways of cats. J Comp Neurol. 1974 Nov 1;158(1):1–18. doi: 10.1002/cne.901580102. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Hoffmann K. P., Stone J. Loss of a specific cell type from dorsal lateral geniculate nucleus in visually deprived cats. J Neurophysiol. 1972 Jul;35(4):532–541. doi: 10.1152/jn.1972.35.4.532. [DOI] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- WIESEL T. N., HUBEL D. H. EFFECTS OF VISUAL DEPRIVATION ON MORPHOLOGY AND PHYSIOLOGY OF CELLS IN THE CATS LATERAL GENICULATE BODY. J Neurophysiol. 1963 Nov;26:978–993. doi: 10.1152/jn.1963.26.6.978. [DOI] [PubMed] [Google Scholar]
- Wiesel T. N., Hubel D. H. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. J Neurophysiol. 1965 Nov;28(6):1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wässle H., Levick W. R., Kirk D. L., Cleland B. G. Axonal conduction velocity and perikaryal size. Exp Neurol. 1975 Oct;49(1 Pt 1):246–251. doi: 10.1016/0014-4886(75)90208-3. [DOI] [PubMed] [Google Scholar]
- Yinon U., Auerbach E. The ocular dominance of cortical neurons in cats developed with divergent and convergent squint. Vision Res. 1975 Nov;15(11):1251–1256. doi: 10.1016/0042-6989(75)90170-4. [DOI] [PubMed] [Google Scholar]
- von Noorden G. K. Histological studies of the visual system in monkeys with experimental amblyopia. Invest Ophthalmol. 1973 Oct;12(10):727–738. [PubMed] [Google Scholar]
- von Noorden G. K., Middleditch P. R. Histology of the monkey lateral geniculate nucleus after unilateral lid closure and experimental strabismus: further observations. Invest Ophthalmol. 1975 Sep;14(9):674–683. [PubMed] [Google Scholar]



