Abstract
1. Five subjects trained for 8 weeks on a bicycle ergometer for an average of 40 min/day, four times a week at a work load requiring 80% of the maximal oxygen uptake (V̇O2 max.). V̇O2 max. determinations were performed, and muscle biopsies from the quadriceps femoris muscle (vastus lateralis) were taken before, as well as repeatedly during, the training period. The muscle biopsies were histochemically stained for fibre-types (myofibrillar ATPase) and capillaries (amylase-PAS method), and analysed biochemically for succinate dehydrogenase and cytochrome oxidase activities.
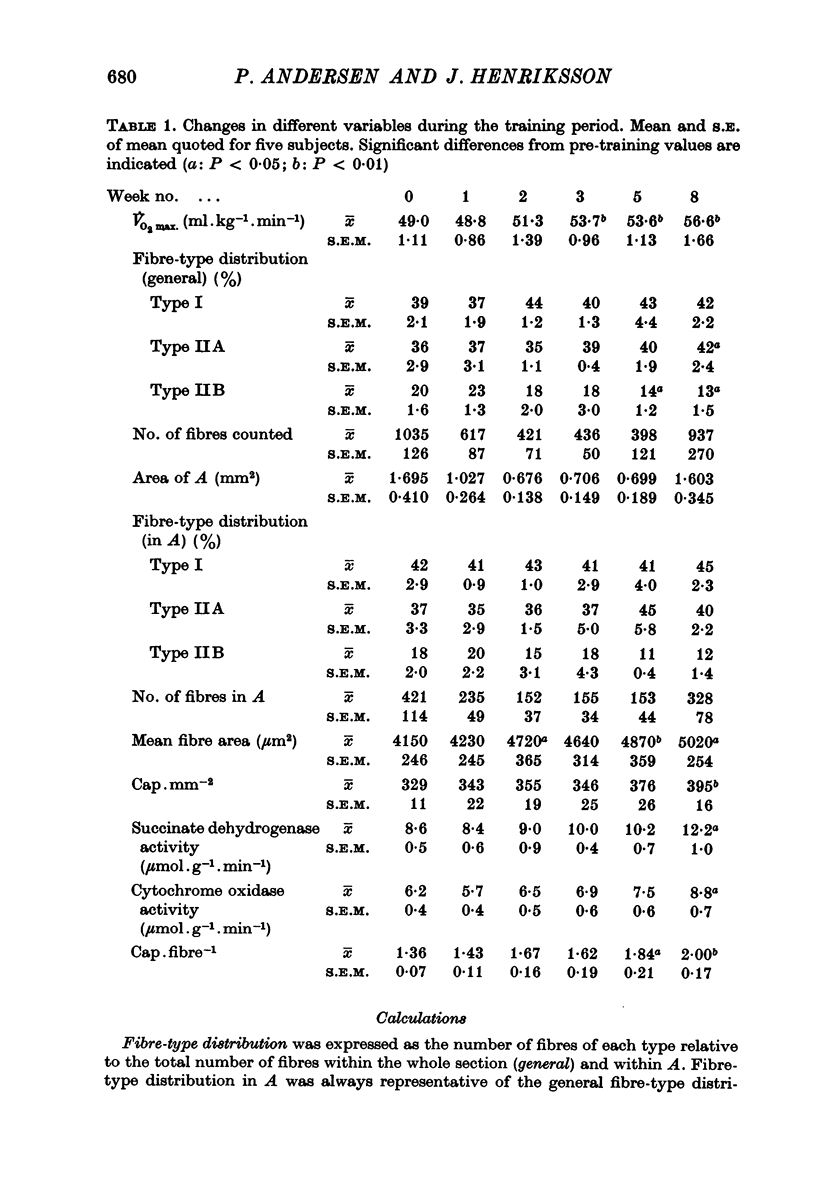
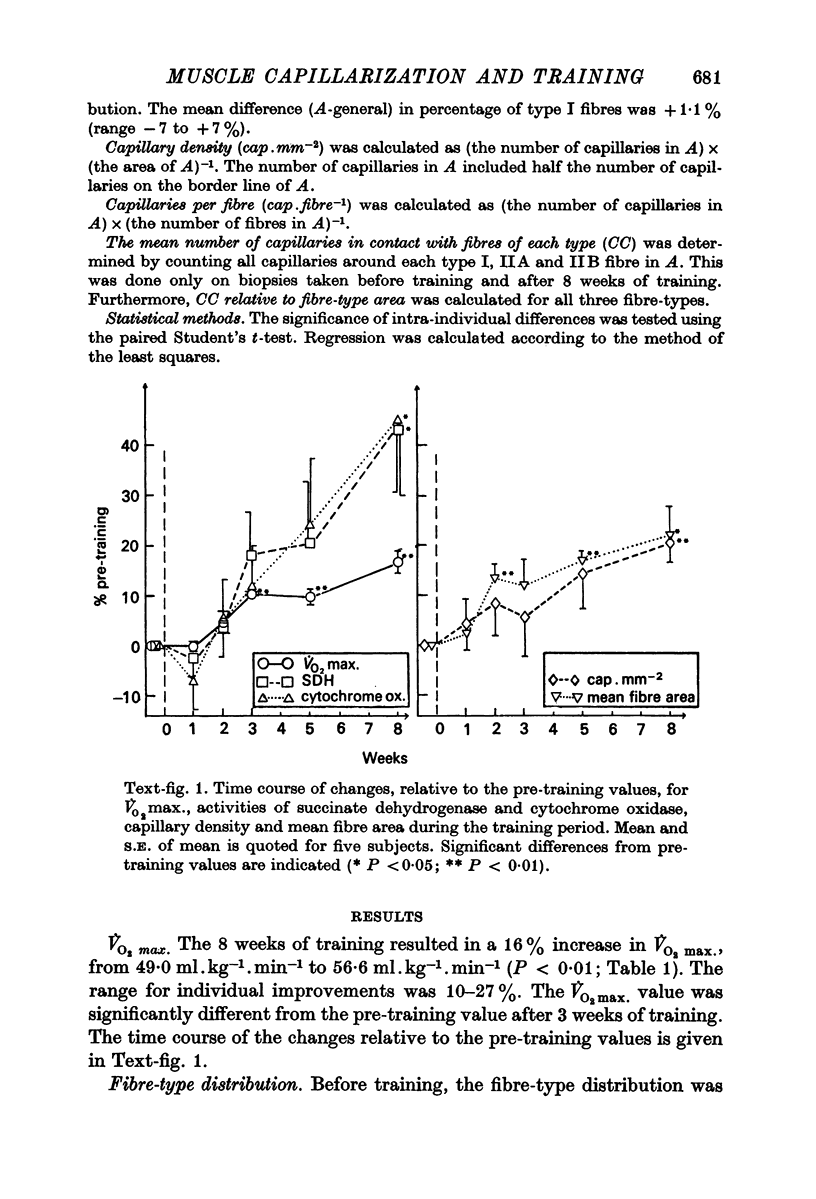
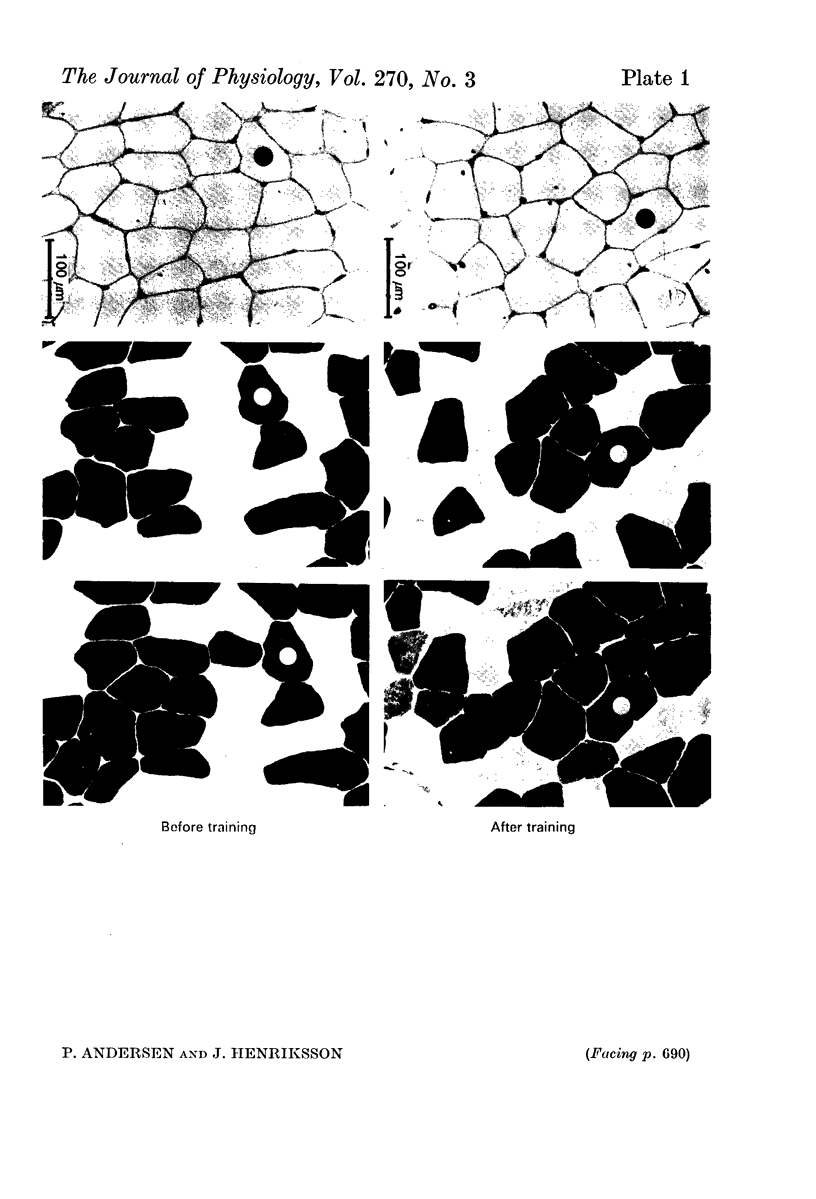
2. The training programme resulted in a 16% increase in V̇O2 max., a 20% increase in capillary density, a 20% increase in mean fibre area, and an approximately 40% increase in the activities of succinate dehydrogenase and cytochrome oxidase.
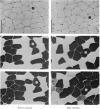
3. The capillary supply to type I, IIA and IIB fibres, expressed as the mean number of capillaries in contact with each fibre-type, relative to fibre-type area, increased equally.
4. The present study shows that endurance training constitutes a powerful stimulus for capillary proliferation in human skeletal muscle.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand. 1975 Oct;95(2):203–205. doi: 10.1111/j.1748-1716.1975.tb10043.x. [DOI] [PubMed] [Google Scholar]
- Andersen P., Henriksson J. Training induced changes in the subgroups of human type II skeletal muscle fibres. Acta Physiol Scand. 1977 Jan;99(1):123–125. doi: 10.1111/j.1748-1716.1977.tb10361.x. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Some comments on the histochemical characterization of muscle adenosine triphosphatase. J Histochem Cytochem. 1969 Jun;17(6):431–432. doi: 10.1177/17.6.431. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Three "myosin adenosine triphosphatase" systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970 Sep;18(9):670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Carrow R. E., Brown R. E., Van Huss W. D. Fiber sizes and capillary to fiber ratios in skeletal muscle of exercised rats. Anat Rec. 1967 Sep;159(1):33–39. doi: 10.1002/ar.1091590106. [DOI] [PubMed] [Google Scholar]
- Costill D. L., Daniels J., Evans W., Fink W., Krahenbuhl G., Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol. 1976 Feb;40(2):149–154. doi: 10.1152/jappl.1976.40.2.149. [DOI] [PubMed] [Google Scholar]
- Cotter M., Hudlická O., Pette D., Staudte H., Vrbová G. Changes of capillary density and enzyme pattern in fast rabbit muscles during long-term stimulation. J Physiol. 1973 Apr;230(1):34P–35P. [PubMed] [Google Scholar]
- Edström L., Ekblom B. Differences in sizes of red and white muscle fibres in vastus lateralis of musculus quadriceps femoris of normal individuals and athletes. Relation to physical performance. Scand J Clin Lab Invest. 1972 Oct;30(2):175–181. doi: 10.3109/00365517209081108. [DOI] [PubMed] [Google Scholar]
- Edström L., Torlegard K. Area estimation of transversely sectioned muscle fibres. Z Wiss Mikrosk. 1969 Jun;69(3):166–178. [PubMed] [Google Scholar]
- Essén B., Jansson E., Henriksson J., Taylor A. W., Saltin B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol Scand. 1975 Oct;95(2):153–165. doi: 10.1111/j.1748-1716.1975.tb10038.x. [DOI] [PubMed] [Google Scholar]
- GIOVACCHINI R. P., SMITH R. D. The vascularity of some red and white muscles of the rabbit. Acta Anat (Basel) 1956;28(4):342–358. doi: 10.1159/000141151. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Etlinger J. D., Goldspink D. F., Jablecki C. Mechanism of work-induced hypertrophy of skeletal muscle. Med Sci Sports. 1975 Fall;7(3):185–198. [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Saltin B., Saubert C. W., 4th, Sembrowich W. L., Shepherd R. E. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973 Jan;34(1):107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Saubert C. W., 4th, Piehl K., Saltin B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol. 1972 Sep;33(3):312–319. doi: 10.1152/jappl.1972.33.3.312. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Piehl K., Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974 Aug;241(1):45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson J., Reitman J. S. Quantitative measures of enzyme activities in type I and type II muscle fibres of man after training. Acta Physiol Scand. 1976 Jul;97(3):392–397. doi: 10.1111/j.1748-1716.1976.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Henriksson J., Reitman J. S. Time course of changes in human skeletal muscle succinate dehydrogenase and cytochrome oxidase activities and maximal oxygen uptake with physical activity and inactivity. Acta Physiol Scand. 1977 Jan;99(1):91–97. doi: 10.1111/j.1748-1716.1977.tb10356.x. [DOI] [PubMed] [Google Scholar]
- Henriksson J. Training induced adaptation of skeletal muscle and metabolism during submaximal exercise. J Physiol. 1977 Sep;270(3):661–675. doi: 10.1113/jphysiol.1977.sp011974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermansen L., Hultman E., Saltin B. Muscle glycogen during prolonged severe exercise. Acta Physiol Scand. 1967 Oct-Nov;71(2):129–139. doi: 10.1111/j.1748-1716.1967.tb03719.x. [DOI] [PubMed] [Google Scholar]
- Hermansen L., Wachtlova M. Capillary density of skeletal muscle in well-trained and untrained men. J Appl Physiol. 1971 Jun;30(6):860–863. doi: 10.1152/jappl.1971.30.6.860. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919 May 20;52(6):409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J. V., Edgerton V. R., Barnard R. J. Capillarity of red, white and intermediate muscle fibers in trained and untrained guinea pigs. Experientia. 1970 Nov 15;26(11):1222–1223. doi: 10.1007/BF01897977. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Plyley M. J., Groom A. C. Geometrical distribution of capillaries in mammalian striated muscle. Am J Physiol. 1975 May;228(5):1376–1383. doi: 10.1152/ajplegacy.1975.228.5.1376. [DOI] [PubMed] [Google Scholar]
- Prince F. P., Hikida R. S., Hagerman F. C. Human muscle fiber types in power lifters, distance runners and untrained subjects. Pflugers Arch. 1976 May 6;363(1):19–26. doi: 10.1007/BF00587397. [DOI] [PubMed] [Google Scholar]
- ROMANUL F. C. CAPILLARY SUPPLY AND METABOLISM OF MUSCLE FIBERS. Arch Neurol. 1965 May;12:497–509. doi: 10.1001/archneur.1965.00460290053007. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-NIELSEN K., PENNYCUIK P. Capillary density in mammals in relation to body size and oxygen consumption. Am J Physiol. 1961 Apr;200:746–750. doi: 10.1152/ajplegacy.1961.200.4.746. [DOI] [PubMed] [Google Scholar]
- VALDIVIA E. Total capillary bed in striated muscles of guinea pigs native to the Peruvian mountains. Am J Physiol. 1958 Sep;194(3):585–589. doi: 10.1152/ajplegacy.1958.194.3.585. [DOI] [PubMed] [Google Scholar]
- Varnauskas E., Björntorp P., Fahlén M., Prerovský I., Stenberg J. Effects of physical training on exercise blood flow and enzymatic activity in skeletal muscle. Cardiovasc Res. 1970 Oct;4(4):418–422. doi: 10.1093/cvr/4.4.418. [DOI] [PubMed] [Google Scholar]