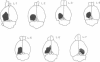Abstract
1. The response characteristics of visual, somatosensory, and auditory neurones in the golden hamster's superior colliculus were investigated.
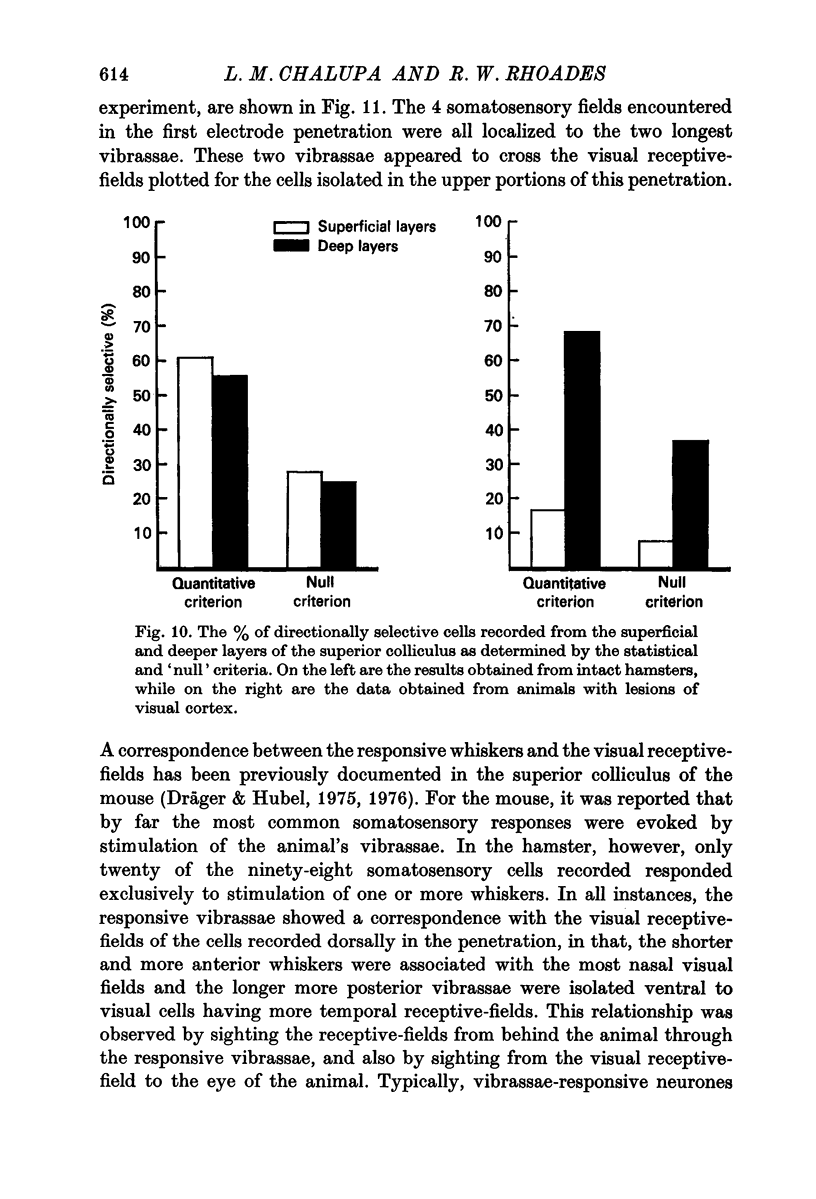
2. As has been noted for other mammalian species, a distinct difference between the functional organizations of the superficial and deeper layers of the superior colliculus was observed.
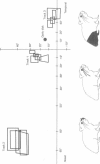
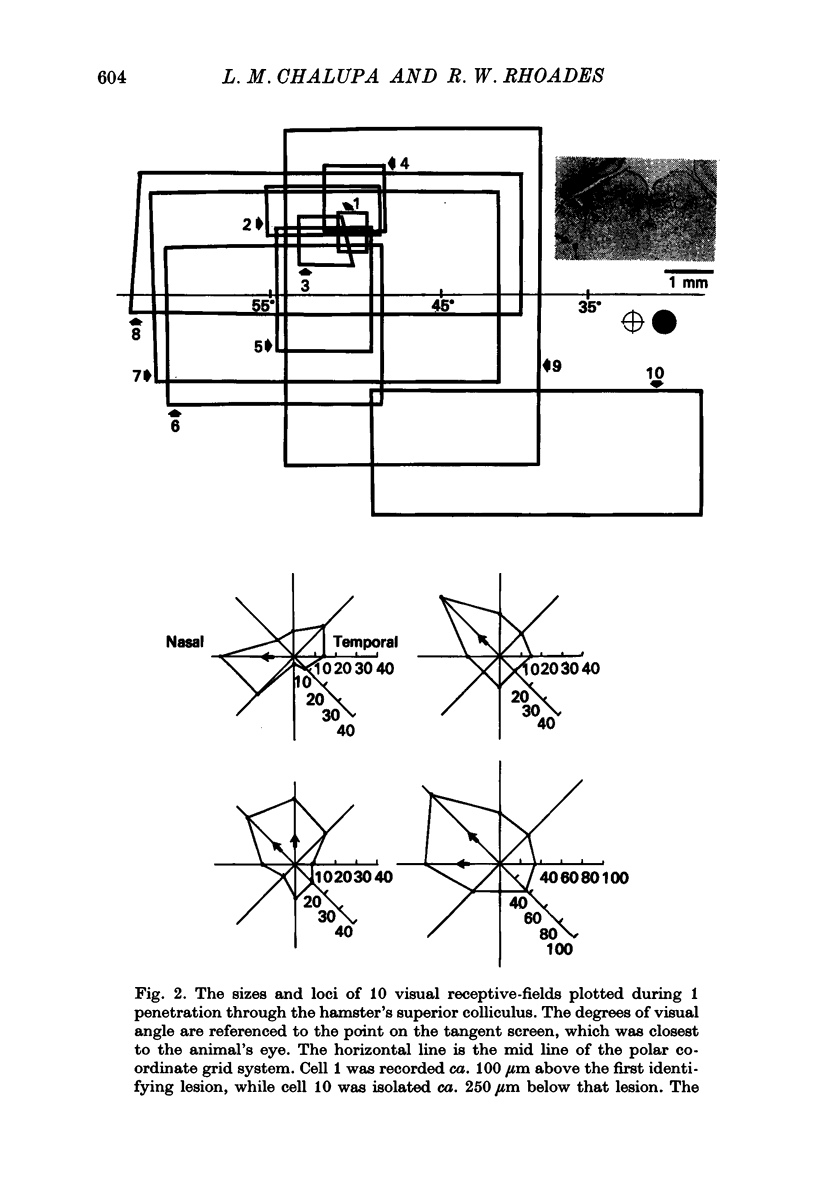
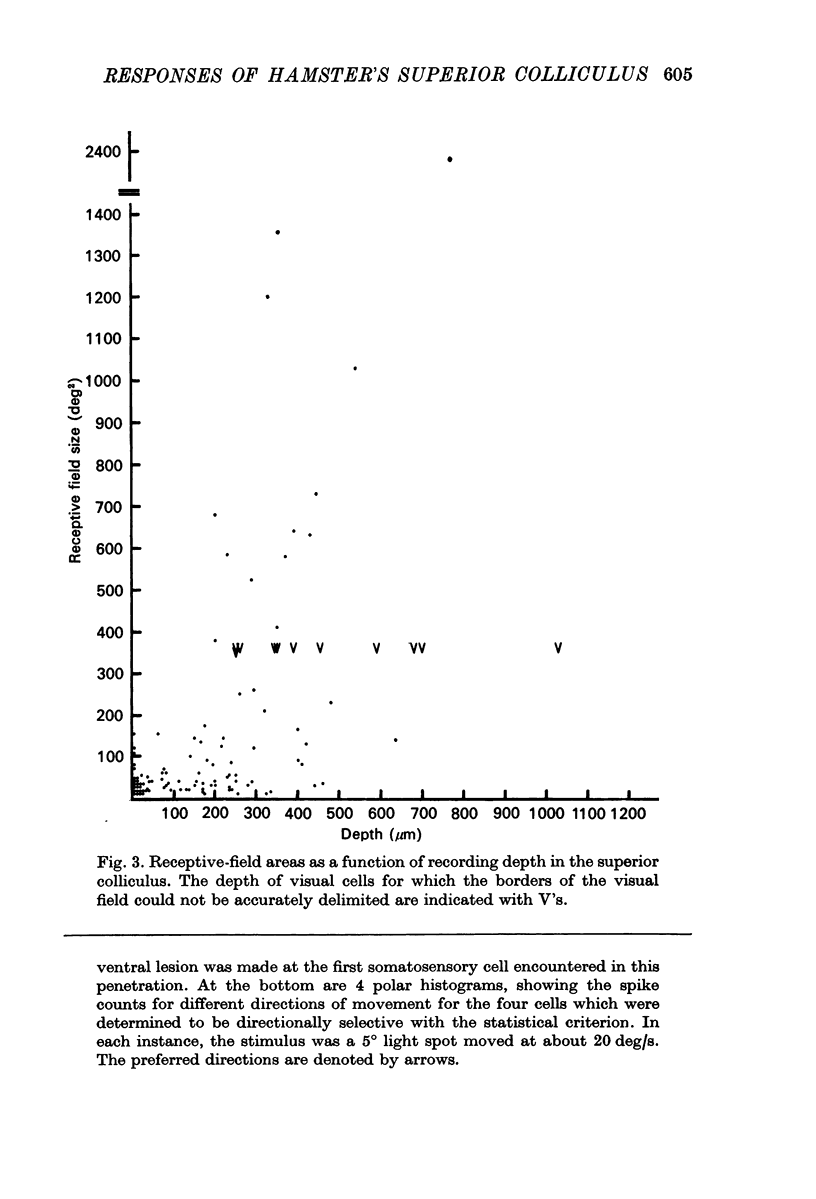
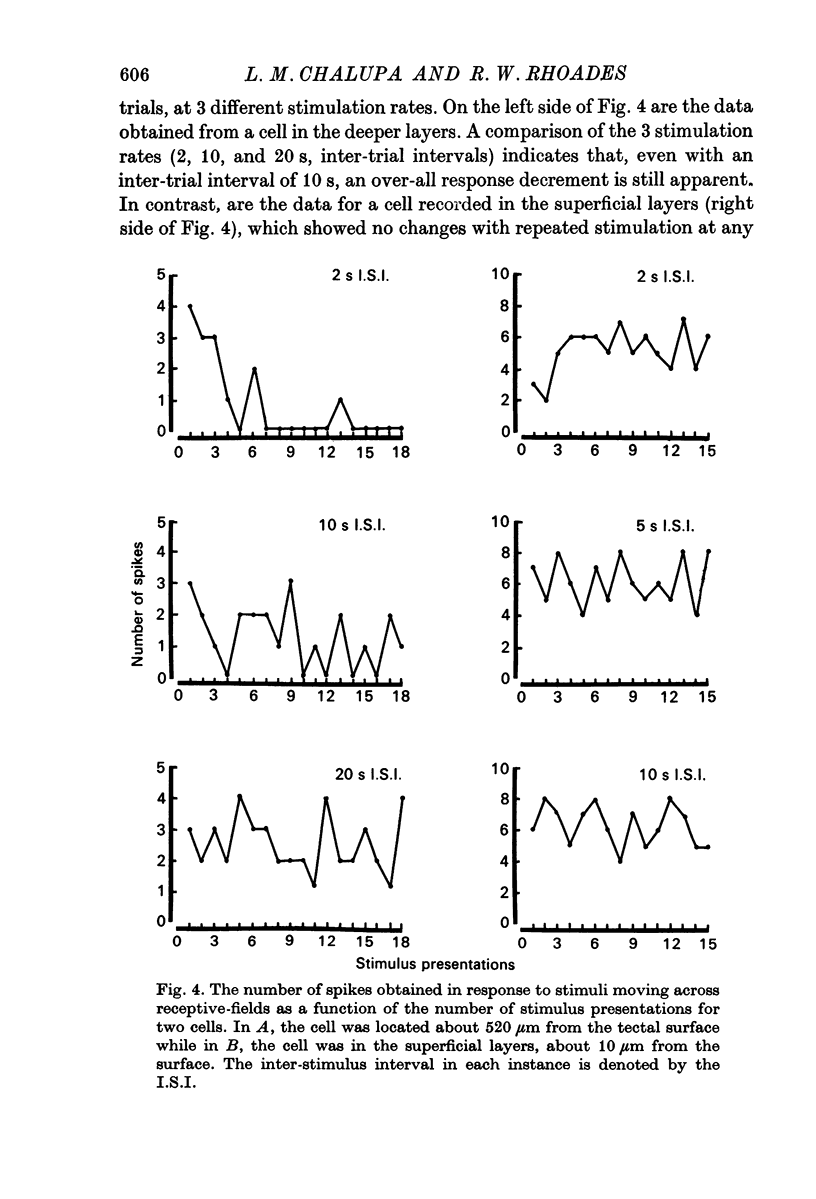
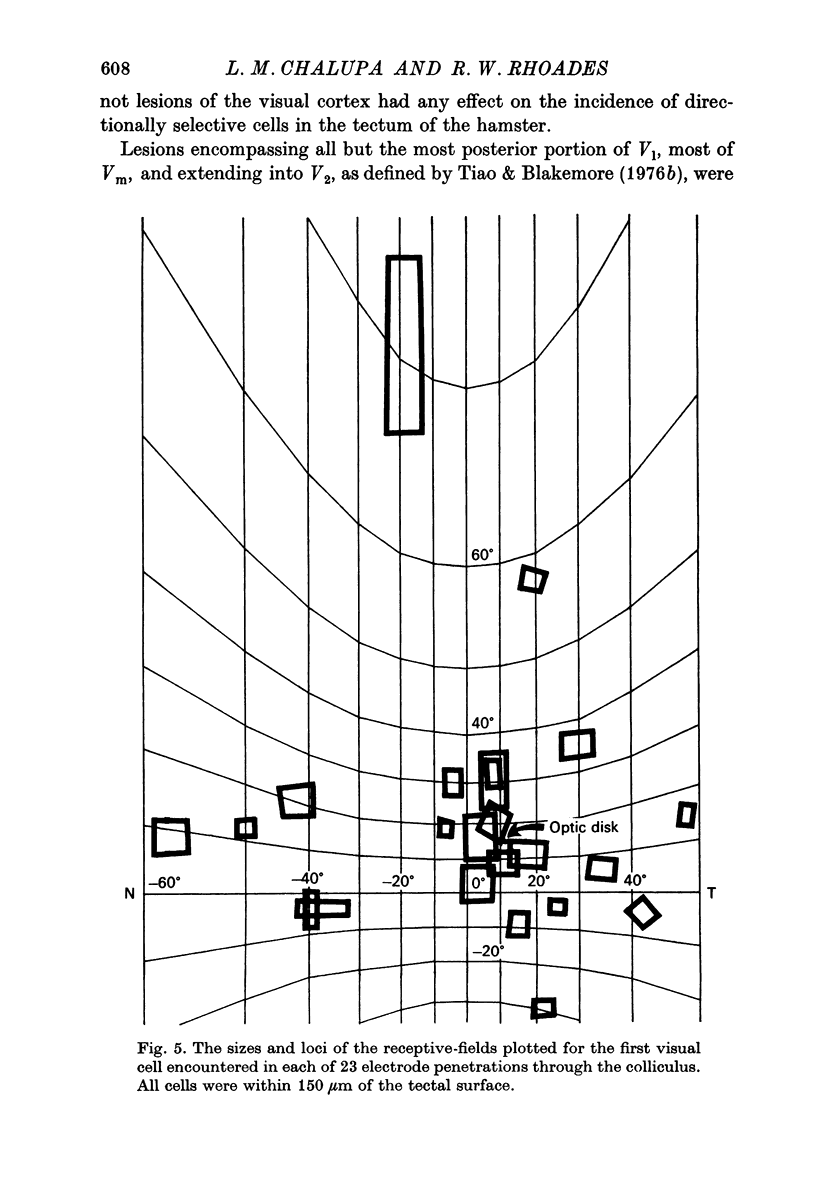
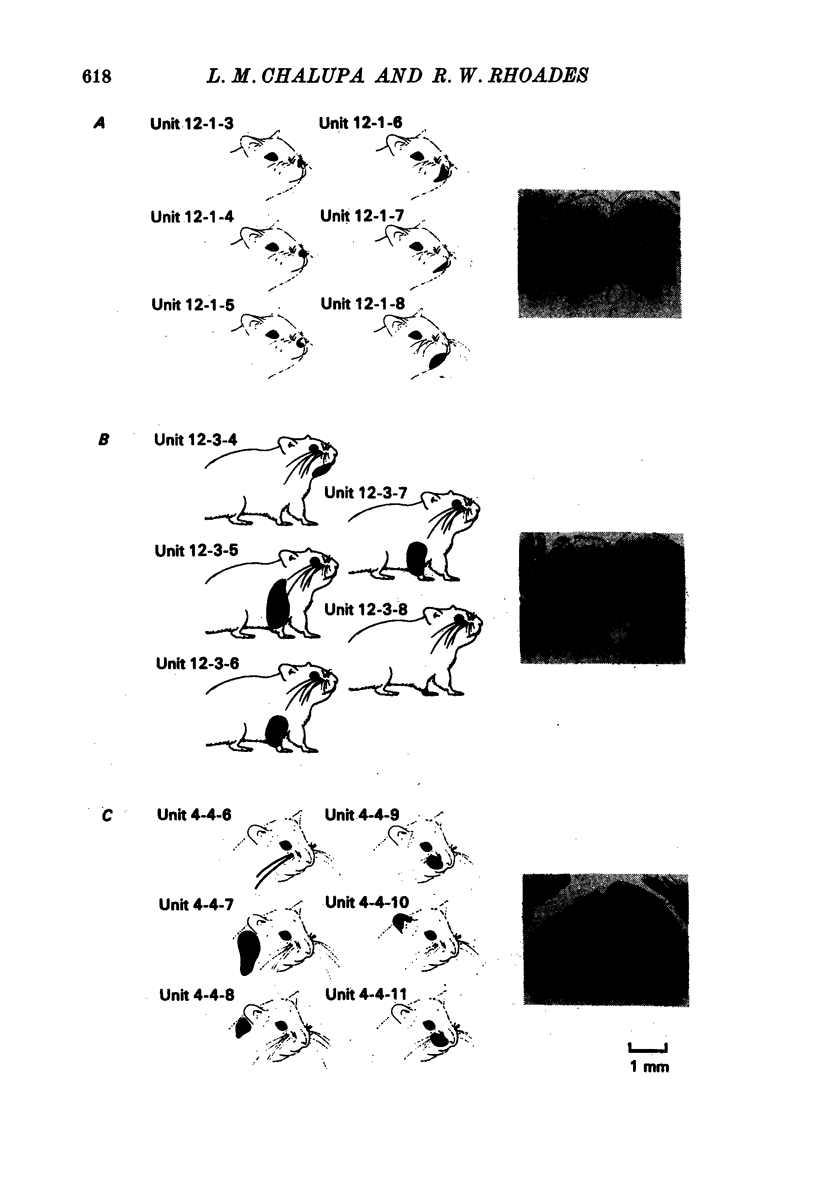
3. Neurones in the superficial layers were exclusively visual, with small receptive-fields, and generally did not show response decrements with repeated stimulation. The sizes of the receptive-fields did not vary appreciably as a function of retinal eccentricity.
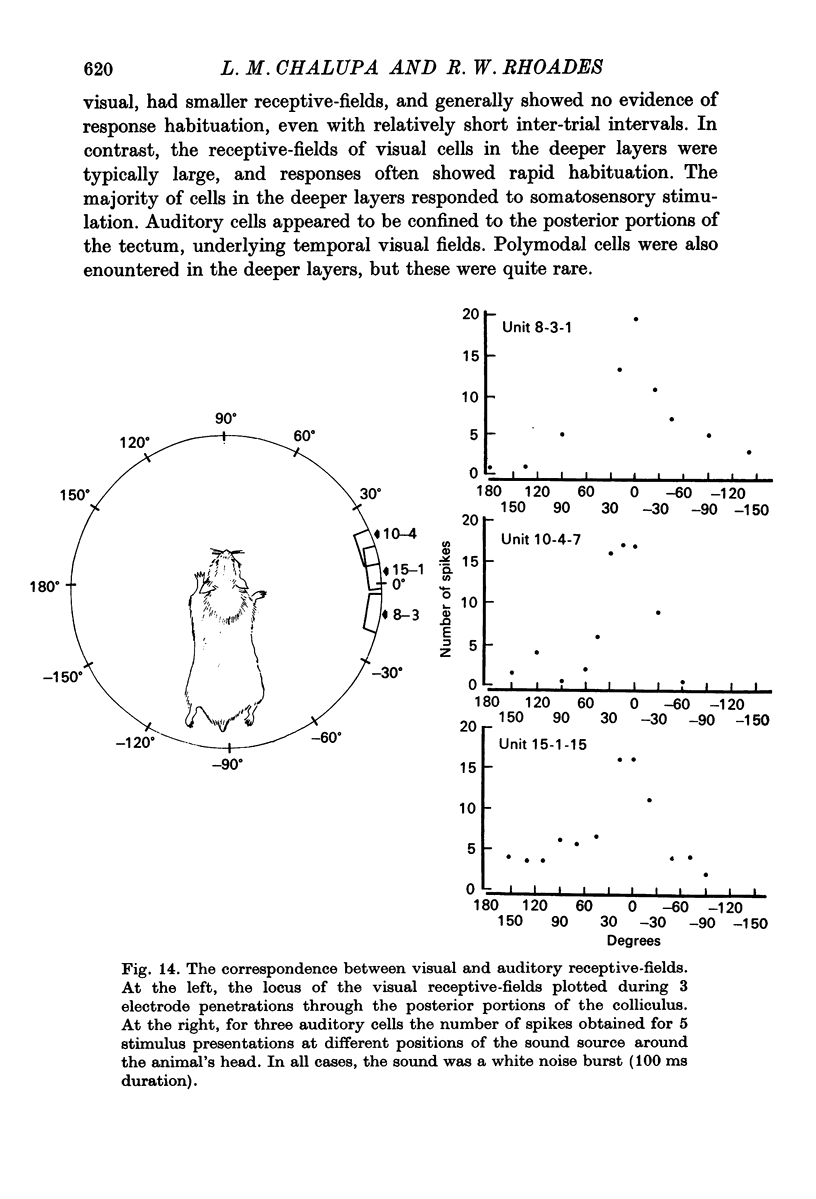
4. In the deeper layers, visual receptive-fields were large, or could not be accurately delimited, and response habituation was often evident. In addition, many cells in the deeper layers of the colliculus responded only to somatosensory stimuli. Far fewer cells, which appeared to be confined to the caudal portions of the colliculus, responded to auditory stimuli. Polymodal cells were also encountered.
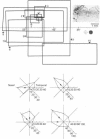
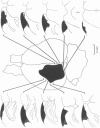
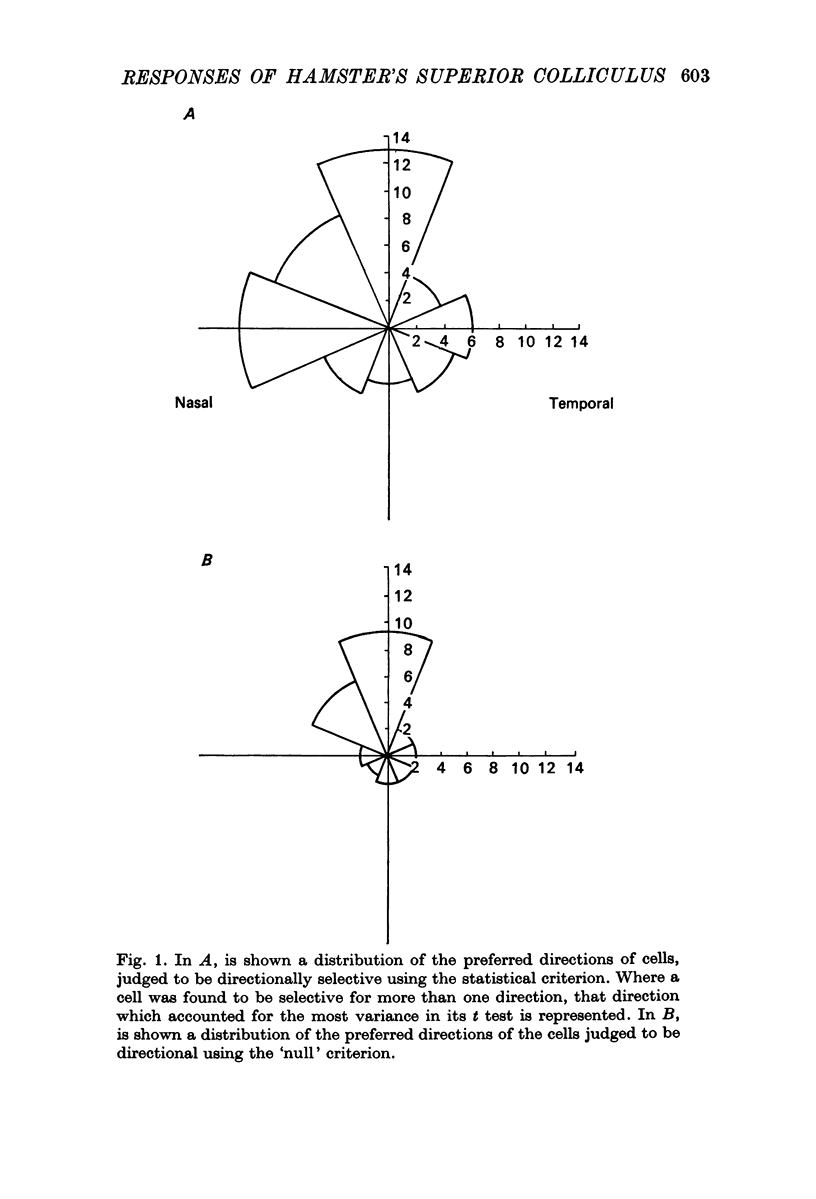
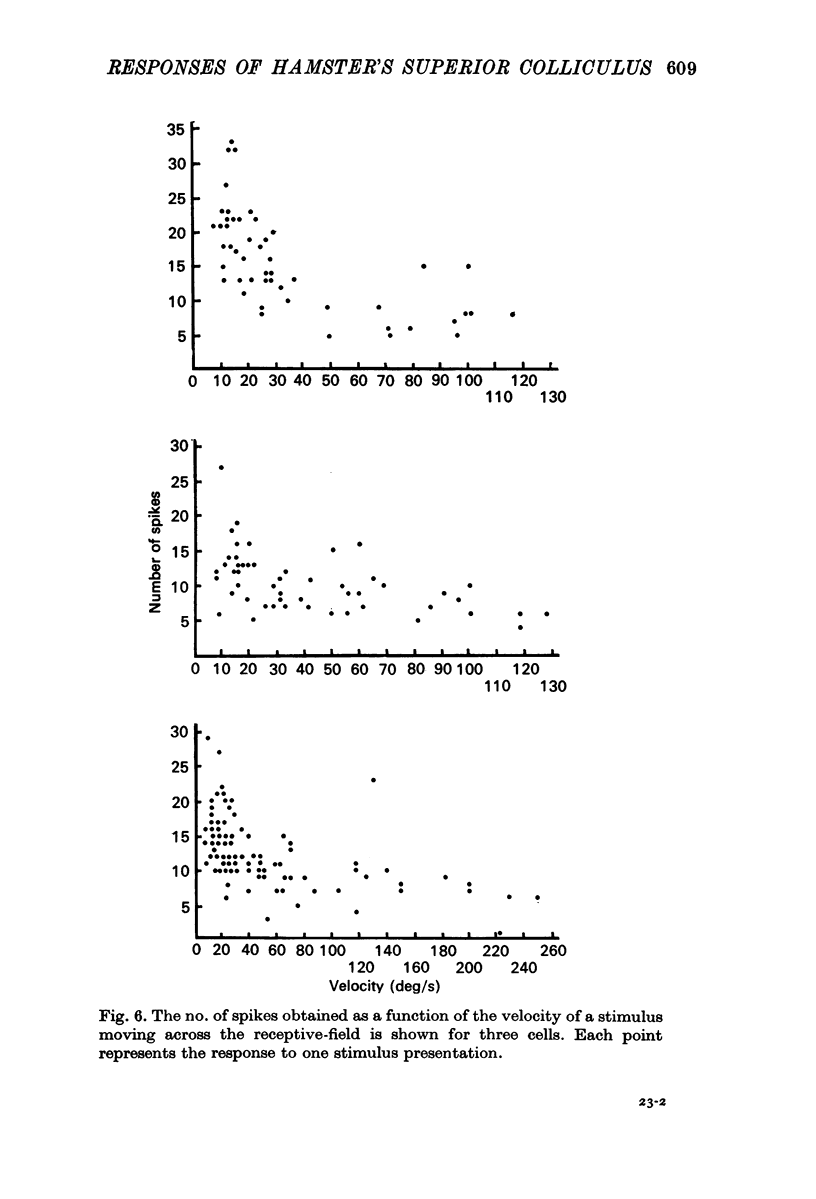
5. Selectivity to opposing directions of movement was tested for ninety-four visual cells. Using a `null' criterion, 27·7% of these cells were judged to be directionally selective. A distribution of the preferred directions of these cells showed a significant preference for movement with an upper-nasal component. With a statistical criterion, 60·6% of these cells were considered to show a significant asymmetry in responding to movement in opposing directions.
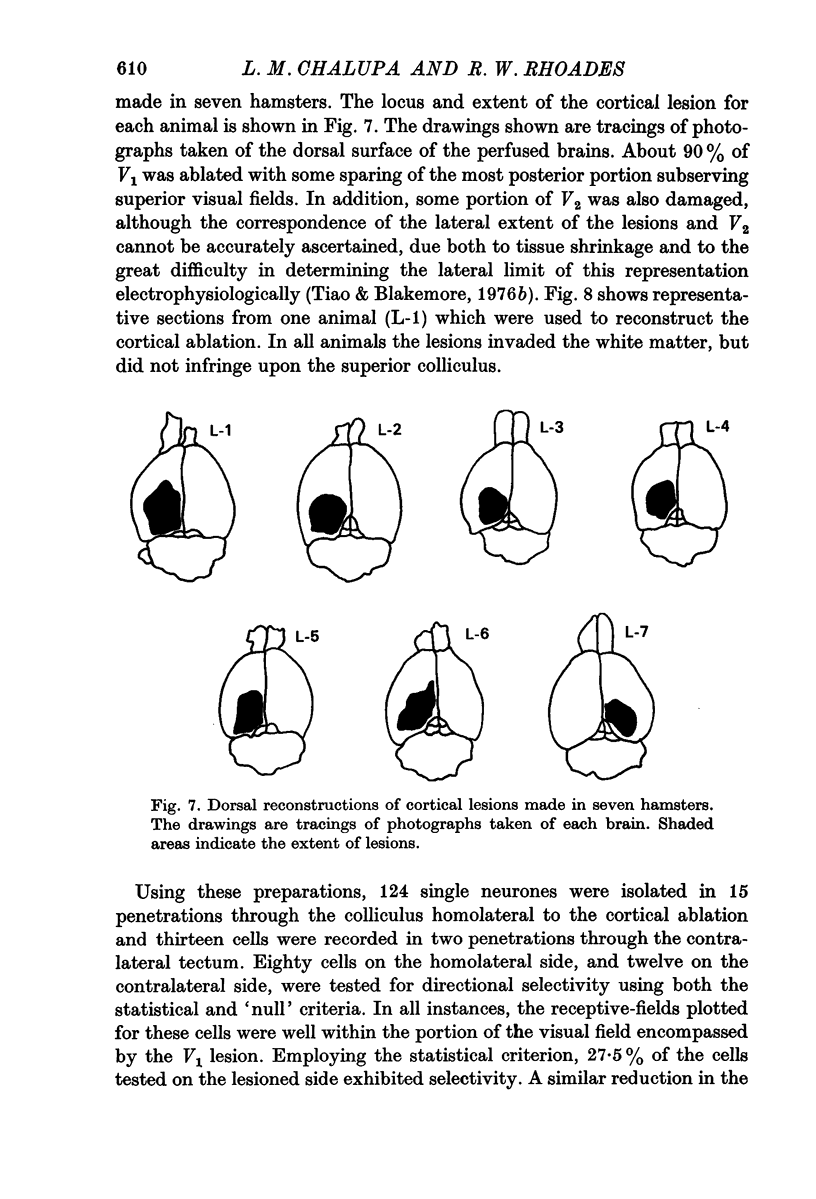
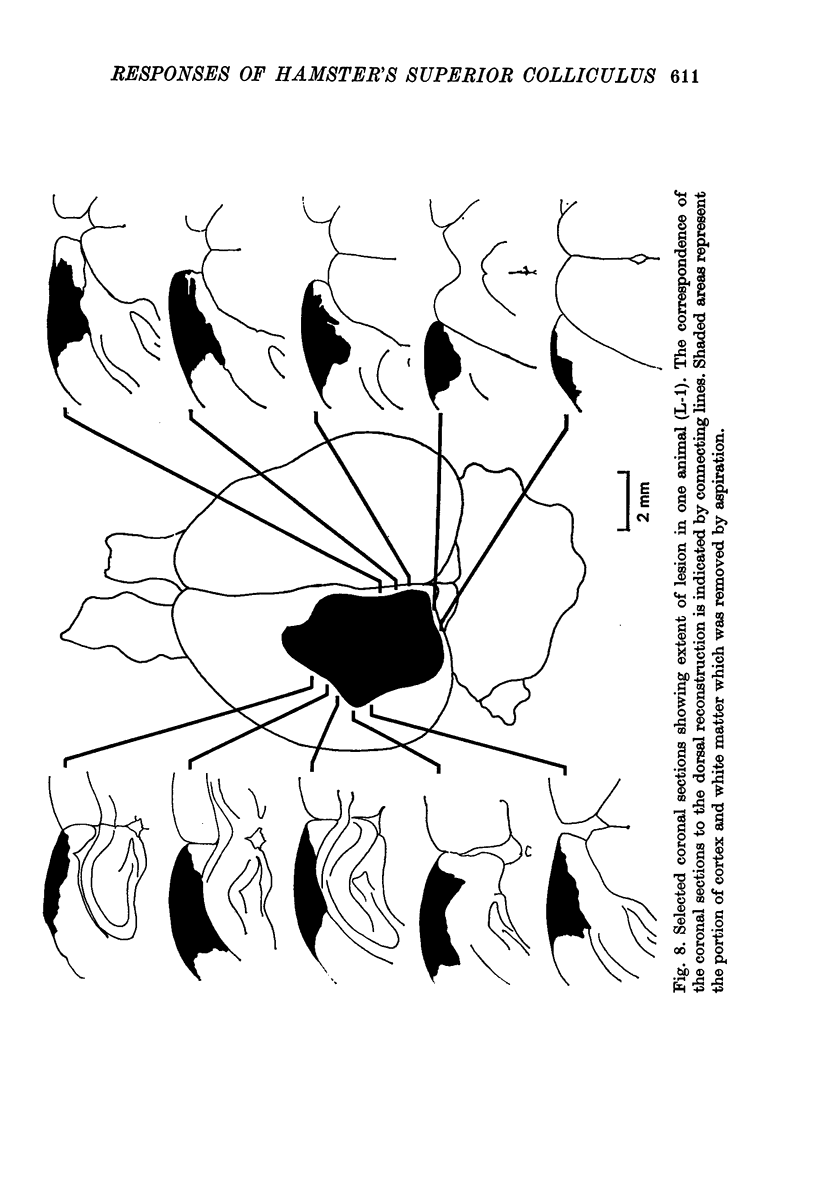
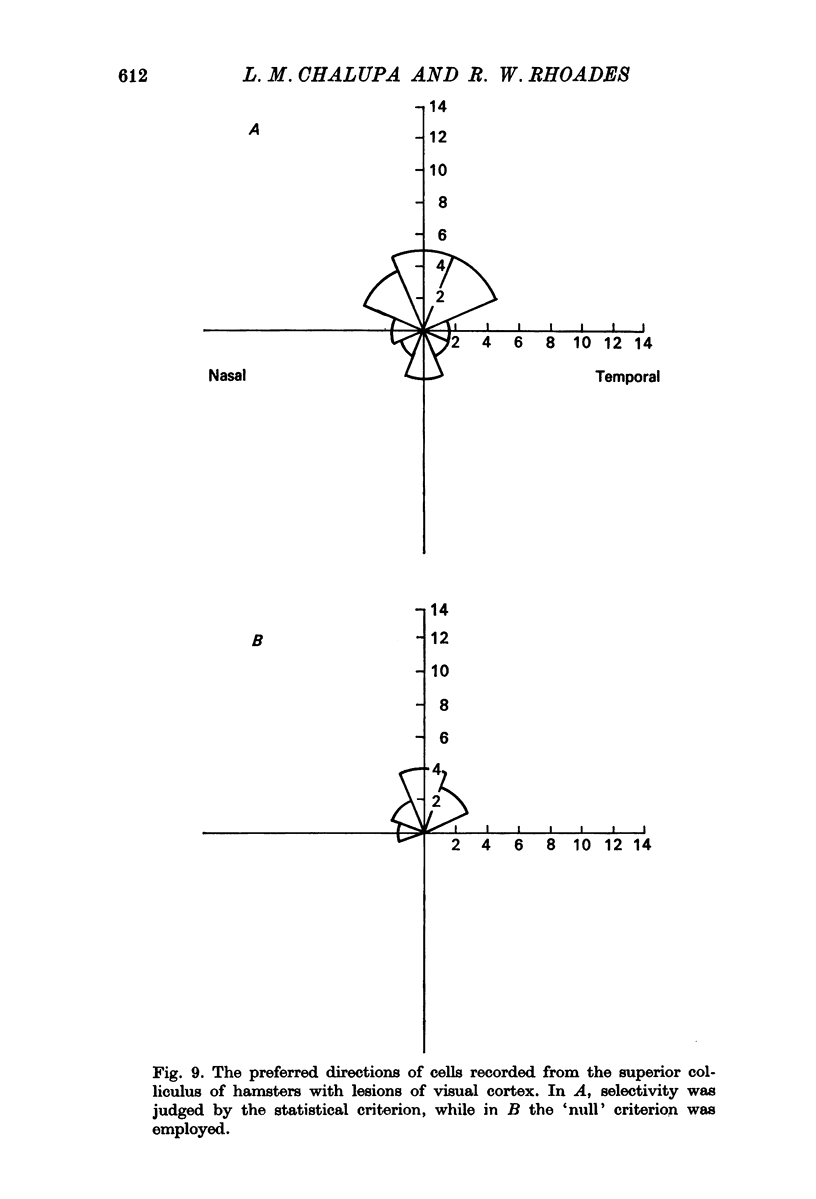
6. Directional selectivity was also tested for ninety-two cells following acute, unilateral, lesions of the visual cortex. For the eighty cells recorded, homolateral to the ablated cortex, 27·5% were judged as directionally selective using the statistical criterion, while 12·5% were selective with the `null' criterion. Of the twelve cells isolated in the colliculus, contralateral to the lesions, seven were judged as directionally selective with the statistical, and three with the `null' criterion.
7. The effects of visual cortical lesions upon directional selectivity appeared to be confined to cells in the superficial layers of the colliculus. It was suggested that directional selectivity of many cells in the superficial layers of the tectum of the hamster is organized cortically.
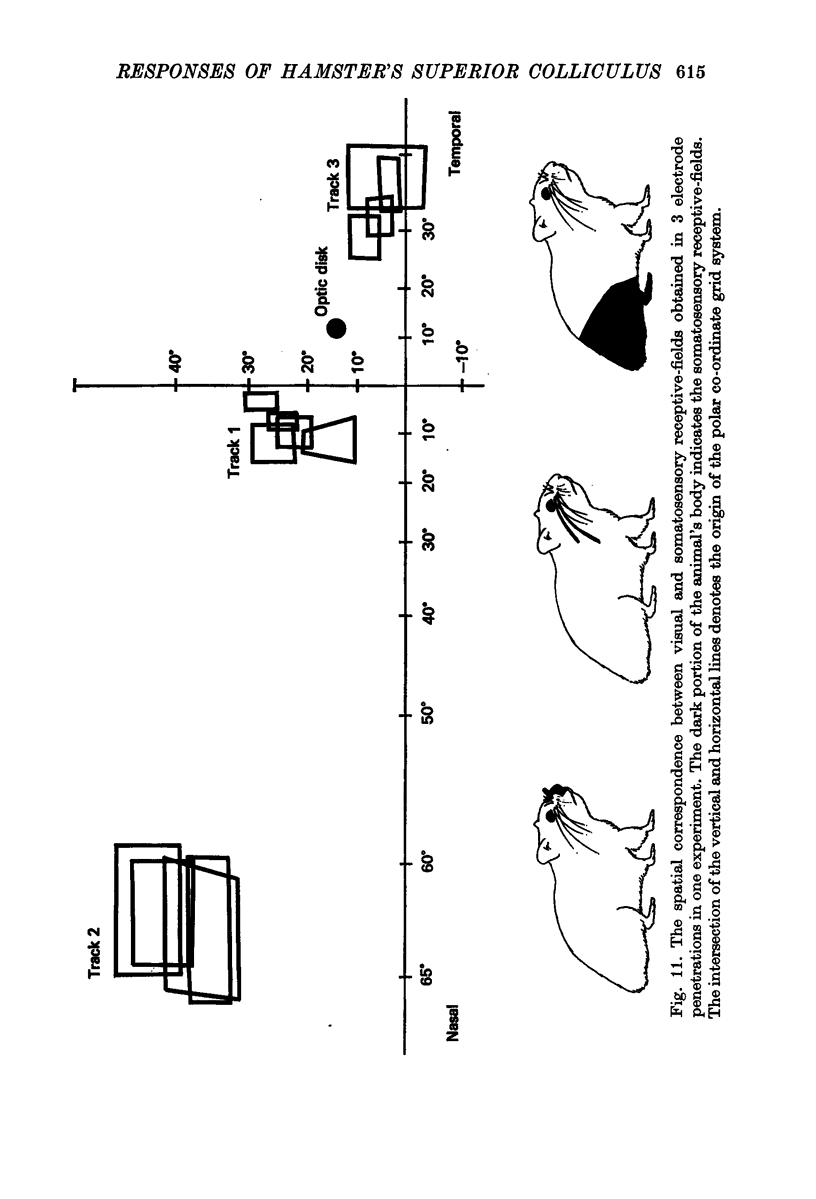
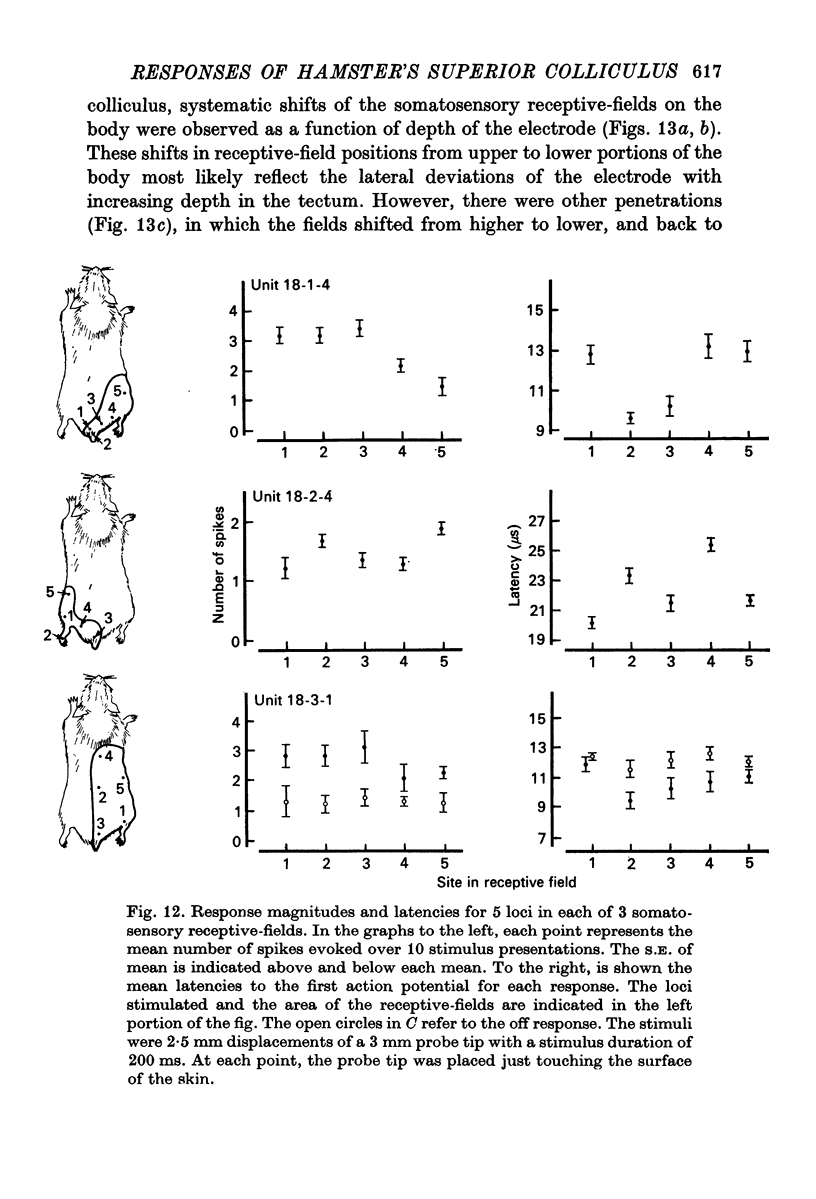
8. A clear spatial correspondence was observed for the receptive-fields of visual, somatosensory, and auditory neurones.
9. As has been suggested for other species, the hamster's superior colliculus appears to play an important role in orienting the animal toward visual, somatosensory, and auditory stimuli.
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. V., Symmes D. The superior colliculus and higher visual functions in the monkey. Brain Res. 1969 Mar;13(1):37–52. doi: 10.1016/0006-8993(69)90142-5. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965 Jun;178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman N., Cynader M. Comparison of receptive-field organization of the superior colliculus in Siamese and normal cats. J Physiol. 1972 Jul;224(2):363–389. doi: 10.1113/jphysiol.1972.sp009900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman N., Cynader M. Receptive fields in cat superior colliculus after visual cortex lesions. J Physiol. 1975 Feb;245(1):261–270. doi: 10.1113/jphysiol.1975.sp010844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynader M., Berman N. Receptive-field organization of monkey superior colliculus. J Neurophysiol. 1972 Mar;35(2):187–201. doi: 10.1152/jn.1972.35.2.187. [DOI] [PubMed] [Google Scholar]
- Dreher B., Hoffmann K. P. Properties of excitatory and inhibitory regions in the receptive fields of single units in the cat's superior colliculus. Exp Brain Res. 1973 Feb 28;16(4):333–353. doi: 10.1007/BF00233427. [DOI] [PubMed] [Google Scholar]
- Dräger U. C., Hubel D. H. Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J Neurophysiol. 1975 May;38(3):690–713. doi: 10.1152/jn.1975.38.3.690. [DOI] [PubMed] [Google Scholar]
- Dräger U. C., Hubel D. H. Topography of visual and somatosensory projections to mouse superior colliculus. J Neurophysiol. 1976 Jan;39(1):91–101. doi: 10.1152/jn.1976.39.1.91. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Jones E. G., Powell T. P. Interrelationships of striate and extrastriate cortex with the primary relay sites of the visual pathway. J Neurol Neurosurg Psychiatry. 1968 Apr;31(2):135–157. doi: 10.1136/jnnp.31.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giolli R. A., Guthrie M. D. Organization of projections of visual areas I and II upon the superior colliculus and pretectal nuclei in the rabbit. Brain Res. 1967 Oct;6(2):388–390. doi: 10.1016/0006-8993(67)90208-9. [DOI] [PubMed] [Google Scholar]
- Glickstein M., Millodot M. Retinoscopy and eye size. Science. 1970 May 1;168(3931):605–606. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- Goldberg M. E., Wurtz R. H. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol. 1972 Jul;35(4):560–574. doi: 10.1152/jn.1972.35.4.560. [DOI] [PubMed] [Google Scholar]
- Gordon B. Receptive fields in deep layers of cat superior colliculus. J Neurophysiol. 1973 Mar;36(2):157–178. doi: 10.1152/jn.1973.36.2.157. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting J. K., Noback C. R. Subcortical projections from the visual cortex in the tree shrew (Tupaia glis). Brain Res. 1971 Jan 8;25(1):21–33. doi: 10.1016/0006-8993(71)90564-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P. Conduction velocity in pathways from retina to superior colliculus in the cat: a correlation with receptive-field properties. J Neurophysiol. 1973 May;36(3):409–424. doi: 10.1152/jn.1973.36.3.409. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Sherman S. M. Effects of early monocular deprivation on visual input to cat superior colliculus. J Neurophysiol. 1974 Nov;37(6):1276–1286. doi: 10.1152/jn.1974.37.6.1276. [DOI] [PubMed] [Google Scholar]
- Humphrey N. K. Responses to visual stimuli of units in the superior colliculus of rats and monkeys. Exp Neurol. 1968 Mar;20(3):312–340. doi: 10.1016/0014-4886(68)90076-9. [DOI] [PubMed] [Google Scholar]
- KLUVER H., BARRERA E. A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol. 1953 Oct;12(4):400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- Kruger L. The topography of the visual projection to the mesencephalon: a comparative survey. Brain Behav Evol. 1970;3(1):169–177. doi: 10.1159/000125469. [DOI] [PubMed] [Google Scholar]
- LaMotte R. H., Mountcastle V. B. Capacities of humans and monkeys to discriminate vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychological measurements. J Neurophysiol. 1975 May;38(3):539–559. doi: 10.1152/jn.1975.38.3.539. [DOI] [PubMed] [Google Scholar]
- Leonard C. M. Degeneration argyrophilia as an index of neural maturation: studies on the optic tract of the golden hamster. J Comp Neurol. 1974 Aug 15;156(4):435–458. doi: 10.1002/cne.901560405. [DOI] [PubMed] [Google Scholar]
- Lund R. D. The occipitotectal pathway of the rat. J Anat. 1966 Jan;100(Pt 1):51–62. [PMC free article] [PubMed] [Google Scholar]
- Masland R. H., Chow K. L., Stewart D. L. Receptive-field characteristics of superior colliculus neurons in the rabbit. J Neurophysiol. 1971 Jan;34(1):148–156. doi: 10.1152/jn.1971.34.1.148. [DOI] [PubMed] [Google Scholar]
- Michael C. R. Visual receptive fields of single neurons in superior colliculus of the ground squirrel. J Neurophysiol. 1972 Nov;35(6):815–832. doi: 10.1152/jn.1972.35.6.815. [DOI] [PubMed] [Google Scholar]
- Mize R. R., Murphy E. H. Alterations in receptive field properties of superior colliculus cells produced by visual cortex ablation in infant and adult cats. J Comp Neurol. 1976 Aug 1;168(3):393–424. doi: 10.1002/cne.901680306. [DOI] [PubMed] [Google Scholar]
- Raczkowski D., Casagrande V. A., Diamond I. T. Visual neglect in the tree shrew after interruption of the descending projections of the deep superior colliculus. Exp Neurol. 1976 Jan;50(1):14–29. doi: 10.1016/0014-4886(76)90231-4. [DOI] [PubMed] [Google Scholar]
- Rhoades R. W., Chalupa L. M. Directional selectivity in the superior colliculus of the golden hamster. Brain Res. 1976 Dec 17;118(2):334–338. doi: 10.1016/0006-8993(76)90721-6. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Tradardi V., Camarda R. Unit responses to visual stimuli in the cat's superior colliculus after removal of the visual cortex. Brain Res. 1970 Dec 1;24(2):336–339. doi: 10.1016/0006-8993(70)90114-9. [DOI] [PubMed] [Google Scholar]
- Rosenquist A. C., Palmer L. A. Visual receptive field properties of cells of the superior colliculus after cortical lesions in the cat. Exp Neurol. 1971 Dec;33(3):629–652. doi: 10.1016/0014-4886(71)90133-6. [DOI] [PubMed] [Google Scholar]
- SPRAGUE J. M., MEIKLE T. H., Jr THE ROLE OF THE SUPERIOR COLLICULUS IN VISUALLY GUIDED BEHAVIOR. Exp Neurol. 1965 Jan;11:115–146. doi: 10.1016/0014-4886(65)90026-9. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Koerner F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1971 Sep;34(5):920–936. doi: 10.1152/jn.1971.34.5.920. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Stryker M., Cynader M., Berman N. Response characteristics of single cells in the monkey superior colliculus following ablation or cooling of visual cortex. J Neurophysiol. 1974 Jan;37(1):181–194. doi: 10.1152/jn.1974.37.1.181. [DOI] [PubMed] [Google Scholar]
- Schneider G. E. Contrasting visuomotor functions of tectum and cortex in the golden hamster. Psychol Forsch. 1967;31(1):52–62. doi: 10.1007/BF00422386. [DOI] [PubMed] [Google Scholar]
- Schneider G. E. Two visual systems. Science. 1969 Feb 28;163(3870):895–902. doi: 10.1126/science.163.3870.895. [DOI] [PubMed] [Google Scholar]
- Schneider G. E. Two visuomotor systems in the Syrian hamster. Neurosci Res Program Bull. 1975 May;13(2):255–257. [PubMed] [Google Scholar]
- Sherman S. M. Visual fields of cats with cortical and tectal lesions. Science. 1974 Jul 26;185(4148):355–357. doi: 10.1126/science.185.4148.355. [DOI] [PubMed] [Google Scholar]
- Sprague J. M. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966 Sep 23;153(3743):1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Sprague J. M. The superior colliculus and pretectum in visual behavior. Invest Ophthalmol. 1972 Jun;11(6):473–482. [PubMed] [Google Scholar]
- Stein B. E., Magalhães-Castro B., Kruger L. Relationship between visual and tactile representations in cat superior colliculus. J Neurophysiol. 1976 Mar;39(2):401–419. doi: 10.1152/jn.1976.39.2.401. [DOI] [PubMed] [Google Scholar]
- Tiao Y. C., Blakemore C. Functional organization in the superior colliculus of the golden hamster. J Comp Neurol. 1976 Aug 15;168(4):483–503. doi: 10.1002/cne.901680404. [DOI] [PubMed] [Google Scholar]
- Tiao Y. C., Blakemore C. Functional organization in the visual cortex of the golden hamster. J Comp Neurol. 1976 Aug 15;168(4):459–481. doi: 10.1002/cne.901680403. [DOI] [PubMed] [Google Scholar]
- Tiao Y. C., Blakemore C. Regional specialization in the golden hamster's retina. J Comp Neurol. 1976 Aug 15;168(4):439–457. doi: 10.1002/cne.901680402. [DOI] [PubMed] [Google Scholar]
- Wickelgren B. G., Sterling P. Influence of visual cortex on receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969 Jan;32(1):16–23. doi: 10.1152/jn.1969.32.1.16. [DOI] [PubMed] [Google Scholar]
- Wickelgren B. G., Sterling P. Influence of visual cortex on receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969 Jan;32(1):16–23. doi: 10.1152/jn.1969.32.1.16. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Toyne M. J. Retino-tectal and cortico-tectal projections in Macaca mulatta. Brain Res. 1970 Dec 18;24(3):395–406. doi: 10.1016/0006-8993(70)90181-2. [DOI] [PubMed] [Google Scholar]
- Wurtz R. H., Goldberg M. E. Activity of superior colliculus in behaving monkey. IV. Effects of lesions on eye movements. J Neurophysiol. 1972 Jul;35(4):587–596. doi: 10.1152/jn.1972.35.4.587. [DOI] [PubMed] [Google Scholar]